新型一维配位聚合物{[Cu(4,4′-bipy)Cl]n}的溶剂热合成及其晶体结构*
2010-11-26葛成敏王寅光张淑华
冯 超, 葛成敏, 王寅光, 张淑华
(桂林理工大学 化学与生物工程学院,广西 桂林 541004)
过渡金属聚合物具有明确有序的一维或者多维微观结构以及独特的宏观特性,选取适当的过渡金属离子、配体以及合成方法可调控配位聚合物的结构,进而调控其性质[1~10]。配位聚合物由于结构的多样性和在催化、化学吸附、磁性和电子导体等方面的功能特性, 近年来已受到研究人员的广泛关注[11]。在配位聚合物的合成研究中,配体是影响结构特征的决定性因素之一。配体的给体基团性质、配体的齿数、配体点间的间距、配体间的连接基团以及配体异构等诸多因素都可能对配位聚合物的最终结构产生影响[12]。但是在设计和合成金属有机骨架时,控制维度仍然是一个重大挑战。除了溶剂、温度、金属与配体的配比、模板和平衡电荷的离子外,最终的结构还会受到如氢键和π-π相互作用[13]等因素的微妙影响。尽管配体的空间结构对配合物领域和催化活性金属中心有巨大的影响力,但其影响力在超分子结构方面还没有得到充分的重视[14,15]。在4,4′-联吡啶(4,4′-bipy)为有机配体的合成体系中,加入客体小分子的研究较多[16,17],但单独以4,4′-bipy构筑的配位聚合物的研究报道较少[18~21]。
本文以4,4′-bipy和CuCl2·2H2O为原料,在DMF中用溶剂热法合成了新型一维配位聚合物——[Cu(4,4′-bipy)Cl]n(1),其结构经IR,元素分析和X-射线单晶衍射表征。
1 实验部分
1.1 仪器与试剂
Bruker Vector 22 FT-IR型红外光谱仪(KBr压片);Perkin-Elmer 240C型元素分析仪;Bruker SMART CCD型X-射线单晶衍射仪。所用试剂均为分析纯,使用前未作进一步纯化处理。
1. 2 1的合成
在聚四氟乙烯内衬不锈钢反应釜(15 mL)中加入CuCl2·2H2O 17 mg(0.1 mmol), 4,4′-bipy 15.6 mg(0.1 mmol)和DMF 10 mL,搅拌下滴加三乙胺,调至pH 8左右。置入烘箱,于120 ℃反应120 h。自然冷却至室温,过滤,滤饼自然晾干得紫红色晶体1,产率51%(基于Cu); IRν: 3 459, 3 044, 1 560, 1 528, 1 478, 1 410, 1 214, 797, 723, 629, 474 cm-1; Anal.calcd for 1: C 40.07, H 3.16, N 10.97; found C 40.03, H 3.22, N 10.89。
1.3 晶体结构测定

2 结果与讨论
1的分子结构见图1,主要键长和键角见表1。由图1可见,在1的结构单元中包括两个Cu+,四个4,4′-bipy分子,两个Cl-。 Cu+具有四面体的配位几何构型,与其配位的4个原子分别是两个4,4′-bipy分子中的两个N原子,两个Cl原子。Cu-N键长从0.198 3(2) nm~0.199 1(2) nm; Cu-Cl键长为0.241 46 (9) nm,相应的键角(N2-Cu1-Cl1,N1-Cu1-N2, N1-Cu1-Cl1和N1-Cu1-Cl1i)分别为99.4°, 126.5°, 111.2°和108.30(8)°。其中两个Cl-起到桥联作用连接两个Cu+, Cu1-Cu1i键长[0.274 7(1) nm]介于羧酸的双核铜(0.259 nm~0.275 nm)的铜铜距离之间[24]。说明两个Cu+间有相互作用。为更好的精密堆积,在4,4′-bipy分子中,两个吡啶环间的二面角是22.06°。
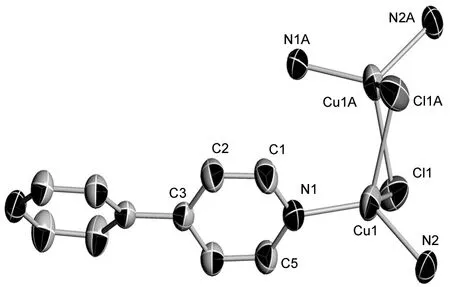
图 1 1的分子结构图Figure 1 Molecular structure of 1
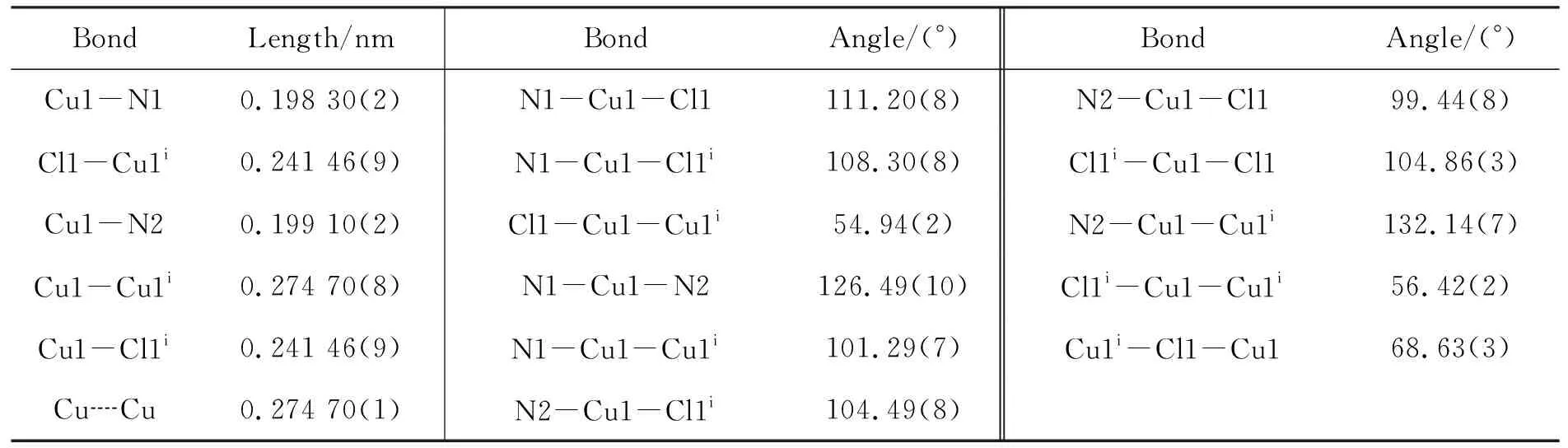
BondLength/nmBondAngle/(°)BondAngle/(°)Cu1-N10.198 30(2)N1-Cu1-Cl1111.20(8)N2-Cu1-Cl199.44(8)Cl1-Cu1i0.241 46(9)N1-Cu1-Cl1i108.30(8)Cl1i-Cu1-Cl1104.86(3)Cu1-N20.199 10(2)Cl1-Cu1-Cu1i54.94(2)N2-Cu1-Cu1i132.14(7)Cu1-Cu1i0.274 70(8)N1-Cu1-N2126.49(10)Cl1i-Cu1-Cu1i56.42(2)Cu1-Cl1i0.241 46(9)N1-Cu1-Cu1i101.29(7)Cu1i-Cl1-Cu168.63(3)Cu┈Cu0.274 70(1)N2-Cu1-Cl1i104.49(8)
i-x, -y+3/2, z
由图2(1的2-D层状结构图)可见,在a+c轴方向,两个Cu+和两个4,4′-bipy分子经桥连作用形成一个大的20元环,并且延伸形成二维层状网格,层与层之间互相平行(图3)。这些由非常规的C-H┈Cl氢键(C7┈Cl1=0.093 00 nm, C7-H17┈Cl1夹角为151.70°)构筑的二维层与另一方向的二维层形成互穿插结构而形成三维网状结构。运用OLEX[25]软件,如果氯桥简化为蓝线,4,4′-bipy简化为红线,四配位的Cu简化为一个棕色点,则二维层可简化为一个4.82的拓扑(图4)。

图 2 1沿b轴方向的2-D层状网格图Figure 2 2-D network structure of 1 along b axis

图 3 1沿c轴方向的堆积图Figure 3 Packing diagram of 1 along c axis
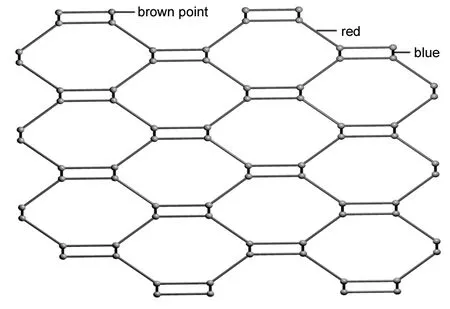
图 4 1的拓扑结构图Figure 4 Topology structure of 1
3 结论
应用溶剂热法合成了新型的一维配位聚合物[Cu(4,4′-bipy)Cl]n,单晶X-射线研究表明,聚合物二维4.82的拓扑通过互相穿插形成三维空间结构。虽然形成了20元大环结构,但由于互相穿插自填补而使整个化合物并没有空洞存在。
[1] Hegetschweiler K, Morgenstern B, Zubieta J,etal. Strong ferromagnetic interaction in [V9O14(H2taci)2]:An unprecedented large spin ground state for a vanyl cluster[J].Angew Chem,Int Ed,2004,43:3436-3439.
[2] Morgenstein B, Steinhauser S, Hegetschweiler K,etal. Complex formation of vanadium(Ⅳ) with 1,3,5-triamino-1,3,5-trideoxy-cis-inositol and related ligands[J].Inorg Chem,2004,43:3116-3126.
[3] Applegrarth L, Goeta A E, Steed J W. Influence of hydrogen bonding on coordinaton polymer assembly[J].Chem Commun,2005:2405-2406.
[4] Manna S C, Ribas J, Zangrando E,etal. Supramolecular networks of dinuclear copper(Ⅱ):Synthesis,crystal structure and magnetic study[J].Inorg Chim Acta,2007,360:2589-2597.
[5] Masaoka S, Tanaka D, Nakanishi Y,etal. Reaction-temprature-dependent supramolecular isomerism of coordination network based on the organometallic building block[CuI2(μ2-BQ)(μ2-OAc)2][J].Angew Chem,Int Ed,2004,43:2530-2534.
[6] Sun D, Ke Y, Mattox T M,etal. Temperature-dependent supramolecular stereoisomerism in porous copper coordination networks based on a design carboxylate ligand[J].Chem Commun,2005:5477-5449.
[7] Chiang R K. Synthesis and structure of a one-dimensional cobalt phosphate:(R,S)-(C5H14H2)Co(HPO4)2[J].J Slid State Chem,2000,153:180-184.
[8] Fujita M, Yoshizawa M, Umemoto K,etal. Molecular paneling via coordination[J].Chem Commun,2001:509-518.
[9] Mana S C, Zangrando E, Ribas J,etal. Cobalt(Ⅱ)-(dpyo)-dicarboxylate networks:Unique H-bonded assembly and rare bridging mode of dpyo in one of them [dpyo=4,4′-dipyridyl-N,N′-dioxde][J].Dalton Trans,2007:1383-1391.
[10] Seidel S R, Stang P J. High-symmetry coordination cages via self-assembly[J].Acc Chem Res,2002,35:972-983.
[11] Ye B H, Tong M L, Chen X M. Metal-organic molecular architectures with 2,2′-bipyridyl-like and carboxylate ligands[J].Coord Chem Rev,2005,249:545-565.
[12] Hong M C, Chen R, Liang W P. Inorganic Chemistry of 21th Century[M].Beijing:Science Press,2005.
[13] Zhao B, Cheng P, Chen X Y,etal. Design and synthesis of 3d~4fmetal-based zeolite-type materials with a 3D nanotubular structure encapsulated “water” pipe[J].J Am Chem Soc,2004,126:3012-3013.
[14] Zhang J P, Chen X M. Crystal engineering of binary metal imidazolate and triazolate frameworks[J].Chem Commum,2006:1689-1699.
[15] Kim J, Chen B, Reineke T M,etal. Assembly of metal-organic frameworks from large organic and ino-ganic secondary building units:New examples and simplifying principles for complex structures[J].J Am Chem Soc,2001,123:8239-8247.
[16] Rao C N R, Natarajan S, Vaidhyanathan R. Metal carboxylates with open architectures angew[J].Chem,Int Ed[J].2004,43:1466-1496.
[17] Hagrman P J, Hagrman D, Zubieta J. Organic-inorganic hybrid materials:From simple coordination polymers to organodiamine-templated molybdenum oxides angew[J].Chem Int Ed,1999,38:2638-2684.
[18] Lu J Y, Lawandy M A, Li J. A new type of two-dimensional metal coordination systems:Hydrothermal synthesis and properties of the first oxalate——bpy mixed-ligand framework[M(ox)(bpy)](M=Fe(Ⅱ),Co(Ⅱ),Ni(Ⅱ),Zn(Ⅱ);ox=C2O42-;bpy=4,4′-bipyridine)[J].Inorg Chem,1999,38:2695-2704.
[19] Zheng L M, Fang X, Li K H,etal. Syntheses,crystal structures and magnetic properties of two novel layered compounds:[Fe3(C2O4)3(4,4′-bpy)4] and [Co(C2O4)(4,4′-bpy)](4,4′-bpy=4,4′-bipyridine)[J].J Chem Soc,Dalton Trans,1999,14:2311-2316.
[20] Hao N, Shen E H, Li Y G. Hydrothermal synthesis and crystal structure of a layered coordination polymer:[Zn3(C2O4)3(4,4′-bipy)4]n(4,4′-bipy=4,4′-bipyridine)[J].J Molecular Structure,2004,691:273-277.
[21] Castillo O, Alonso J, Garcia-Couceiro U,etal. A 2D polymer constructed through bridging oxalato and 4,4′-bipyridine ligands:Crystal structure and magnetic behavior of [Cu3(μ-ox)3(μ-4,4′-bpy)2(4,4′-bpy)2]n[J].Inorg Chem Commun,2003,6:803-806.
[22] Bruker SMART(Version 5. 622) , SAINT(Version 6.02a), SADABS(Version 2. 03 ) and SHELXTL(Version 6.10)[K].Bruker AXS Inc,Madison,Wisconsin,USA,2000.
[23] Sheldrick G M. SHELXS97 and SHELXL97 Software,Reference Manual[K].University of Göttingen,Germany,1997.
[24] Catterick J, Thornton P. Crystal and molecular structure of tera-μ-benzoato-bisquinolinedicobalt(Ⅱ),a binuclear cobalt(Ⅱ) carboxylate[J].Adv Inorg Chem Radiochem,1977,20:291.
[25] Dolomanov O V, Blake A J, Champness N R. Schöder M. OLEX:New software for visualization and analysis of extended crystal structures[J].J Appl Crystallogr,2003,36:1283-1284.
