MR多参数成像评估慢性胰腺炎临床分级的价值
2010-11-23柏梅陆建平赖晓伟
柏梅 陆建平 赖晓伟
·论著·
MR多参数成像评估慢性胰腺炎临床分级的价值
柏梅 陆建平 赖晓伟
目的探讨应用多种MRI技术评估慢性胰腺炎(CP)临床分级的价值。方法纳入经病理和临床随访证实的65例CP患者,按M-annheim分级分为轻度组(14例)、中度组(37例)和进展组(14例),并以20例健康志愿者作为对照。在上腹部常规T1WI及T2WI抑脂扫描后,进行胰腺MRCP检查及胰腺动态MR检查。测量T1WI、T2WI加权扫描的胰腺实质信号及肝脏信号,获取它们的比值(rT1、rT2),根据MRCP测量主胰管最大直径(MPD),并对胰腺病变进行评估、分类;测量动态MR增强时胰腺实质信号值,并计算强化率;ROC分析MRI表现与CP临床分级的相关性。结果正常、轻度、中度和进展组rT1分别为0.98±0.27、0.84±0.12 、0.81±0.16和0.75±0.24,中度、进展组较正常组明显降低(P﹤0.01);rT2分别为1.28±0.30、1.46±0.44、1.46±0.55和1.76±0.72,各组间无统计学差异;MPD为(2.0±0.6)mm、(5.4±2.4)mm、(6.5±3.3)mm和(8.1±4.1)mm,各组间差异显著(P值均﹤0.01)。轻度、中度和进展组的剑桥重度分级分别有4例(29%)、33例(90%)和13例(93%),差异显著(P﹤0.01);胰管结石分别有2例(14%)、11例(30%)和5例(36%),胰腺假性囊肿分别有0例、6例(16%)和3例(21%),胰腺萎缩分别有4例( 29%)、 22例(60%)和10例(71%),各组间均无统计学差异。正常、轻度、中度和进展组的胰腺动态增强扫描实质期与动脉期胰腺信号强化率比值(P/A)分别为0.88±0.08、1.10±0.08、1.37±0.15和1.48±0.53,各组间差异显著(P﹤0.05)。rT1值、剑桥分级、胰管直径及P/A比值与临床分级均有相关性(r值分别为0.34、0.41、0.62、-0.43)。ROC分析显示,MPD>2.5 mm、rT1<0.8、P/A>0.8诊断CP均有较好的敏感性和特异性,三者结合时诊断CP的特异性可提高到95%。结论应用磁共振的 T1WI、MRCP及动态增强检查能准确、良好地评估CP的严重程度,其中MRCP的敏感性及特异性最高,其次是动态增强检查与T1平扫。
胰腺炎,慢性; 磁共振成像; 动态增强
随着慢性胰腺炎(CP)的病因学、流行病学、遗传学以及影像学技术的发展,CP的临床分类越来越完善[1]。德国海德堡大学Schneider等[2]建立的M-ANNHEIM分类系统能简单、客观、精确和相对非侵害性地对CP进行临床分级,并采纳了CP剑桥分级系统[3]。随着成像速度的加快和图像信噪比的提高,MRI在CP的影像检查中所起的作用逐渐加大,其多参数成像更是为胰腺病变提供了丰富的检查序列。本研究分析临床不同严重程度的慢性胰腺炎(CP)的各种MRI表现,评估MRI多种成像技术在CP临床分级中的价值。
材料与方法
一、临床资料
收集第二军医大学长海医院2008年4月至2009年10月确诊的CP患者65例,以20例健康者作为正常组。按M-annheim评分将CP患者分为轻度、中度、进展3组,分别有14例、37例和14例。
二、扫描方法
所有扫描均采用Siemens Avanto 1.5T MR扫描仪。扫描序列和参数:T1WI采用三维容积内插快速扰相梯度回波序列,横断面扫描,脂肪抑制,TR 5.8 ms,TE 2.6 ms,激励次数1,FA10,矩阵142×256,FOV 300 mm×400 mm,层厚3.0 mm。T2WI采用快速自旋回波序列,横断面扫描,脂肪抑制,TR 7000~9000 ms,TE 104 ms,激励次数2,矩阵173×384,FOV 300 mm×400 mm,层厚5 mm,层间距10%。MRCP采用单次激发半傅里叶采集快速自旋回波序列,厚层MRCP TR 4500 ms,TE 754 ms,激励次数1,FA180,矩阵308 mm×384 mm,FOV 350×350,模块厚69.8 mm;薄层MRCP TR 1210 ms,TE 114 ms,激励次数1,FA 125,矩阵192×320,FOV 329×329,层厚3.5 mm,层间距10%。横断面屏气抑脂T1加权动态增强扫描,完全重复增强前的序列。使用高压注射器经静脉注射Gd-DTPA,剂量0.2 mmol/kg,流速3 ml/s,注射后18、26和38 s重复采集,获取动脉期、实质期及延迟期图像。
三、图像评价及分析
由两位对胰腺疾病诊断有丰富经验的高级职称医师共同阅片。影像评价指标:测量正常组和CP组T1WI、T2WI扫描的胰腺实质信号及肝脏信号,获得它们的比值(rT1、rT2);测量主胰管最大直径(MPD);记录胰管结石、假性囊肿、胰腺萎缩状况;进行剑桥程度分级;测量胰腺动脉期、实质期及延迟期的信号强度,并计算各期的胰腺强化率,强化率=(强化后的胰腺信号均值- T1平扫的胰腺信号均值)/T1平扫的胰腺信号均值;ROI的选择尽可能大,但不能达到脏器边缘,胰腺各部位ROI还要避开肉眼可见的大血管,以减少部分容积效应对结果准确性的影响,肝脏选取肝右叶,避开胆管、血管及肝内病变,部分病例胰腺某部位明显萎缩而无法测量则为空缺值。
四、数据统计分析
采用SPSS17.0软件包。计量资料采用单因素方差分析,计数资料采用χ2检验;各项MRI表现与CP临床分级的关系采用spearman秩相关分析;有统计学差异的变量作为评估参数行ROC分析。P﹤0.05有统计学意义。
结 果
一、MRI扫描图征象及参数的变化
65例CP患者中,T1信号降低37例,其中32例全程不均匀降低,1例局限于胰尾,4例局限于胰腺头颈部;T2信号的改变多样,感染时可轻度增高或呈不均匀信号改变。正常、轻度、中度、进展组的rT1值分别为0.98±0.27、0.84±0.12、0.81±0.16和0.75±0.24,中度和进展组较正常组明显降低(P﹤0.01)。rT1值与CP临床分级相关(r=0.34,P<0.01)。正常、轻度、中度、进展组的rT2值分别为1.28±0.30、1.46±0.44、1.46±0.55和1.76±0.72,各组间无显著差异。
正常、轻度、中度、进展组的MPD分别为(2.0±0.6)mm、(5.4±2.4)mm、(6.5±3.3)mm和(8.1±4.1)mm,CP组较正常组明显增大(P﹤0.01),进展组又较轻度组明显增大(P﹤0.05)。MPD与CP临床分级相关(r=0.62,P<0.01)。
根据CP剑桥分级,轻度、中度、进展组的中度分级者分别有7例(50%)、2例(5%)和1例(7%);重度分级者有4例(29%)、33例(89%)和13例(93%)。中度和进展组的剑桥重度分级者较轻度组显著增加(P<0.01)。剑桥分级与CP临床分级相关(r=0.41,P<0.01)。
主胰管扩张59例,主胰管狭窄并远端扩张1例,主胰管未见异常5例。胰管结石18例,其中轻度组2例(14%),中度组11例(30%),进展组5例(36%)。胰腺假性囊肿9例,其中中度组6例(16%),进展组有3例(21%)。胰腺萎缩36例,轻、中、进展组分别有4例(29%)、22例(60%)和10例(71%)。各组间均无显著差异。
rT1、MPD的ROC见图1和表1。以MPD>2.5 mm为界,诊断CP的敏感性94%,特异性79%;以rT1<0.8为界,敏感性90%,特异性48%。
二、MRI动态增强图征象及参数的变化
正常组的胰腺强化峰值在注射造影剂18 s后的动脉期出现,实质期和延迟期造影剂缓慢退出,实质期与动脉期胰腺信号强化率比(P/A)<1;CP组的胰腺强化峰值在注射造影剂26 s后的实质期出现,轻度、中度和进展组的P/A值分别为1.10±0.08、1.37±0.15和1.48±0.53,各组间差异明显(P﹤0.05,表2)。P/A值与CP临床分级相关(r=-0.43,P<0.01)。以P/A值>0.8为界,诊断CP的敏感性95%,特异性47%,结合MPD>2.5 mm及rT1<0.8诊断CP的特异性可提高到95%。
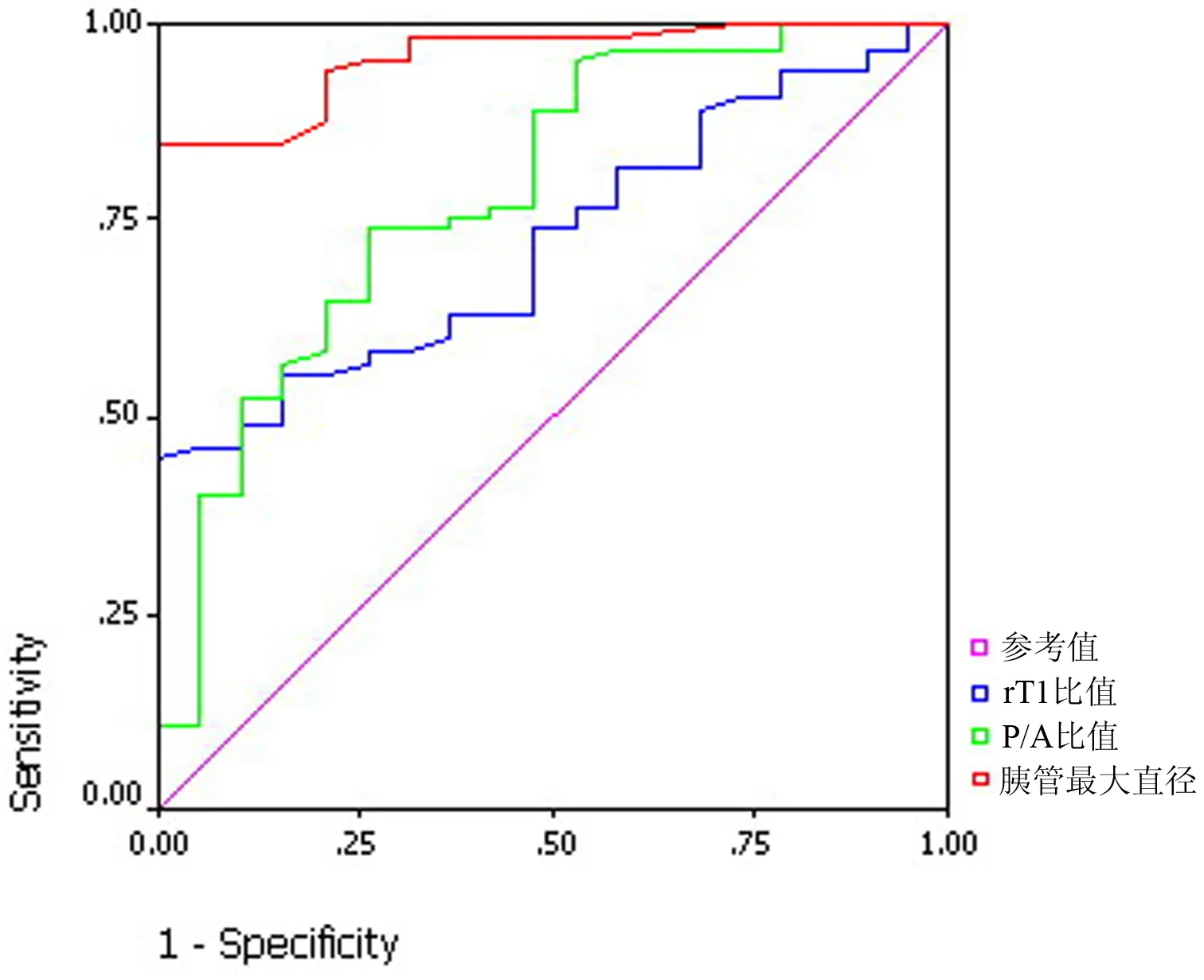
图1 rT1、MPD及P/A的ROC曲线图
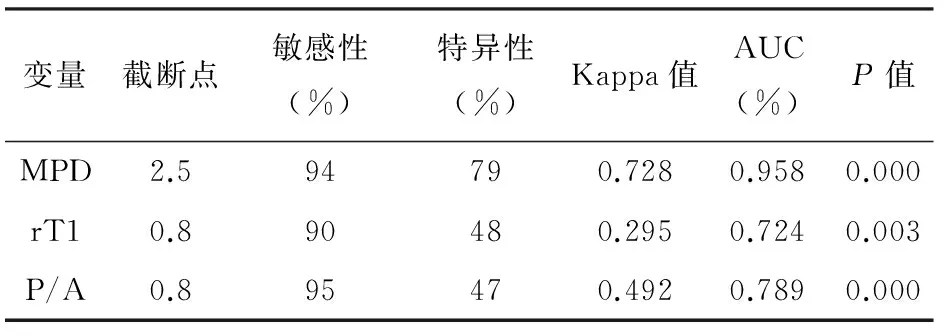
变量截断点敏感性(%)特异性(%)Kappa值AUC(%)P值MPD2.594790.7280.9580.000rT10.890480.2950.7240.003P/A0.895470.4920.7890.000
注:AUC为曲线下面积
讨 论
早期确诊CP及对其严重程度进行分级对于CP的治疗、患者的预后、生活质量的提高等均有重要的影响。多年来人们制定了不同的CP临床分级方式,如Marseilles系统[4-5]、Amman的CP分期标准[6]、Chari系统[7]、Ramesh的ABC系统[4]。M-ANNHEIM评分系统[2]等影像学检查可为CP患者提供多项参数。

表2 正常组和CP各组胰腺信号强化率及P/A值
本组采用MRCP技术测量MPD,发现CP临床分级越重,主胰管直径扩张越明显。当主胰管直径>2.5 mm时诊断CP的敏感性达94%,特异性为79%。此外,MRCP能发现直径在2 mm以上的结石[8]以及与胰管不相通的假性囊肿。CP临床分级越重,结石或囊肿的出现概率也越高。
胰腺实质T1及T2信号的改变是CP的影像学表现之一。临床经验显示,胰肝信号强度的比是鉴别胰腺实质正常与否最好的指标[9]。本组结果发现,CP组T1加权像上胰腺信号常有不同程度的降低,且CP临床分级越重,T1信号降低越明显。当T1信号比值<0.8时诊断CP有较高的敏感性和一定的特异性,有一定的临床应用价值。T2加权成像可以显示感染而造成的胰腺T2信号轻度增高或不均匀信号改变[7]。磁共振平扫的不足之处在于对CP钙化的显示远不及CT。本组有3例CT显示的胰腺钙化,在磁共振检查时未发现。
Zhang等[10]研究发现,Gd-DTPA动态增强时,T1WI上正常胰腺信号动脉期升高最明显,而CP时胰腺信号则在静脉早期或延迟期增加最明显,胰腺静脉早期与动脉早期的信号强度比﹤1.7以及胰腺峰值强化延迟诊断早期CP的敏感性为92%,特异性为75%,明显高于仅靠胰腺形态学改变诊断的50%的敏感性,提示血供改变有时在CP早期无明显主胰管形态学改变之前发生。Johnson等[11]研究亦证实,Gd-DTPA动态增强T1WI脂肪抑制像上CP患者胰腺实质呈逐渐强化,与正常胰腺在动脉期或门脉期明显强化不同,从而有利于CP的发现。本组结果发现CP患者的胰腺强化峰值较正常组推迟,常出现在实质期,且随着CP程度加重,胰腺强化延迟越明显,当P/A值>0.8时诊断CP的敏感性高达95%,特异性也有47%。
[1] Etemed B,Whitcomb DC.Chronic pancreatitis:diagnosis,classification,and new genetic developments.Gastroenterology,2001,120:682-707.
[2] Schneider A,Löhr JM,Singer MV.The M-ANNHEIM classification of chronic pancreatitis:introduction of a unifying classification system based on a review of previous classifications of the disease.J Gastroenterol,2007,42:101-119.
[3] Sarner M,Cotton PB.Classification of pancreatitis. Gut, 1984, 25:756-759.
[4] Ramesh H.Proposal for a new grading system for chronic pancreatitis:the ABC system.J Clin Gastroenterol,2002,35:67-70.
[5] Singer MV,Gyr K,Sarles H.Revised classification of pancreatitis:Report of the Second International Symposium on the Classification of Pancreatitis in Marseille,France,March 28-30,1984.Gastroenterology,1985,89:683-685.
[6] Aroman RW,Akovbiantz A,Largiader F,et al.Course and out-come of chronic panreatitis.Longitudinal study of a mixed medical-surgical series of 245 patients.Gastroenterology,1984,86:820-828.
[7] Chari ST,Singer MV.The problem of classification and staging of chronic pancreatitis:Proposals based on current knowledge and its natural history.Scand J Gastroenterol,1994,29:949-960.
[8] Vitellas KM,Keogan MT,Sprtzer CE,et al.MR cholangiopancreatography of bile and pancreatic duct abnormalities with emphasis on the single-shot fast spin-echo technique.Radiographics,2000,20:939-957.
[9] 周诚,叶晓华,杨正汉.小胰腺癌的CT/MRI诊断与鉴别诊断.中国医学计算机成像杂志,2002,8:256-259.
[10] Zhang XM,Shi H,Parker L,et al.Suspected early or mild chronic pancreatitis: enhancement patterns on gadolinium chelate dynamic MRI Magnetic resonance imaging.J Magn Reson Imaging,2003,17:86-94.
[11] Johnson PT,Outwater EK.Pancreatic carcinoma versus chronic pancreatitis:dynamic MR imaging.Radiology,1999,212:213-218.
2010-07-04)
(本文编辑:吕芳萍)
EvaluationofMRmultiparameterimagingforclinicalclassificationofchronicpancreatitis
BAIMei,LUJian-ping,LAIXiao-wei.
DepartmentofRadiology,ChanghaiHospital,SecondMilitaryMedicalUniversity,Shanghai200433,China
LUJian-ping,Email:cjr.lujianping@vip.163.com
ObjectiveTo investigate the value of MR multiparameter imaging for the clinical classification of chronic pancreatitis.Methods65 patients with confirmed chronic pancreatitis by follow-up and pathologic examinations (14 mild, 37 moderate and 14 severe according to MANNHEIM system) and 20 healthy volunteers were included in this study. MR examination including routine T1WI, T2WI, MRCP and dynamic enhanced MRI. The data were measured and statistical analysis was applied in four groups. Two radiologists assessed pancreatic duct diameter, pancreatic size, pancreatic cyst, pancreatic stone and pancreatic signal intensity on MRCP, T1-weighted and T2-weighted images. Pancreatic signal intensity were also measured on dynamic enhanced MR.ResultsMean values of pancreatic signal intensity ratio on T1WI (rT1) in the pancreas were significantly reduced in patients with moderate and severe CP compared with volunteers.
There was significant difference among four groups (normal, 0.98±0.27; mild, 0.84±0.12; moderate, 0.81±0.16; severe, 0.75±0.24). Mean values of pancreatic signal intensity ratio on T2WI (rT2) in the pancreas were no difference among four groups (normal, 1.28±0.3; mild,1.46±0.44, moderate, 1.46 ±0.55; severe, 1.76 ±0.72). Pancreatic duct diameters were significantly increased in mild, moderate and severe CP groups [mild (5.3±2.4)mm; moderate (6.5 ±3.3)mm; severe (8.1 ±4.1)mm] compared with patients without CP [(2.0±0.6)mm;P﹤0.01]. Severe degree of Cambridge classification was graded as mild in 4 (29%), moderate in 33 (89%), severe in 13 (93%). Pancreatic calcification was graded as mild in 2 (14%), moderate in 11 (30%), severe in 5 (36%). Pancreatic pseudocyst was graded as mild in 0, moderate in 6 (16%), severe in 3 (21.43%). Pancreatic parenchymal atrophy was graded as mild in 4 (29%), moderate in 22 (59%), severe in 10 (71%). They did not vary among CP groups. Parenchymal/arterial phase enhanced ratio (P/A) in the pancreas were significantly increased in patients with mild, moderate and severe CP (mild, 1.10±0.08; moderate, 1.37±0.15; severe, 1.48±0.53) compared with patients without CP (0.88±0.08,P﹤0.05). Significant correlation was present between the severity level of CP and the change of rT1, severe degree of Cambridge classification, the pancreatic duct diameter and P/A (r=0.34, 0.41, 0.62, -0.43;P﹤0.01). ROC analysis showed the presence of pancreatic duct diameters more than 2.5mm, rT1 less than 0.8 and P/A more than 0.8 had a sensitivity and specificity of diagnosing chronic pancreatitis of 94% and 79%, 90% and 48%, 95% and 47% respectively. Combined with the three variables, the specificity of diagnosing chronic pancreatitis can be improved to 95%.ConclusionsT1-weighted, MRCP and dynamic enhanced MRI imaging can accurately evaluate the clinical severity of chronic pancreatitis. MRCP had the highest sensitivity and specificity, followed by T1-weighted and dynamic enhanced MRI imaging.
Pancreatitis,chronic; Magnetic resonance imaging; Dynamic enhanced MRI
10.3760/cma.j.issn.1674-1935.2010.05.001
国家自然科学基金(2006BAI02A12)
200433 上海,第二军医大学长海医院放射科(柏梅、陆建平),消化内科(赖晓伟)
陆建平,Email: cjr.lujianping@vip.163.com
