Expression of FLICE-inhibitory Protein in Synovial Tissue and Its Association with Synovial Inflammation in Juvenile Idiopathic Arthritis△
2010-11-22FengxiaWuLijunWuXiongyanLuoMinghuiYangZhongTangChuanmeiXieJingguoZhouJianlongGuanandGuohuaYuan
Feng-xia Wu,Li-jun Wu,Xiong-yan Luo,Ming-hui Yang*,Zhong Tang,Chuan-mei Xie,Jing-guo Zhou,Jian-long Guan,and Guo-hua Yuan*
1Institute of Rheumatology and Immunology,Affiliated Hospital of North Sichuan Medical College,Nanchong,Sichuan 637000,China
2Department of Rheumatology,People’s Hosptial of Xinjiang Uygur Autonomus Region,Urumchi 830001,China
3Department of Rheumatology and Immunology,Changhai Hospital,Second Military Medical University,Shanghai 200433,China
JUVENILE idiopathic arthritis (JIA),one of the most common rheumatic diseases in children,is defined as chronic arthritis developing in a child at 16 years of age or younger and lasting for 6 or more weeks in the absence of any known cause.1It is a heterogenous group of conditions consisting of several subtypes.Although different in onset and disease course,the subtypes of JIA share the common features of chronic inflammation of the joints:hyperplastic synovial tissue with accumulation of inflammatory cells.2It has been proposed that dysregulation of apoptosis may result in synovial hyperplasia and prolong survival of infiltrated inflammatory cells in synovial tissue in JIA.
Death receptors (DR) of the tumor necrosis factor (TNF)receptor superfamily are known to be important for the regulation of cell proliferation and cell death.The first step induced by activation of DR is the recruitment of a complex of different proteins termed death-inducing signaling complex (DISC) to the intracellular death domain of these receptors through the Fas-associated death domain protein(FADD).3DISC formation results in the homodimerization and proteolytic activation of caspase 8.Once activated,caspase 8 is released into the cytosol,inducing apoptosis through direct activation of terminal caspases or by prior activation of caspase 9 through the mitochondrial apoptotic pathway.4,5Specific inhibition of Fas signaling by inhibitors of caspase 8 restrains Fas-mediated apoptosis in fibroblast-like synoviocytes (FLS) derived from rheumatoid arthritis (RA) patients.6,7The process of caspase 8 activation is counter balanced by the parallel recruitment of cellular FLICE (Fas-associated death domain-like IL-1-converting enzyme)-inhibitory protein (c-FLIP,also called CASPER,CLARP,FLAME-1,I-FLICE,CASH,or MRIT),a novel Fas pathway-inhibitory protein.8-12FLIP exists in long(FLIPL) and short (FLIPS) isoforms.FLIPLpossesses two death effector domains (DEDs) and a caspase-like domain in which tyrosine is substituted for the active cysteine residue necessary for enzymatic activity,9while the caspase-like domain is absent in FLIPS.Both the isoforms form heterodimers with procaspase 8 within DISC and block its activation.13,14FLIP has been demonstrated to be a dominant inhibitor of caspase 8 activation and Fas-induced apoptosis in a large variety of human cells.12,15,16
In RA,the expression of FLIP has been detected in areas of synovial tissue,bone,and cartilage invasion.17Enforced FLIP expression has been shown to partially decrease Fas-induced apoptosis in RA FLS,18while downregulation of FLIP sensitizes RA FLS and SF macrophage to Fas-mediated apoptosis.19,20Also,high expression of FLIP had been noticed in synovial fluid lymphocytes in JIA.21However,FLIP expression in synovial tissue in JIA is still matters of debate.
In the present study,we investigated the expression of FLIP in JIA and normal synovial tissues using reverse transcription-polymerase chain reaction (RT-PCR) and immunohistochemical methods,and analyzed its correlation with the degree of synovial inflammation.
PATIENTS AND METHODS
Patients and samples
Samples of synovial tissues were obtained from 11 children(8 females,3 males) with JIA who underwent arthroscopic examination in our hospitals between May 2003 and August 2008.Serial cryostat sections (5-7 μm) were fixed with 4% (v/v) formaldehyde in phosphate-buffered saline(PBS) containing 0.1% saponin at room temperature for 30 minutes and stored at-70°C.All patients met the International League Against Rheumatism (ILAR) criteria for the classification of JIA.22The study was approved by the ethics committee of the three hospitals participated in.Full informed consent was obtained from parents of each enrolled child.All samples were processed within one hour of removal from the patient.Samples of synovial tissues from 3 trauma patients (1 female,aged 13 years old;2 males,aged 10 and 15 years old) undergoing knee arthroscopic examination were taken as controls.
Reverse transcriptase-polymerase chain reaction(RT-PCR) analysis
Synovial tissue was minced and lyzed by adding 1 mL of TRIzol Reagent (Invitrogen,San Diego,CA,USA).RNA was precipitated in ethanol from the aqueous phase,recovered by centrifugation,resuspended in 10 μL of sterile water,and evaluated spectrophotometrically for quantity and purity.First-strand complementary DNA (cDNA) was synthesized in a 20 μL reaction system containing 500 ng of total RNA,2.5 mmol/L of each dNTP,1 μmol/L of random hexamer primers,40 units of ribonuclease inhibitor (RNasin;Toyobo,Tokyo,Japan),and 200 units of Superscript II RT (Gibco BRL) by incubation at 42°C for 2 hours.The resulting cDNA (1-2 μL) was subjected to PCR using Taq DNA polymerase (TaKaRa Shuzo,Shiga,Japan)and the specific primers for c-FLIPL/S.23,24In each experiment,amplification of cDNA for the housekeeping gene GAPDH was used as an internal standard.The primer sequences and expected product sizes were as follows.c-FLIPL/S:sense,5'-TGTTGCTATAGATGTGG-3';antisense:5'-AAGGATCCTTGAGACTCT-3',512 bp;GAPDH:sense,5'-CCACCCATGGCAAATTCCATGGCA-3';antisense,5'-TCTAGACGGCAGGTCAGGTCCACC-3',598 bp.The reaction was performed at 95°C for 2 minutes,followed by 35 cycles of denaturing at 95°C for 45 seconds,annealing at 55°C for 45 seconds,and extension at 72°C for 1 minute.PCR products and a molecular weight marker (Marker 5,Φξ174/Hind II digest;Nippon Gene,Tokyo,Japan) were electrophoresed on 1.5% agarose gels,stained with ethidium bromide (10 mg/mL),and bands were visualized and photographed under ultraviolet illumination.
Immunohistochemical analysis
Sections of synovial tissues were preincubated with heatinactivated 10% normal rabbit serum at room temperature for 30 minutes to prevent nonspecific binding and with blocking solution (KPL) for 45 seconds to quench endogenous peroxidase activity.Then sections were incubated at 4°C for 16 hours with affinity-purified polyclonal rabbit anti-human antibodies (2-5 μg/mL) with specificity for FLIP(NeoMarkers,USA).Positive staining was visualized using the HistoMark Enhance-Orange Peroxidase System kit (KPL)and counterstained with hematoxylin.Samples incubated with normal rabbit IgG (Sigma) were used as negative controls.
Cell types present in synovial tissue were identified by their morphology after haematoxylin counterstaining.25The specific cell types evaluated in the synovial tissue were lining cells,subsynovial macrophages,fibroblasts,vascular endothelium,and lymphocytes.The degree of FLIP staining were evaluated by a pathologist who was blinded to the elements of the study,and graded by semi-quantitative evaluation and categorized by the extent and intensity of staining as follows.(1) The extent of positive cells was estimated as 0:no staining;1:positive staining cells <25%,2:positive staining cells 25%-75%,3:positive staining cells >75%.(2) The intensity of staining was scored as 0:achromatic,1:light yellow,2:yellow,3:brown.Five 400-fold magnification fields were examined on each section.The percentage of positive cells and staining intensity were multiplied to produce a weighted score for each case.In addition,synovial tissue samples were assigned scores for the degree of inflammation on a relative scale of 1 to 3,which was estimated by the degree of sublining inflammatory cell infiltration composed of macrophages,lymphocytes,and neutrophils (inflammatory score of 1,2,and 3 represented < 25,25-100,and >100 inflammatory cells per 400-fold magnification microscopic field,respectively).25Histopathological scoring was conducted on an Olympus BX41 microscope(1 000×).Photographs were taken on a Nikon (Tokyo,Japan) microscope equipped with the Nikon digital camera DMX1200.
Caspase 8 activity analysis
The caspase 8 activity assay was performed using the BD ApoAlert caspase fluorescent assay kit,according to the manufacturer's instructions.Synovial tissues were minced and digested by rotating overnight at 37°C in Dulbecco's modified Eagle's medium (DMEM;Gibco BRL,Grand Island,NY,USA) containing 1 mg/mL of bacterial collagenase (Sigma,St.Louis,MO,USA).The cells released by enzymatic digestion were filtered through a sterile nylon strainer (Becton Dickinson,Franklin Lakes,NJ,USA),washed,harvested,counted,and stored in liquid nitrogen.For caspase 8 activity assay,the pellet was lysed in manufacturer-provided cell lysis buffer.The cell supernatants were transferred to a 96-well plate,and 2×reaction buffer/dithiothreitol and the fluorescent caspase 8 substrate (IETD-AFC) were added and incubated for 1-3 hours at 37°C before reading with a fluorometer at 400/505 nm.
Statistical analysis
Data were expressed as mean±SD or median values.A multi-way Pearson correlation analysis was performed between FILP expression and synovial inflammation score or caspase 8 activity.A value ofP<0.05 was considered significant.
RESULTS
Demographic and clinical characteristics of subjects
This study included 11 active JIA patients and 3 controls without inflammatory arthritis.The demographic characteristics,clinical and laboratory profiles,and current medications of the 11 patients are summarized in Table 1.
c-FLIPL/SmRNA expression in synovial tissue
We confirmed the presence of c-FLIPL/SmRNA in synovial tissue derived from JIA.c-FLIPL/SmRNA expression was found in all 11 JIA samples tested.In contrast,c-FLIPL/SmRNA was weakly detected in 2 of 3 normal synovium.A representative RT-PCR analysis of synovial tissue from JIA patients is shown in Figure 1.
Immunolocalization of c-FLIP in JIA synovial tissue
To evaluate the production and localization of c-FLIP in synovial tissue from JIA,immunohistochemistry analysis was performed.A representative result,depicting positive immunostaining of c-FLIP in the cytosol and/or plasma membranes of synovial cells,is shown in Figure 2.FLIPpositive and -negative areas were found within the sametissue,and the intensity of positive staining was also found to vary within a case tested.After multiplying the weighted intensity scores,the mean FLIP expression scores in synovial tissues from JIA patients was 5.27 (ranged from 2 to 9),which was higher than that in normal synovial tissues(0.67,ranged from 0 to 2).
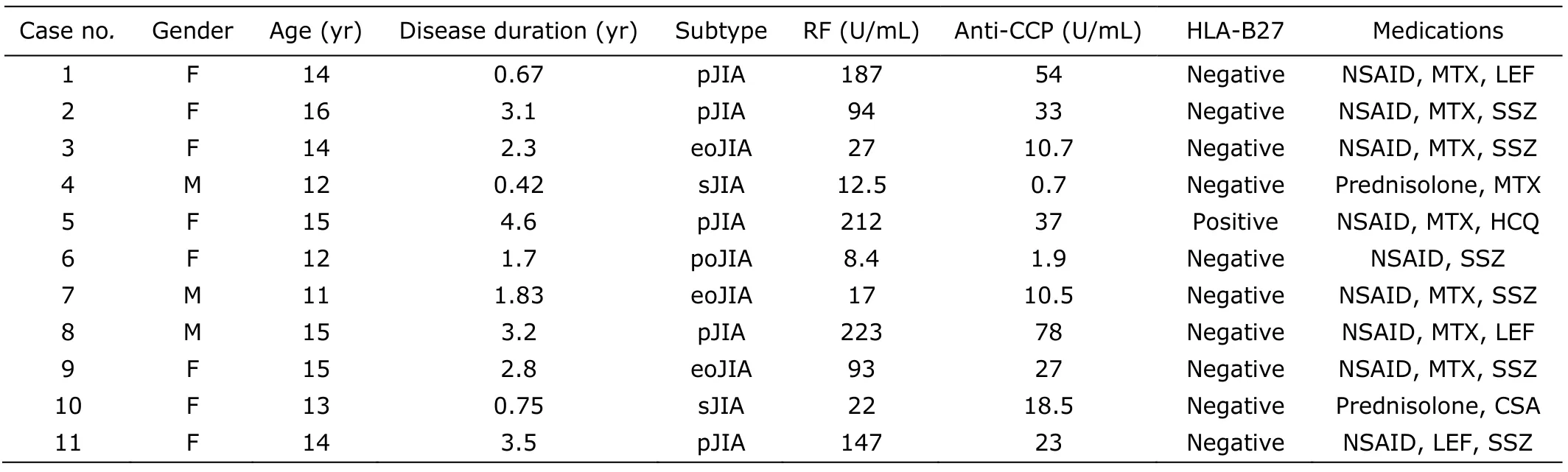
Table 1.Demographic and clinical characteristics of patients with juvenile idiopathic arthritis included in this study
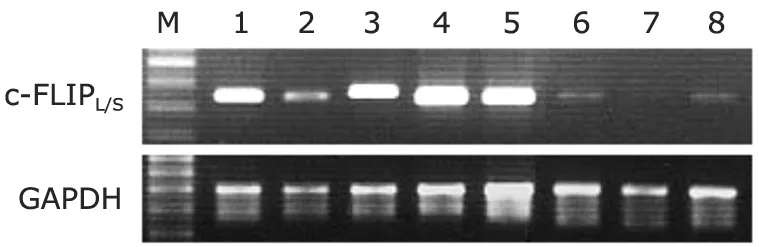
Figure 1.c-FLIP mRNA expression in synovial tissue from juvenile idiopathic arthritis patients (lanes 1-5) and control subjects (lanes 6-8) analyzed by reverse transcriptasepolymerase chain reaction.
FLIP positive cells were present in lining and sublining layers,mainly in the macrophage-like cells and to a less extent in lining and subsynovial fibroblast-like synoviocytes (Fig.3).And a positive correlation between FLIP expression in synovial tissue from JIA and inflammatory scores was also found (r=0.563,P<0.05) (Fig.4).
Caspase 8 proteolytic activity in JIA synovial tissue
The c-FLIP mRNA expression and protein production observed in synovial tissue from JIA led to further investigation on caspase 8 activity.Therefore,all 11 JIA samples were analyzed for the proteolytic activity of caspase 8.And the result showed that caspase 8 activity had a tendency to be negatively correlated with c-FLIP expression in JIA synovial tissues (r=-0.418,P>0.05) (Fig.5).
DISCUSSION
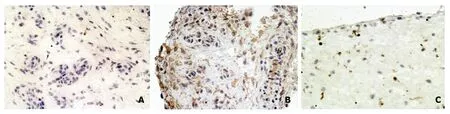
Figure 2.Immunostaining of FLIP in synovial biopsy specimens counterstained with haematoxylin.× 400
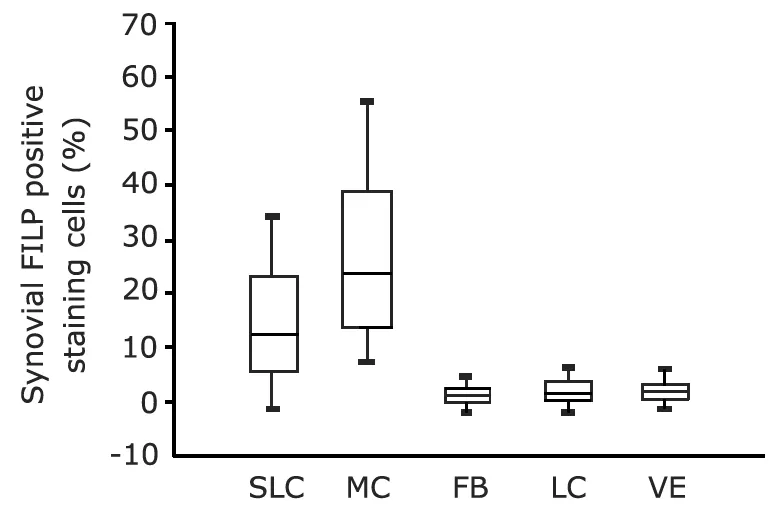
Figure 3.FLIP expression in the specific cell types evaluated in synovial tissue from juvenile idiopathic arthritis patients.
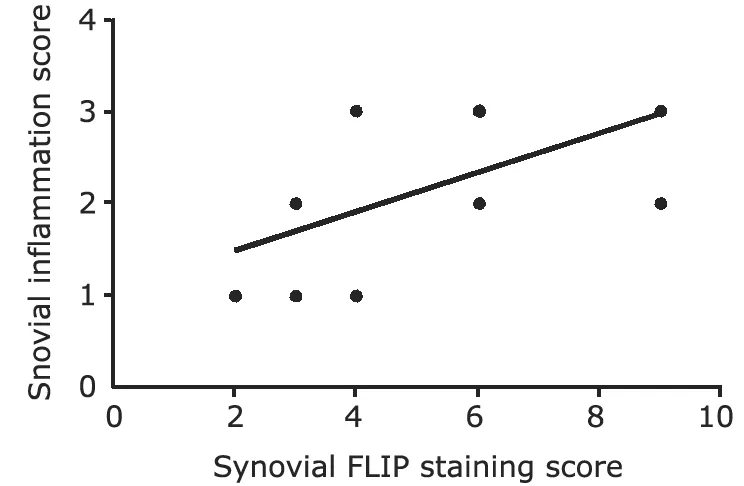
Figure 4.Correlation between FLIP expression in synovial tissue and degree of synovial inflammation from 11 patients with juvenile idiopathic arthritis (r=0.563,P<0.05).
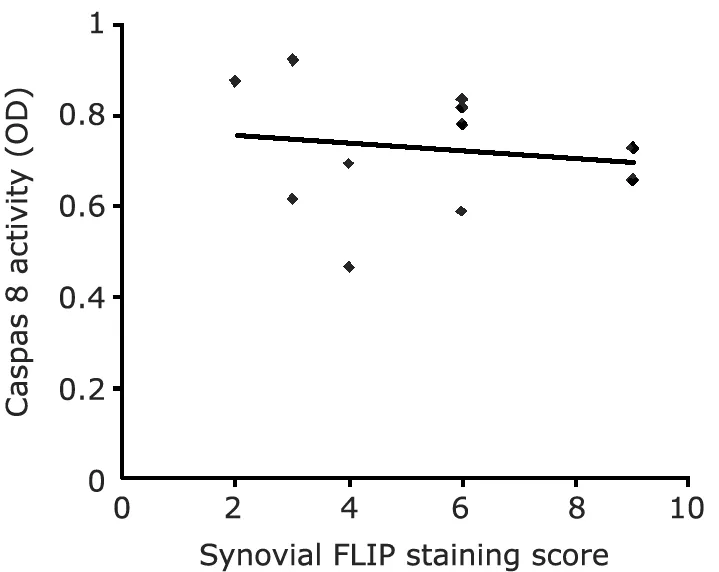
Figure 5.Correlation between FLIP expression and caspase 8 activity in synovial tissue from 11 patients with juvenile idiopathic arthritis (r=-0.418,P>0.05).
JIA is a chronic inflammatory disease,and one of its characteristics is hyperplastic synovial tissue.Hyperplasia is ascribed to both increased cellular activation and impaired apoptosis of synovial cells.In adult RA,the investigation of cellular proliferationin vivohas revealed controversial results which suggest that the rate of proliferation is not increased in RA synovium,26several lines of evidence favor an altered apoptosis as the underlying mechanism of hypercellularity in the synovial tissue.27-29Although the expression of Fas had been demonstrated in a variety of synovial cells in RA synovial tissue,synovial cells actually very rarely undergo apoptosisin vivo,30,31indicating that apoptotic pathways appear to be blocked functionally by an overexpression of antiapoptotic molecules.FLIP contains a caspase-like domain which has significant homology to caspase 8 but lacks its catalytic domain.FLIP prevents the binding of caspase 8 and,presumably,caspase 10 with FADD,and thus exerts its antiapoptotic function through inhibition of death receptor-mediated apoptosis.9The overexpression of FLIP had been demonstrated in RA synovial tissue,17and high FLIP expression correlated with low levels of apoptosis in early RA synovium had been reported by Catrina et al.32Moreover,Bai et al33found that FLIP expression protected RA synovial fibroblasts from TNFα-mediated apoptosis,and it had been also reported that down-regulation of FLIP expression would sensitize rheumatoid synovial fibroblasts to Fas-mediated apoptosis.20,33
The pathological changes of JIA synovium are much similar to that of RA,and abnormalities in regulation of apoptosis also have a role in the pathogenesis of JIA.Inhibition of apoptosis,with higher expression of antiapoptotic Bcl-2 protein in synoviocytes,was found in patients with JIA.34Reports about FLIP expression in JIA were scarce.In the study by Smolewska et al,21they examined the expression of FLIP in synovial fluid (SF) lymphocytes from JIA patients,and found that FLIP expression was distinctly higher in SF lymphocytes than in JIA peripheral blood lymphocytes.However,FLIP expression in synovial tissue in JIA is still matters of debate.
To examine the expression of FLIP in JIA synovial tissue,we first confirmed the presence of c-FLIPL/S mRNA in synovial tissue derived from JIA,because this has been alluded to in adult RA in previous studies.17,24As a result,c-FLIPL/S mRNA expression was found in all 11 JIA samples tested.In contrast,c-FLIPL/SmRNA was detected in none of 3 normal synovium.To verify the positive FLIP expression in JIA synovial tissue found by RT-PCR,immunohistochemistry analysis was also performed.FLIP expression score in synovium from JIA patients was significantly higher than that in control synovial tissues.FLIP positive cells were present in lining and sublining layers,mainly in the macrophage-like cells and to a less extent in fibroblast-like synovitocytes,which is consistent with results reported in adult RA.17,32
Accumulation of inflammatory cells in synovial tissue is another hallmark of JIA.Inflammatory cells accumulation may be due to both increased leucocyte ingress into synovial tissue and prolonged survival of those cells.In adult RA,SF monocytes/macrophages were resistant to Fas-mediated apoptosis,with up-regulation of FLIP expression in SF monocytes/macrophages.19Perlman et al19found that RA synovial macrophages expressed FLIP and were refractory to Fas-mediated apoptosis.Those findings suggest that the impaired apoptosis pathway in inflammatory infiltration,resulted by antiapoptotic properties of FLIP,may lead to their increased survival in RA.In this study,we also found a positive correlation of FLIP expression on synovial tissue from JIA with inflammatory scores,indicating that FLIP expression may enhance survival of inflammatory cells by inhibiting apoptosis and subsequently increasing the numbers of inflammatory cells in JIA synovial tissue.
As mentioned above,FLIP may exert its antiapoptotic properties through its ability to bind directly to the protease domain of caspase 8 and to prevent the activation of caspase 8.In RA synovial fibroblasts,Bai et al33confirmed that TNFα-induced apoptotic cell death mediated by caspase 8 activation was prevented by the ectopic expression of FLIP.We analyzed the relationship between the expression of FLIP and the activity of caspase 8 in JIA synovial tissue,and found that they were negatively correlated,but with no statistical significance due to the small number of specimens.However,even when FLIP does not block Fas-mediated apoptosis due to insufficient levels of expression,it could still regulate apoptosis by other DR.5,35In a recent study,Zhang et al36reported that c-FLIP(L)-deficient T cells displayed defective TCR-mediated proliferation,which indicated that FLIP itself played an important role in cell proliferation.
In conclusion,expression of FLIP demonstrated in JIA synovial tissue suggests that this antiapoptotic molecule might increase the lifespan of synovial cells including inflammatory cells infiltrated into synovial tissue.Therefore,the inhibition or suppression of FLIP may lead to a decrease in synovial hyperplasia and inflammatory infiltration,resulting in amelioration of JIA.
1.Haines KA.Juvenile idiopathic arthritis:therapies in the 21st century.Bull NYU Hosp Jt Dis 2007;65:205-11.
2.Schneider R,Passo MH.Juvenile rheumatoid arthritis.Rheum Dis Clin North Am 2002;28:503-30.
3.Chinnaiyan AM,O'Rourke K,Tewari M,et al.A novel death domain-containing protein,interacts with the death domain of Fas and initiates apoptosis.Cell 1995;81:505-12.
4.Hu WH,Johnson H,Shu HB.Activation of NF-κB by FADD,casper,and caspase-8.J Biol Chem 2000;275:10838-44.
5.Tschopp J,Irmler M,Thome M.Inhibition of fas death signals by FLIPs.Curr Opin Immunol 1998;10:552-8.
6.Okamoto K,Kobayashi T,Kobata T,et al.Fas-associated death domain protein is a Fas-mediated apoptosis modulator in synoviocytes.Rheumatology (Oxford) 2000;39:471-80.
7.Perlman H,Pagliari LJ,Volin MV.Regulation of apoptosis and cell cycle activity in rheumatoid arthritis.Curr Mol Med 2001;1:597-608.
8.Hu S,Vincenz C,Ni J,et al.I-FLICE,a novel inhibitor of tumor necrosis factor receptor-1-and CD-95-induced apoptosis.J Biol Chem 1997;272:17255-7.
9.Irmler M,Thome M,Hahne M,et al.Inhibition of death receptor signals by cellular FLIP.Nature1997;388:190-5.
10.Peter ME.The flip side of FLIP.Biochem J 2004;382:e1-3.
11.Hyer ML,Samuel T,Reed JC.The FLIP-side of Fas signaling.Clin Cancer Res 2006;12(20 Pt 1):5929-31.
12.Safa AR,Day TW,Wu CH.Cellular FLICE-like inhibitory protein (C-FLIP):a novel target for cancer therapy.Curr Cancer Drug Targets 2008;8:37-46.
13.Xiao C,Yang BF,Asadi N,et al.Tumor necrosis factor-related apoptosis-inducing ligand-induced death-inducing signaling complex and its modulation by c-FLIP and PED/PEA-15 in glioma cells.J Biol Chem 2002;277:25020-5.
14.Yang JK.FLIP as an anti-cancer therapeutic target.Yonsei Med J 2008;49:19-27.
15.Krueger A,Baumann S,Krammer PH,et al.FLICE-inhibitory proteins:regulators of death receptor-mediated apoptosis.Mol Cell Biol 2001;21:8247-54.
16.Thome M,Tschopp J.Regulation of lymphocyte proliferation and death by FLIP.Nat Rev Immunol 2001;1:50-8.
17.Schedel J,Gay RE,Kuenzler P,et al.FLICE-inhibitory protein expression in synovial fibroblasts and at sites of cartilage and bone erosion in rheumatoid arthritis.Arthritis Rheum2002;46:1512-8.
18.Kobayashi T,Okamoto K,Kobata T,et al.Differential regulation of Fas-mediated apoptosis of rheumatoid synoviocytes by tumor necrosis factor alpha and basic fibroblast growth factor is associated with the expression of apoptosis-related molecules.Arthritis Rheum 2000;43:1106-14.
19.Perlman H,Pagliari LJ,Liu H,et al.Rheumatoid arthritis synovial macrophages express the Fas-associated death domain-like interleukin-1beta-converting enzyme-inhibitory protein and are refractory to Fas-mediated apoptosis.Arthritis Rheum 2001;44:21-30.
20.Palao G,Santiago B,Galindo M,et al.Down-regulation of FLIP sensitizes rheumatoid synovial fibroblasts to Fasmediated apoptosis.Arthritis Rheum 2004;50:2803-10.
21.Smolewska E,Stanczyk J,Robak T,et al.Inhibited apoptosis of synovial fluid lymphocytes in children with juvenile idiopathic arthritis is associated with increased expression of myeloid cell leukemia 1 and XIAP proteins.J Rheumatol 2006;33:1684-90.
22.Petty RE,Southwood TR,Manners P,et al.International League of Associations for Rheumatology.International League of Associations for Rheumatology classification of juvenile idiopathic arthritis:second revision,Edmonton,2001.J Rheumatol 2004;31:390-2.
23.Zhou XD,Yu JP,Chen HX,et al.Expression of cellular FLICE-inhibitory protein and its association with p53 mutation in colon cancer.World J Gastroenterol 2005;11:2482-5.
24.Masuko-Hongo K,Sakata M,Yuan GH,et al.Expression of Fas-associated death domain-like interleukin-1beta-converting enzyme (FLICE) inhibitory protein (FLIP) in human articular chondrocytes:possible contribution to the resistance to Fas-mediated death of in vitro cultured human articular chondrocytes.Rheumatol Int 2001;21:112-21.
25.Szekanecz Z,Haines GK,Koch AE.Differential expression of the urokinase receptor (CD87) in arthritic and normal synovial tissues.J Clin Pathol 1997;50:314-9.
26.Kim WU,Yoo SA,Min SY,et al.Hydroxychloroquine potentiates Fas-mediated apoptosis of rheumatoid synoviocytes.Clin Exp Immunol 2006;144:503-11.
27.Aicher WK,Heer AH,Trabandt A,et al.Overexpression of zinc-finger transcription factor Z-225/Egr-1 in synoviocytes from rheumatoid arthritis patients.J Immunol 1994;152:5940-8.
28.Firestein GS,Yeo M,Zvaifler NJ.Apoptosis in rheumatoid arthritis synovium.J Clin Invest 1995;96:1631-8.
29.Ceponis A,Hietanen J,Tamulaitiene M,et al.A comparative quantitative morphometric study of cell apoptosis in synovial membranes in psoriatic,reactive and rheumatoid arthritis.Rheumatology (Oxford) 1999;38:431-40.
30.Baier A,Meineckel I,Gay S,et al.Apoptosis in rheumatoid arthritis.Curr Opin Rheumatol 2003;15:274-9.
31.Matsumoto S,Müller-Ladner U,Gay RE,et al.Ultrastructural demonstration of apoptosis,Fas and Bcl-2 expression of rheumatoid synovial fibroblasts.J Rheumatol 1996;23:1345-52.
32.Catrina AI,Ulfgren AK,Lindblad S,et al.Low levels of apoptosis and high FLIP expression in early rheumatoid arthritis synovium.Ann Rheum Dis 2002;61:934-6.
33.Bai S,Liu H,Chen KH,et al.NF-kappaB-regulated expression of cellular FLIP protects rheumatoid arthritis synovial fibroblasts from tumor necrosis factor alpha-mediated apoptosis.Arthritis Rheum 2004;50:3844-55.
34.Palao G,Santiago B,Galindo MA,et al.Fas activation of a proinflammatory program in rheumatoid synoviocytes and its regulation by FLIP and caspase 8 signaling.Arthritis Rheum 2006;54:1473-81.
35.Scaffidi C,Schmitz I,Krammer PH,et al.The role of c-FLIP in modulation of CD95-induced apoptosis.J Biol Chem 1999;274:1541-8.
36.Zhang N,He YW.An essential role for c-FLIP in the efficient development of mature T lymphocytes.J Exp Med 2005;202:395-404.
杂志排行
Chinese Medical Sciences Journal的其它文章
- Sex Hormones and Androgen Receptor:Risk Factors of Coronary Heart Disease in Elderly Men△
- Comparison between Ophthalmologists and Community Health Workers in Screening of Shallow Anterior Chamber with Oblique Flashlight Test△
- Factors Influencing Pleural Effusion after Fontan Operation:an Analysis with 95 Patients
- Relationship between Carotid Atherosclerosis and Cerebral Infarction
- A Case of Large“Silent”Extra-adrenal Retroperitoneal Paraganglioma Resected Laparoscopically
- Revascularization for Iliac-femoral Artery Pseudoaneurysm with Greater Saphenous Vein
