A Case of Large“Silent”Extra-adrenal Retroperitoneal Paraganglioma Resected Laparoscopically
2010-11-22JinWenHanZhongLiZhigangJiQuanzhongMaoBingbingShiandWeigangYan
Jin Wen,Han-Zhong Li*,Zhi-gang Ji,Quan-zhong Mao,Bing-bing Shi,and Wei-gang Yan
Department of Urology,Peking Union Medical College Hospital,Chinese Academy of Medical Sciences &Peking Union Medical College,Beijing 100730,China
SILENT extra-adrenal retroperitoneal paragangliomas (PGLs) arise from dispersed paraganglias which tend to be symmetrically distributed in close relation to the aorta and sympathetic nervous system.They are rarely encountered in everyday surgical practice.Together with the absence of typical symptoms of catecholamine excess such as headache,palpitations,diaphoresis,and anxiety,the diagnosis of such tumors is a significant challenge,and it is usually delayed until an advanced stage of the disease.This delay may contribute to the development of malignant disease.As a result,silent extra-adrenal retroperitoneal PGLs are rare tumors causing considerable difficulty in both diagnosis and treatment.They can be unicentric or multicentric,tend to be locally invasive,and therefore have a high incidence of local recurrence.Histological and immunological phenotypes of silent extra-adrenal retroperitoneal PGLs are not significantly different with functional ones.However,they have a potential risk of catecholamine syndrome due to surgery and severe infection,which endanger the lives of patients severely.Herein,we reported a case of silent extra-adrenal retroperitoneal PGLs in our hospital to highlight the diagnosis and treatment of silent extra-adrenal PGLs which usually lack the manifestations of catecholamine hypersecretion and hypertension.
CASE DESCRIPTION
A 45-year-old Chinese man who complained of slight right-side upper abdominal pain for 2 months was hospitalized.He had no symptoms related to catecholamine excess such as headache,palpitations,and diaphoresis.No abodominal mass was palpable.He had no history of hypertension and his blood pressure was 120/75 mm Hg at the time of admission.There was nothing peculiar about his family history.Endocrine secretion examinations,B-mode ultrasound (B-US),computed tomography (CT),and octreotide were performed for preoperative diagnosis.At the same time,urine catecholamine,urinary-free cortisol,serum F,blood adrenocorticotropic hormone,dexamethasone suppression test,blood renin-angiotensin-aldosterone,and other tests were used to rule out other diseases.Hand microcirculation test was used to evaluate systemic microcirculation.
B-US revealed a tumor near renal hilum with size of 9 cm × 8 cm × 8 cm.CT showed a similarly round retroperitoneal tumor with inner central hemorrhage and necrosis.The tumor shows obvious intensification after injection of constrast medium (Fig.1).Endocrinic test results including urine catecholamine were within normal range.Octreotide examination showed tumor with high expression of octreotide and it was diagnosed as silent extra-adrenal retroperitoneal PGL.
The patient was given phenoxybenzamine,an α-receptor blocker,for 2 weeks preoperatively.He was then operated laparoscopically and the tumor was removed successfully.
The cut surface of the tumor showed integrated amicula and abundant blood supply.There was a remote hemorrhage and necrosis inside the tumor (Fig.2).Pathological evaluation was performed with HE staining and S-P immunohistochemical test.Under light microscope,the tumor cells were same in size with distinct boundary (Fig.3A).Neoplastic cells were diffusely positive for cgA,syn,NSE,and s-100 by immunohitochemical staining (Figs.3B-E).These chief cells generally had central nuclei,but tumor cells occasionally showed nuclear pleomorphism or bizarre nuclei.

Figure 1.Coronal contrast-enhanced reformatted computed tomography (CT) scan shows a similarly round retroperitoneal tumor with inner central hemorrhage and necrosis.The tumor shows obvious intensification after injection of constrast medium,and is presented as a lobulated soft-tissue-attenuation mass (arrow) near the right renal hilum.
DISCUSSION
Silent extra-adrenal retroperitoneal PGLs are neoplasias of the chromaffin cells which locate in the para-aortic sympathetic chain and aortic bifurcation.They seldom manifest themselves by symptoms of episodic freeing of catecholamines,such as high blood pressure,migraines,sweating,and palpitations.They are extremely rare because incidence of all extra-adrenal PGLs is only 2-8 per million,which occur either sporadically or as part of a hereditary syndrome.There have been few reports of patients with PGLs of considerable size,yet normal plasma and urine catecholamine concentrations.1
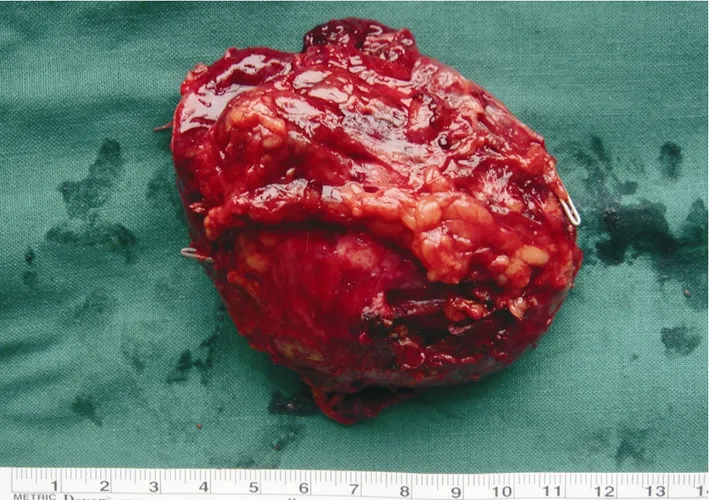
Figure 2.The speciman of paraganglioma obtained after operation shows integrated amicula and abundant blood supply,and there is a remote hemorrhage and necrosis inside the tumor.
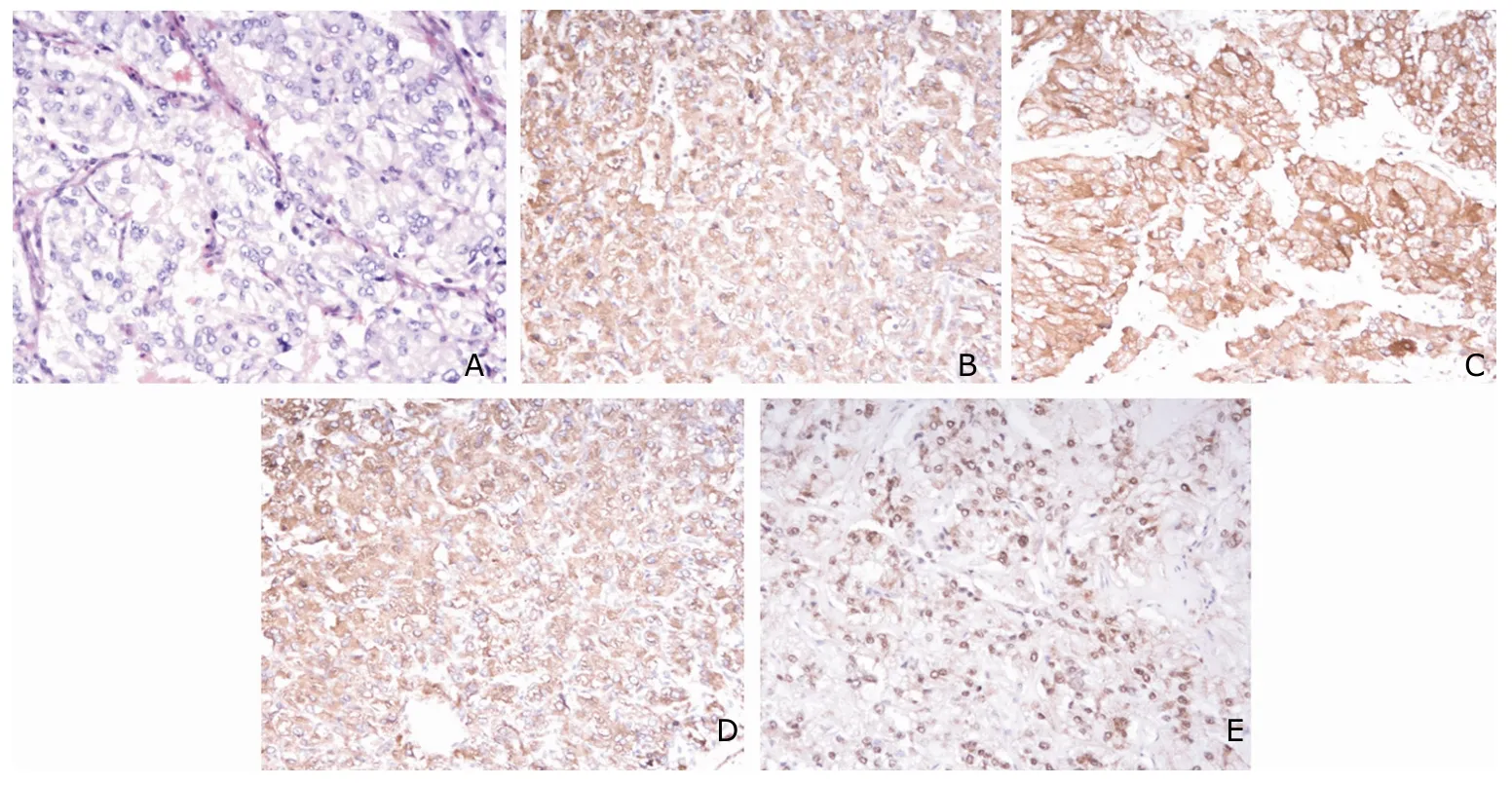
Figure 3.Pathological evaluation of the paraganglioma under light microscope.
B-US can be a simple and effective diagnostic method.The diameter of adrenal masses as measured by B-US correlates closely with mass diameter measured by CT.However,B-US depends to a large extent on operator’s skills.Furthermore,obesity and overlying gas are obstacles for the visualization.Not surprisingly,B-US does not detect tumors with the same sensitivity as CT or magnetic resonance imaging (MRI).
The tumors usually appear as round or oval masses on unenhanced CT.Larger lesions may show a cystic component due to central necrosis or hemorrhage.Calcification is present in some cases.Owing to their hypervascularization,the tumors usually exhibit intense enhancement.The tumors are generally characterized by low T1 and high T2 signal intensities.Central necrosis is frequently observed.There are no signal changes from out-of-phase to in-phase images.For the localization and identification of retroperitoneal PGLs,the radio-pharmaceuticals131I-metaiodobenzylguanidine (MIBG) and111In-octreotide have been most commonly used.2Any retroperitoneal focal uptake 24-72 hours after administration of131I-MIBG should be suspected of PGLs.The sensitivity of MIBG for detecting pheochromocytoma ranges between 80% and 90%,with a specificity of 90%-100%.3The synthetic somatostatin analog111In-octreotide seems less sensitive but is able to visualize tumors that are undetected by MIBG scan.4
Most malignant tumors show an enhanced glycolytic metabolism with increased uptake of deoxyglucose that can be visualized by18F-fluorodeoxyglucose (FDG).FDGpositron emission tomography (PET) has been suggested for the characterization of adrenal masses in patients.However to date,there are insufficient data to justify the use of PET to diagnose clinically silent extra-adrenal retroperitoneal PGLs.We are engaged in the relevant research with the help of PET center of our hospital.
The diagnosis of biochemically silent extra-adrenal retroperitoneal PGLs that either do not produce or do not secrete catecholamines,is usually delayed until advanced metastatic disease emerges,or when complications of space-occupying lesions occur.Hypertension and urinary catecholamine was usually normal or elevated lightly in silent extra-adrenal retroperitoneal PGLs.The measurement of plasma catecholamines is not recommended because this method has poor sensitivity and specificity,which often leads to false-positive results.Urine metanephrines test has a higher specificity,and the receiver operating characteristic curve reveals a better test performance than other biochemical tests.Special attention should be given to acetaminophen use,which interferes with assays and is a source of false-positive testing.
Thorough preoperative pharmacological preparation,attentive intraoperative monitoring,and aggressive surgical therapy all have an important role in achieving the safest and most successful outcome.Operative mortality is down to below one percent with adequate pharmacological preparation.Preoperative management is aimed at prevention of possible catecholamine-induced complications like hypertensive crisis,cardiac arrhythmias,pulmonary edema,and cardiac ischemia due to manipulation of the tumor,and especially to prevent hypotensive crisis after tumor removal.
We prefer to use an adrenoceptor blocking agent for 2-4 weeks.Blood pressure of the patient was observed,and it rose slightly for a moment during operation.This indicates that silent extra-adrenal retroperitoneal PGLs are non-functional relatively.In practical clinical work,it is possible to result in severe consequences if the preoperative awareness is not enough.Randomized controlled trials for preoperative drug treatment are lacking temporarily.
Surgery remains the mainstay of the treatment for silent extra-adrenal retroperitoneal PGLs.Once the diagnosis and drug preparation are accomplished,attempt should be made to perform a complete surgical resection.Resection is often challenging as these highly vascular tumors are located near multiple vital blood vessels.If a tumor is felt to be unresectable at surgery,attempts to reduce its size by chemotherapy,radiation,or embolization may be indicated.
With the development of laparoscopic technique,we applied the technique to silent retroperitoneal PGLs successfully.The laparoscopic approach may have advantages over the open approach when performed by a surgical team experienced in advanced laparoscopic techniques,including decreased postoperative pain,reduced time to return of bowel function,decreased length of hospital stay,and the potential for earlier return to work.When residual tumor cannot be resected,medical therapy for symptomatic relief is preferred.Both radiotherapy and chemotherapy have some effectiveness.
Silent extra-adrenal retroperitoneal PGLs have a more aggressive course than their adrenal counterparts,which are called pheochromocytomas.The prognosis of PGLs can be very difficult to predict.However,their anatomical location may influence their biological behaviour:PGLs in the carotid body have a relatively low rate of malignant behaviour while retroperitoneal PGLs tend to have a higher incidence of malignant transformation.Approximately 20%-42% of PGLs metastasize,compared with only 2%-10% of adrenal pheochromocytomas.Dissemination occurs both lymphatically and hematogenously,with the most common sites of metastasis being the regional lymph nodes,bone,liver,and lung.Distant metastasis to bones,lymph nodes,and lungs has been reported as a unique feature of the metastatic spread of extra-adrenal retroperitoneal PGLs.Diagnosis of malignant PGLs is based on evidence of extensive local invasion,or more reliably on documentation of metastasis to one or more sites where nonchromaffin tissue is not normally present.5According to current WHO criteria,local invasion alone does not define malignancy because it is not always present in tumors that metastasize.6Consequently,not only metastasis but also local invasion should be carefully evaluated when making a diagnosis of sympathetic PGL.Intimate follow-up is necessary and very important.Treatment with therapeutic doses of131I-MIBG or combination chemotherapy (cyclophosphamide,vincristine,and darcabazine) may induce(partial) responses to malignant PGLs.7External beam irradiation can be useful in the treatment of local tumor.
In conclusion,a heterogeneous,hypervascular,retroperitoneal mass with areas of necrosis and without typical clinical menifestations of headaches,hypertension,and tremor,is highly predictive of the presence of a silent extra-adrenal PGLs.131I-MIBG and octreotide have high sensitivity and accuracy in diagnosis of silent extra-adrenal PGL.Once the correct diagnosis is acquired,surgical treatment should be carried out after using alpha-adrenergic blockade for 2-4 weeks in order to prevent and treat a syndrome of possible intraoperative catecholamine release.Tumors should be resected laparoscopically if possible.Future efforts should be directed toward obtaining a larger database to define the true natural history of silent extra-adrenal retroperitoneal PGLs.More prospective clinical trials are needed to provide the most reliable evidence regarding the management of patients.
1.Timmers HJ,Kozupa A,Eisenhofer G,et al.Clinical presentations,biochemical phenotypes,and genotypephenotype correlations in patients with succinate dehydrogenase subunit B-associated pheochromocytomas and paragangliomas.J Clin Endocrinol Metab 2007;92:779-86.
2.Rubello D,Bui C,Casara D,et al.Functional scintigraphy of the adrenal gland.Eur J Endocrinol 2002;147:13-28.
3.Mozley PD,Kim CK,Mohsin J,et al.The efficacy of iodine-123-MIBG as a screening test for pheochromocytoma.J Nucl Med 1994;35:1138-44.
4.Tenenbaum F,Lumbroso J,Schlumberger M,et al.Comparison of radiolabeled octreotide and meta-iodobenzylguanidine (MIBG) scintigraphy in malignant pheochromocytoma.J Nucl Med 1995;36:1-6.
5.Lack EE,Lloyd RV,Carney JA,et al.Recommendations for the reporting of extra-adrenal paragangliomas.Hum Pathol 2003;34:112-3.
6.Young WF Jr.Paragangliomas:clinical overview.Ann N Y Acad Sci 2006;1073:21-9.
7.Fitzgerald PA,Goldsby RE,Huberty JP,et al.Malignant pheochromocytomas and paragangliomas:a phase II study of therapy with high-dose 131I-metaiodobenzylguanidine (131I-MIBG).Ann N Y Acad Sci 2006;1073:465-90.
杂志排行
Chinese Medical Sciences Journal的其它文章
- Sex Hormones and Androgen Receptor:Risk Factors of Coronary Heart Disease in Elderly Men△
- Comparison between Ophthalmologists and Community Health Workers in Screening of Shallow Anterior Chamber with Oblique Flashlight Test△
- Factors Influencing Pleural Effusion after Fontan Operation:an Analysis with 95 Patients
- Relationship between Carotid Atherosclerosis and Cerebral Infarction
- Expression of FLICE-inhibitory Protein in Synovial Tissue and Its Association with Synovial Inflammation in Juvenile Idiopathic Arthritis△
- Revascularization for Iliac-femoral Artery Pseudoaneurysm with Greater Saphenous Vein
