一维链状配位聚合物{[Ni(4-abaH)(2,2′-bipy)(NO3)(H2O)](NO3)}n的合成与晶体结构
2010-11-10黄妙龄
黄妙龄
(泉州师范学化学与生命科学学院,泉州 362000)
研究简报
一维链状配位聚合物{[Ni(4-abaH)(2,2′-bipy)(NO3)(H2O)](NO3)}n的合成与晶体结构
黄妙龄
(泉州师范学化学与生命科学学院,泉州 362000)
镍配合物;2,2′-联吡啶;4-氨基苯甲酸;配位聚合物;晶体结构
0 Introduction
The design and synthesis of coordination polymers has been a subject of intense research due to their novel structures such as diamond,square network,brick wall network,octahedral network and so on[1-7],and unique properties in magnetic,catalytic activity and biological activity[6-9].4-aminobenzoic acid is a good linear bridging ligand with oxygen and nitrogen donors on opposite sides,and it is also a good donor and/or acceptor of hydrogen bonds in assembly of supermolecular systems[9-11].In this paper,we report a novel structure of complex with 4-aminobenzoic acid and 2,2′-bipyridine.
1 Experimental
1.1 Materials and instruments
All the regents and solvents were used as commercial sources without further purification.Elemental analyses were performed on an Elementary Vario EL analyzer.The IR spectra were recorded on AVATAR 360 spectrophotometer using KBr discs.The crystal was determined on a Bruker SMART APEXⅡCCD diffractometer equipped with a graphitemonochromatized Mo Kα radiation(λ=0.071073 nm).
1.2 Preparation of the title compound
An aqueous solution(5 mL)of Ni(NO3)2·2H2O(1 mmol)was added dropwise to an aqueous solution(5 mL)of 4-amionbenzoic acid(1.5 mmol).After refluxing for 1 h at 60~70 ℃,an ethanol solution(5 mL)of 2,2′-bipyridine (1 mmol)was added slowly to the mixed solution,and continue refluxing for 2 h,the mixture was filtered off while hot.The green single crystals suitable for X-ray analysis were obtained by slow evaporation of the above filtrate at room temperature after two months.Yields based on Ni:41%.Ana.Calcd.for C17H17N5NiO9(%):C 41.25,H 3.40,N 14.10;found(%):C 41.33,H 3.47,N 14.18.Main IR bands(cm-1)3321(s),3276(s),1658(s),1603(s),1575(vw),1516(w),1465(m),1444(m),1 384(vs),1 295(s),1 253(w),1 175(m),1 097(w),1 062(vw),1 029(m),1 016(m),1 001(w),855(w),772(m),735(m),700(w),652(w),621(w),539(w).
1.3 X-ray crystallography and structure determination
A green block crystal with dimensions of 0.37 mm×0.27 mm×0.21 mm was selected for the measurement.The diffraction data were collected at 293(2)K on a Bruker SMART APEXⅡCCD fractometer equipped with a graphite-monochromatized Mo Kα radiation(λ=0.071 073 nm).The structure was solved by direct methods with SHELXS-97.The hydrogen atoms were assigned with common isotropic displacement factors and included in the final refinement by use of geometrical restrains.A fullmatrix least-squares refinement on F2was carried out using SHELXL-97.The final R=0.024 9,wR=0.058 6 (w=1/[σ2(Fo2)+(0.027 3P)2+0.836 8P]where P=(Fo2+2Fc2)/3),S=1.024,(Δ/σ)max=0.002,(Δρ)max=207 and (Δρ)min=-216 e·nm-3.Table 1 shows crystallographic crystal data of the title complex.
CCDC:692857.
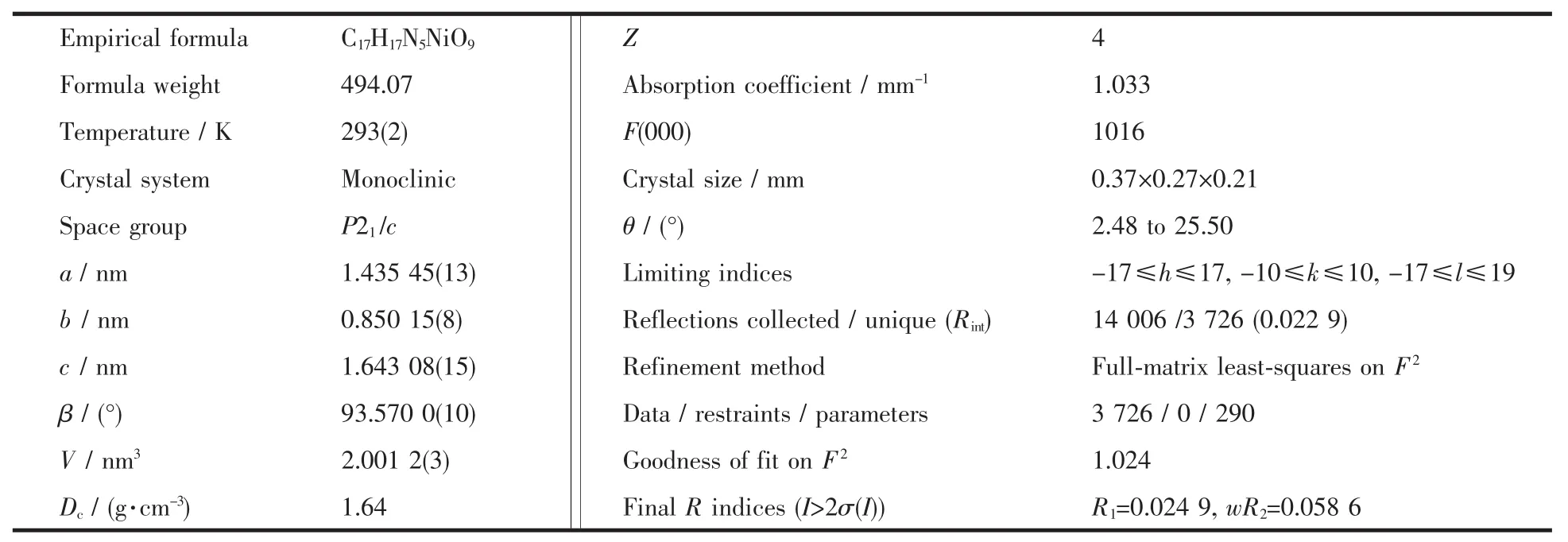
Table 1 Crystal data and structure parameters for the title complex
2 Results and discussion
2.1 Crystal structure of the title complex
The molecular structure of the title complex is shown in Fig.1,and the 1D chain structure in Fig.2.The selected bond lengths and bond angles are given in Table 2.


Table 2 Selected bond lengths(nm)and bond angles(°)for the title complex
It is interesting that the 4-abaH acts as a bidentate bridging ligand in the title complex,which uses only one of the carboxyl oxygen atoms to bond the Ni atom,while the other oxygen atom did not take part in the coordination and not deprotonation,which is very different from the reported 4-aba complexes[9-11].On the other hand,the 1D chains are further formed into a 2D layerstructure (Fig.3)through hydrogen bonds interactions between coordination nitrate and 4-abaH ligand,uncoordinated nitrate and coordination waters(Table 3).Furthermore,there are other hydrogen bonds such as O(6)-H(1W)…O(9)D,N(1)-H(1A)…O(4)C,which extend the complex into a 3D network structure.

Fig.3 2D layers structure for the complex formed by hydrogen bonds
2.2 Spectra characteristics
The infrared spectra of the title complex has been recorded and some important assignments are shown above.In the IR spectra,the band at 3 321 cm-1,due to the ν(O-H)absorptions of water molecules.On the other hand,the title complex displays characteristic bands of carboxylate groups(4-aba)at 1 658 cm-1for asymmetric vibrations and at 1 444 cm-1for symmetric vibrations,respectively.The value of Δν(as-s)is 214 cm-1,comparatively larger than 200 cm-1,indicating the monodentate coordination mode of the carboxyl group to the metal ion[12]which is in good according with the X-ray crystal analysis result.The strong absorption at 1 384 cm-1indicates that the dissociative nitrate ion exists,while the bands of 1465,1295,855 and 735 cm-1are ascribing to the coordinated nitrate[13].
[1]Zhang R F,Wang Q F,Yang M Q,et al.Polyhedron,2008,27:3123-3131
[2]Dybtsev D N,Yutkin M P,Peresypkina E V,et al.Inorg.Chem.,2007,46:6843-6845
[3]Zhang J,Liu R,Feng P Y,et al.Angew.Chem.Int.Ed.,2007,46:8388-8391
[4]Zhang J,Chen S M,Valle H,et al.J.Am.Chem.Soc.,2007,129:14168-14169
[5]Zhu Y Y,Li J,Sun Z G,et al.Inorg.Chem.Com.,2009,12:38-40
[6]Liu B L,Xiao H P,Nfor E N,et al.Inorg.Chem.Com.,2009,12:8-10
[7]Zhang D J,Song T Y,Wang L,et al.Inorg.Chim.Acta,2009,362:299-302
[8]Lu X Q,Bi W Y,Chai W L,et al.Polyhedron.,2009,28:27-32
[9]Wang Y,Okabe N B.Inorg.Chim.Acta,2005,357:3407-3416
[10]Wang R H,Hong M C,Luo J H,et al.Inorg.Chim.Acta,2004,357:103-114
[11]Wang R H,Jiang F L,Zhou Y F,et al.Inorg.Chim.Acta,2005,358:545-554
[12]Zhan D J,Song T Y,Wang L,et al.Inorg.Chim.Acta,2009,362:299-302
[13]Braibanti A,Dallavalle F,Pellinghelli M A,et al.Inorg.Chem.,1968,7:1430-1433
Synthesis and Crystal Structure of One-Dimensional Coordination Polymer:{[Ni(4-abaH)(2,2′-bipy)(NO3)(H2O)](NO3)}n
HUANG Miao-Ling
(College of Chemistry and Life Sciences,Quanzhou Normal University,Quanzhou,Fujian 362000)
The title compound,{[Ni(4-abaH)(2,2′-bipy)(NO3)(H2O)](NO3)}n,where 4-abaH=4-aminobenzoic acid and 2,2′-bipy=2,2′-bipyridine,was synthesized and its crystal structure was determined by X-ray diffraction analysis.The crystal is of monoclinic,space group P21/c with a=1.43545(13)nm,b=0.85015(8)nm,c=1.64308(15)nm,β=93.570 0(10)°,V=2.0012(3)nm3,Z=4,Mr=494.07,Dc=1.640 g·cm-3,μ=1.033 mm-1,F(000)=1 016,R1=0.024 9,wR2=0.058 6.The Ni atoms are octahedrally coordinated by three N atoms from 2,2′-bipyridine and 4-abaH ligand,and three O atoms from nitrate,4-abaH ligand and water molecule,respectively.The complex shows a one-dimensional zigzag chain bridged by 4-abaH molecules.CCDC:692857.
nickel complex;2,2′-bipyridine;4-aminobenzoic acid;coordination polymer;crystal structure
O614.81+3
A
1001-4861(2010)10-1912-04
2010-03-22。收修改稿日期:2010-06-01。
福建省科技厅项目(No.2008F5053)和泉州师范学院硕士点建设立项资助。
E-mail:hml301@163.com
作者:黄妙龄,女,34岁,硕士,副教授;研究方向:配位化学。
