木荷及其与无患子皂甙抽提物单复配体外抗稻瘟病活性
2010-10-09霍光华詹五根付日辉陈明辉
霍光华,詹五根,付日辉,陈明辉
江西农业大学生物科学与工程学院,南昌 330045
木荷及其与无患子皂甙抽提物单复配体外抗稻瘟病活性
霍光华*,詹五根,付日辉,陈明辉
江西农业大学生物科学与工程学院,南昌 330045
来自木荷叶和无患子中果皮的皂甙抽提物对抗稻瘟病原活性被试验。皂甙抽提物体外抑制活性显示了皂甙剂量与抗真菌效果高度相关性。它们的抗稻瘟有效中浓度 EC50:木荷叶乙醇抽提物为 42.95μg/mL,木荷叶水抽提物为 452.91μg/mL,无患子中果皮的甲醇抽提物为 95.65μg/mL。一个重要的结果是当上述木荷叶和无患子中果皮醇抽提物以 3∶4到 15∶8的质量配比时产生了显著的增效抗稻瘟作用,而在其它配比时有相加作用。通过乙醚/丙酮沉析、硅胶柱色谱分离,以及酸水解和薄层过程,检测出木荷抽提物皂甙中含有 2种三萜类皂甙元和 5种单糖。纯化后的皂甙在 150μg/mL时显示了 98.28%的抑制率。此研究结果表明:木荷叶抽提物,或木荷叶和无患子中果皮抽提复配物对毁灭性的稻瘟病害具有重要地抗病效果。
皂甙抽提物,复配,木荷,无患子,稻瘟病
Abstract:Saponin extracts fromSchim a superbaleaves(SSL)andSapindus m ukorossifruit mesocarp(S MFM)have been tested against the pathogenPiricularia oryzae.The inhibitory effectof these extractsmeasuredin vitroshow a nearly relationship between the saponin dose and the antifungal effect.Their valuesof EC50(effective concentrations at 50%inhibitory rate)are 42.95μg/mL for an ethanolic extract of SSL,452.91μg/mL for a water extract of SSL and 95.65 μg/mL for amethanolic extractof S MFM respectively.An important resultwas that combination of extracts from SSL and S MFM have notable synergistic effect at ratios ranged from 3∶4 to 15∶8(m/m)and additive effect at other ratio.In addition,antifungal components of SSL were established as triterpenoid saponins composed of 2 aglycones and 5 monosaccharides after purification by ethyl ether/acetone precipitation,silica gel chromatography,hydrolysis and TLC procedure.The purified saponin complex showed up to a 98.28%inhibitory rate at 150μg/mL.The results suggested that selected extracts from SSL or a combination of SSL and S MFM can play an important role in resistancePiricularia oryzae,a destructive disease of rice.
Key words:Saponin-extract;Combination;Schim a superba;Sapindus m ukorossi;Piricularia oryzae
Introduction
Rice blast is the most destructive disease of rice caused by the fungal pathogen,PiriculariaoryzaeCavaral[M agnaporthe grisea(Hebert)Barr].P.oryzaecan infect rice from the seedling stage to maturity.Infection results in lesions on most plant parts including leaves,leaf collar,stems,nodes,panicles and grains.It is estimated for the disease to reduce annualworldwide yield by 157 million tons(Shi andWang,2008).Controlling this disease has largely relied on breeding resistant cultivars and applying chemical fungicides.However,the effectiveness of these strategies is limited because of the frequent emergence ofP.oryzaeisolates that are able to overcome specific resistance genesor fungicides.Antifungal chemicals from natural products exhibit ben-eficial effect(Grayer and Harboune,1994;Grayer and Kokubun,2001;Mukhopadhyay,2004;Rodrigueset al,2005;Dawet al,2008;).Therefore,we carried out experiments to isolate new natural bioactive products from plants,which can be used against rice blast.
Sch im a superbaGardn.et Champ(Theaceae)ismainly distributed in Central and Southern China.The plant is commonly cultivated as an ornamental tree,for t imber production and as a firebreak planting.Its bark powder is used as a repellant agent to dispel wild pigs and birds from crops in Fujian mountain,to poison fish in Taiwan,and as an arrow tip toxin by traditional hunters.A methanolic extract of its stem bark is used to kill snails(Zeng and Ju,2005),to induce apastia inPlutella xylostellaandPieries rapae(Denget al,2007),and to control intestinal parasite.We recently find a strong antifungal effect of an ethanolic extract ofSch im a superbleaves onP.oryzae(Huoet al,2008).
The objective of thispaperwas to test,in vitro,the antifungal activity of saponin-rich extractsof leavesofSchim a superba,and its combination with extract of a mesocarp ofSapindus m ukorossiGaertn.The chemical components of these extractswere also investigated.The overall objective of the study was to search formore selective,more environmentally friendly and toxicologically safe fungicides.
Materials andM ethods
Plantmaterial and pathogen ic fungal stra ins Leaves ofSch im a superba(SSL)and fruits ofSapindus m ukorossi(S MF)were collected from Jiangxi Province of China in November 2007.They were identified by Prof.X.H.Shi of the College of Forestry,University of JiangxiAgriculture.Voucher specimens have been deposited at the herbarium of thisCollege.The pathogenic fungal strain isPyricularia oryzae05Z11 obtained from the Research School of Plant Protection,Academy of JiangxiAgricultural Science.
Preparation of sapon in-rich extracts
Sch im a superbaThe air-dried and powdered leaveswere extracted with a series of solvents with increasing polarities.These were petroleum,chlorofor m,ethyl acetate,ethanol and water successively.The ethanolic phase was further purified by precipitationwith anhydrous ethyl ether/acetone 1:1(v/v),centrifugation at 3000 rpm for 15 min,silica-gel(100~200 mesh)chromatography,elution with CH3OH/CHCl3/H2O 5:2:1(v/v/v).The eluted fraction was dried under a steam ofN2.
Sapindus m ukorossi
After removing the seed and epicarp from the fruit,the mesocarp was dried in an oven at 50℃for 48 h,and then powdered in a grinder.The powdered sample was extracted with methanolwhich gave a brown syrup.The suspended particleswere separated by centrifugation at 8000 rpm for 20 min,followed by vacuum drying of the extract at 70℃,which gave a pale yellowish-white powder.
Identification of sapon in-rich fractions fromSchim a superba
Fractions fromSchim a superbawere tested by using a foam test,concentrated sulphuric acid reaction and Rosen-heimer reaction.The fraction isolated using silica gelwas hydrolyzed with 1 M H2SO4,at 90℃under reflux conditions for 15 h.The mixture was extracted with ethyl acetate to obtain the aglycone part,and the aqueous layer was neutralized with BaCO3,centrifuged at 4500 rpm for 10 min and filtered.The ethyl acetate layer and the filtrate from the H2O layerwere analyzed on TLC plate with silica gel G using a CH3OH/CHCl33∶1 solvent with 10%H2SO4color reagent,and using n-butanol/ethyl acetate/isopropanol/acetic acid/H2O/pyridine 12∶34∶21∶12∶10∶10 solvent with aniline-phthalic acid color reagent respectively.The following standard saccharides(galactose,glucose,mannose,arabinose,rhamnose,xylose)were used for identification of the color spots.
Growth inhibition measurements
Extracts of SSL,S MFM in different concentrations(50,100,200,400,800,1600μg/mL)and a negative control(0μg/mL,CK)were added to separate Erlenmeyer flasks containing sterilized PDA(121℃,1.2 atm,20 min)and mixed thoroughly,then poured separately into sterilized Petri dishes and allowed to solidify.3 plugswith 8 mm diameters of active growing mycelium colony ofPiricularia oryzaewere placed in a triangularshape in the each plate(replicated,two plate,6 plugs per treatment).These plateswere then incubated at 27℃.Measurement of the colony diameter of the radial mycelium growthwas carried outon the 4thday.The inhibitory rate of each treatment was expressed as the percent growth inhibition compared to the negative control using the following formula(Test rule,2006),where OCDt=observed colony diameter with treatment,OCDc=observed colony diameterwithout treatment,IPD = initial plug diameter,G MCt= growth measurement of colonywith treatment,G MCc=growth measurement of colony without trea tment,I R= inhibitory rate.
G MCt=OCDt–IPD
G MCc=OCDc–IPD
IR(%)=(G MCc– G MCt)/G MCc×100 The relationship between saponin dose(x)and antifungal effect(probability value about inhibitory rate of reducing the colony diameter,Y)was drawn out.
Comb ination test of sapon in-rich ingredient from Schim a superbaandSapindus m ukorossi
Five different extractmixtures[(a+5b)/6、(a+2b)/3、(a+b)/2、(2a+b)/3、(5a+b)/6]were tested,where a and b are the calculated effective concentrations at 50% inhibitory rate(EC50)of extracts fromSchim a superbaandSapindus m ukorossirespectively.The presence of any synergistic or antagonistic effect was evaluated using the following formula,where CTC=co-toxicity coefficient,O IRM=observed inhibitory rate of mixture,PIRM=predicted inhibitory rate of mixture.
CTC(%)=(O I RM– PIRM)/PIRM ×100 and where CTC>20 indicates a synergistic effect,CTC from-20 to 20 indicates an additive effect,CTC<-20 indicates an antagonistic effect(Wei,1999;Chen,2000).
Statistical analysis
Statistical analysis of the data was performed with software DPS3.01 for means in every table,linear regression equations and determining significant difference levels at p=0.01 level of significance.
Results
Effect of different solvent extraction fractions of SSL and SM FM on mycelium growth of P.oryzae in vitro

Table 1 Extractive rate(500 g plantmaterial)and antifungal activity of extractsin different polar solvent of SSL and methanol extract of SM FMat 800μg/mL
The total amount of extractive obtained (extractive rate)from SSL using 6 solvents with different polarity is 33.13% (Table 1),including 19.11%hydrophilic fractions(ethanolic and water extractions),12.87%hydrophobic fractions(chlorofor m and petroleum extractions)and 1.15%moderate hydrophobic fractions(ethyl acetate and ethyl ether extractions).The antifungal components mainly exist in hydrophilic fractions.The strongest antifungal activity against blast(99.02%inhibitory rate at 800μg/mL)is in the ethanolic extract.The antifungal activity(63.5% inhibitory rate)of the water extractwas less than for the ethanolic extract,while other components had little antifungal activity,or even promoted mycelium growth.There was also good antifungal activity against blast with the methanolic extract of S MFM having 96.34%inhibitory rate at 800μg/mL(Table 2).
Effective concentration of active fractions inhibit ing mycelium growth of P.oryzaein vitro
The comparison between logarithm values of concentration(logx)and probability values of inhibitory rate(y)(Table 2)indicates a nearly relationship between dose of extracts and activity effect using the software DPS3.01.Their linear relationships are expressed by the equations y=2.00+1.84 logx,R=0.999 for ethanolic extract of SSL;y=1.61+1.28 logx,R=0.992 for the water extract of SSL and y=0.97+2.04 logx,R=0.997 for methanolic extract of S MFM respectively.The calculated effective concentrations at 50% inhibitory rate(EC50)using above equations are 42.95 μg/mL,452.91μg/mL,and 95.65μg/mL for the ethanolic extract of SSL,the water extract of SSL and the methanolic extract of S MFM respectively.The inhibitory concentration and active range show the strongest antifungal extract is ethanolic fraction of SSL and second ismethanolic fraction of S MFM,while the water fraction of SSL shows only weak antifungal activity.

Table 2 The inhibitory effect of ethanolic extract and water extract of SSL,and methanolic extract of SM FM in different concentrations onP.oryzae
Comb ined effect of ethanolic extract of SSL and methanolic extract of SM FM on inhibit ing mycelium growth of P.oryzaein vitro
The combined behavior of the ethanolic extract of SSL and methanolic extract of S MFM systems are shown in Table 3.Their mass ratios are 3:40,3:16,3:8,3:4,15:8 based on the concentration ratios from five different extractmixtures:(a+5b)/6,(a+2b)/3,(a+b)/2,(2a+b)/3,(5a+b)/6 where a=42.95μg/mL,b=95.65μg/mL respectively.It is observed that cotoxicity coefficient values are above 20 with the extract combination ratios 3:4 and 15:8.The best effect at the ratio of 15:8 gives rise to 55.09% synergistic effect.The other combinations all show an additive effect because their cotoxicity coefficient values are in the range from 1.7 to 9 on the standard range from-20 to 20.No antagonistic effects were observed.This indicates that using a combination of SSL and S MFM ismore effective in inhibitingmycelium growth ofP.oryzaethan using either SSL or S MFM extracts alone.

Table 3 Cotoxicity coefficient of extract combinations of SSL and SM FM I nhibitting mycelium growth ofP.oryzae
The effect of purification of the ethanolic SSL extract on inhibition of mycelium growth of P.oryzae in vitro
The results in Table 4 show that the inhibitory rate of the ethanolic extract of SSL is increased with its increasing purity.Solvent precipitation is a good technique amenable to large scale operation and industrialization.It improves relative purity from 7.04%to 45.74%,and enhances the inhibitory rate from 81.20% to 93.27%at 150μg/mL.Silica gel chromatography is a high resolution method,but its isolative efficiency is limited because of column volume and adsorption.It increases relative purity from 45.74%to 69.41%,and inhibitory rate from 93.27% to 98.28%at 150μg/mL.The inhibitory rate is increased gradually with the increasing purity of isolation of SSL.This indicates a non-linear relationship between dose and inhibitory effect.At the same time,it shows that antifungal active components are being concentrated in the successive fractions isolated.
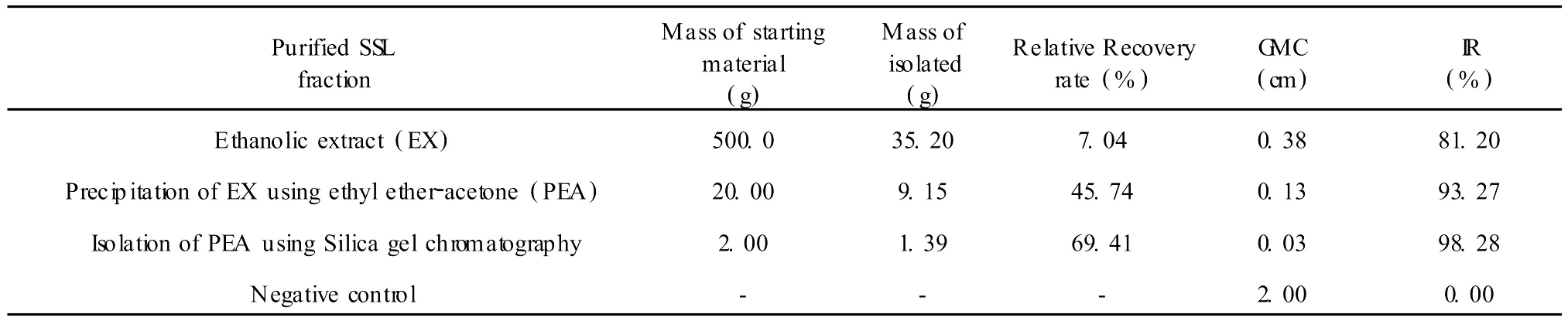
Table 4 The relative recovery rate and inhibitory rate in each fraction from SSLat 150μg/mL
Identification of antifungal component fromS.superba
A foam testwas carried out on aqueous solutions of the SSL ethanolic extract,and on its ethyl ether/acetone precipitation fraction and its silica gel elution after 10 minutes of boiling.Each fraction gave similar persistent foam and there was no difference in foam height of foam measurement with same concentration and tube size between an acidic solution pH 1.0(adjusted with HCl)and an alkaline solution pH 13.0(adjusted with NaOH).The result is consistent with the presence of triterpenoid saponins.The reaction color of the extracts when tested with concentrated sulfuric acid changed from yellow to red,then purple;while the reaction color when tested with trichloroacetic acid at 100℃gave a red color,again consistentwith the presence of triterpenoid saponins.
To further identify the saponins,the final isolate was hydrolyzed with 1 M sulphuric acid,the resulting products separated with an ethyl acetate/water separation and applied to a TLC plate.The aqueous phase gave five spots that were identified as the monosaccharides(glucose withRf0.23,galactose withRf0.15,arabinose withRf0.36,xylose withRf0.45,rhamnose withRf0.55).The ethyl acetate phase gave two spots considered to be aglycones(Rf0.25,0.54 respectively).It is likely that the SSL extract has at least two different triterpenoid saponins,and that these saponins fromS.superbaare antifungal againstP.oryzae.
D iscussion
Seven triterpenoid saponins have been isolated from a methanolic extract of S MFM and shown molluscicidal effects againstPom acea canaliculata(Huang,2003).Some saponins exhibitmoderate cytotoxicity against human tumor cells(Huang,2008).In addition,The pericarp of S.mukorossi has been traditionally used as a source of natural surfactants(Kommalapatiet al,1997;Kommalapatiet al,1998;Rao and Paria,2009).This is the first report of SSL and S MFM having antifungal effects againstP.oryzae.
While alcohol extracts of SSL and S MFM have antifungal properties,mixturesof the two extracts can have enhanced,or synergistic,effects and result in more efficient use of extractants.The synergistic and additional effects of the combination of SSL and S MFM extracts may be related to differences in association of different saponin molecules with sterols(mainly cholesterol)present in the fungal cell.Differences in detoxifying glycosidases(Bouarabet al,2002)from pathogenic fungi is difficult to aportion to different saponin molecules.It is possible to increase antifungal activity of some saponins with partial deglycosylation(Strardo andMartin,2008).
To completely control the mycelium growth ofP.oryzae,it is necessary to use more than 800μg/mL of ethanolic SSL extract,ormore than 370μg/mL of purified SSL extract or 1600μg/mL methanolic S MFM ex-
tract.However,in combination it is only necessary to use 300μg/mL of ethanolic SSL extract plus 60μg/mL ofmethanolic S MFM extract,or150μg/mL of ethyl ether/acetone SSL precipitation plus 60μg/mL of methanolic S MFM extract(at the optimum dose-effect ratio of 15∶8)from ourin vitroexperiments.Future field applicationswill be required to validate these resultsin vivo.
Extracts of active antifungal ingredients isolated from SSL and S MFM or combinations of these extracts can play an important role in controllingP.oryzae.The results of this studymay ultimately help in search for environmental approaches to control crop disease and avoid chemical contamination.Saponin extracts may be useful as an attractive alternative antifungal agent,especially under organic management.
Acknowledgements Authors thank Dr Bala Thumma and Charlie Bell from plant industry of CSIRO in Australia for their helpful advice and proofreading the English style of our manuscript.This work was supported by Science Project from Jiangxi Education Depar tment(GJJ09162), Jiangxi Natural Science Fund(2009GZN0029)and Chinese National Natural Science Fund(31060250).
1 Bouarab K,Melton R,Peart J,et al.A saponin-detoxifying enzyme mediates suppression of plant defences.Nature,2002,418:889-892.
2 Chen L,Xu HH,Li YY,et al.The screening methods to obtain the optimal synergistic mixture ratio of combined pesticides.J Plant Prot,2000,27:349-354.
3 Daw BD,Zhang LH,Wang ZZ.Salicylic acid enhances antifungal resistance toM agnaporthe griseain rice plants.Aust Plant Pathol,2008,37:637-644.
4 Deng ZY,Deng YC,Liu YH.Antifeedant activities of the extracts fromShim a superbaetChamp.againstPlutella xylostellaandPieries rapae.Pesticide,2007,46:854-856.
5 Grayer RJ,Harborne JB.A survey of antifungal compounds from higher plants,1982-1993.Phytochem istry,1994,37:19-42.
6 Grayer RJ,Kokubun T.Plant-fungal interactions:the search for phytoalexins and other antifungal compounds from higher plants.Phytochem istry,2001,56:253-263.
7 Huang HC,Liao SC,Chang FR,et al.Mollusicicidal saponins fromSapindus m ukorossi,inhibitory agents of golden apple snails,Pomacea canaliculata.J Agric Food Chem,2003,51:4916-4919.
8 Huang HC,Wu MD,Tsai WJ,et al.Triterpenoid saponins from the fruits and galls ofSapindusm ukorossii.Phytochem istry,2008,69:1609-1616.
9 Huo GH,ZhanWG,ChenMH.Antifungal activities from twoTheaceae Schima superbaandCam ellia oleiferaagainst Pyricular oryzae.Acta Agric Univ Jiangxiensis,2008a,30:48-52,72
10 Huo GH,Zhan WG,Chen MH,et al.Compound preparation and its manufacture method for resistance blast fromShima superba.CN Patent,2008b,application No.200810070483.7
11 Kommalapati RR,Valsaraj KT,ConstantWD,et al.Aqueous solubility enhancement and desorption of hexachlorobenzene from soil using a plant-based surfactant.W at Res,1997,31:2161-2170.
12 Kommalapati RH,Valsaraj KT,ConstantWD,et al.Soil flushing using colloidal gas aphron suspensions generated from a plantbased surfactant.J Hazard Mater,1998,60:73-87.
13 MukhopadhyayB,Field RA.Synthesis of L-arabinose-containing fragments of the oat root saponin avenacin.Carbohyd Res,2004,339:1285-1291.
14 Rodrigues FA,JurickIIWM,Datnoff LE,et al.Silicon influences cytological and molecular events in compatible and incompatible rice-M agnaporthe griseainteractions.PhysiolM ol Plant Pathol,2005,66:144-159.
15 Rao KJ,Paria S.Solubilization of naphthalene in the presence of plant-synthetic mixed surfactant systems.J Phys Chem B,2009,113:474-481.
16 ShiBJ,Wang GL.Comparative study of genes expressed from rice fungus-resistant and susceptible lines during interactions withM agnaporthe oryzae.Gene,2008,427:80-85.
17 StuardoM,Martin RS.Antifungal properties of quinoa(Chemopodium quinoqW illd)alkali treated saponins againstBotrytis cinerea.Ind Crops and Prod,2008,27:296-302.
18 The test rule of pesticide biological determination in room.ge rmicide,the second part:growth test inhibitting pathogen fungal mycelium.method of culture dish,NY/T 1156.2-2006,China.
19 Wei L.Manufacture on Pesticide Mixture and Their Types.Beijing:Chemical Industry Press,1999.22-24.
20 Lu Y,Zeng XN,Ju JH.Molluscidal activity of the methanol extracts of 40 species of plants.Plant Prot,2005,31:31-34.
In vitro Antifungal Activity of Sapon in Extracts from Schim a superbain Combination withSapindus mukorossiaga inst Piricularia oryzae
HUO Guang-hua*,ZHAN Wu-gen,FU Ri-hui,CHEN Ming-hui
College of B ioscience and Engineering,University of Jiangxi Agriculture,Nanchang 330045,China
S482.2+92
A
1001-6880(2010)05-0755-06
Received September 27,2009;Accepted November 21,2009
Foundation Item:Thisworkwas supported by Science Project from Jiangxi Education Department(GJJ09162),Jiangxi Natural Science Fund(2009GZN0029) and Chinese National NaturalScience Fund(31060250)
*Corresponding author Tel:86-791-3828079;E-mail:hgh3813899@sohu.com