Microwave-Hydrothermal Synthesis of Nanoporous MetalPhosphate CoVSB-1
2010-09-05XIELiliWANGLijunYUANHaoTIANZhenSchoolofUrbanDevelopmentandEnvironmentalEngineeringShanghaiSecondPolytechnicUniversityShanghai201209China
XIE Li-li, WANG Li-jun, YUAN Hao, TIAN Zhen(School of Urban Development and Environmental Engineering, Shanghai Second Polytechnic University, Shanghai 201209, P.R.China)
Microwave-Hydrothermal Synthesis of Nanoporous Metal
Phosphate CoVSB-1
XIE Li-li, WANG Li-jun, YUAN Hao, TIAN Zhen
(School of Urban Development and Environmental Engineering, Shanghai Second Polytechnic University, Shanghai 201209, P.R.China)
Nanoporous metal phosphate CoVSB-1 was prepared via the microwave-hydrothermal synthesis, and the yield of about 89 % has been achieved for 30 min at 453 K under microwave irradiation. The crystallization time of CoVSB-1 was reduced by about 8 times compared with the conventional electric heating. The fast crystallization favors more uniform particle size distribution and smaller aspect ratios of CoVSB-1 product. By the microwave-hydrothermal synthesis, the product of VSB-1 formed for at least 60 min.
CoVSB-1; VSB-1; microwave-hydrothermal synthesis
0 Introduction
Microwave hydrothermal synthesis has been widely applied to the preparation of various aluminosilicate zeolites and aluminophosphate molecular sieves (AlPO) materials[1-3]. It has advantages of rapid crystallization[2], narrow particle size distribution[4], increased phase purity and phase selectivity[5]and decreased size of crystal[6-7]. Metal ions can also be introduced into the framework of AlPOs/SAPOs molecular sieves through microwave hydrothermal synthesis[3]. Recently, it has been reported that microwave heating produces large pore CoAPOs molecular sieves (type VFI of 18MR and type AFI of 12MR)[8]due to the rapid crystallization of microwave method. It was suggested that microwave synthesis is useful in the preparation of relatively unstable materials especially with large pore.
A large pore open-framework nickel phosphate VSB-1 (24MR) has higher thermal stability than other nanoporous metal phosphates[9]. Many studies involving the synthesis, structure characterization, and properties (ion-exchange, adsorption and catalysis) of VSB-1 itself have been carried out during the past several years[10-14]. Meanwhile, modification in framework[15]or channel[16]and host-guest assemble of functional nanometer semiconductor species[17-18]based on VSB-1 bring many composites with good physical and chemical properties. Through isomorphous substitution of framework Ni2+with Co2+, nanoporous metal phosphate CoVSB-1 has been successfully fabricated[19-20]. CoVSB-1 has large adsorption capacity for NH3(23.7 %) and H2NCH2CH2NH2(24.3 %)[21]. Different from VSB-1, CoVSB-1 can be taken as a potential catalyst with the selectivity of 60.8% to benzaldehyde in styrene oxidation reaction[22]. However, the general preparation of CoVSB-1 is hydrothermal method at 443 K under conventional electric heating. It requires relatively long crystallization time of 4 hours to form crystals with high quality[20]. The wide distribution of crystal size has also been observed. Thus, fast crystallization and facile morphology control of CoVSB-1 is very important from the standpoint of application and characterization.
In this work, we reported the rapid microwave synthesis of nanoporous metal phosphate CoVSB-1 using VSB-1as a reference compound. CoVSB-1 prepared through microwave-hydrothermal method was well characterized, the crystallization kinetic of CoVSB-1 and VSB-1 was discussed.
1 Experimental
1.1 Synthesis of CoVSB-1
Pure VSB-1 and CoVSB-1 were prepared using the molar composition of xCoO:yNiO:200H2O:4.25P2O5:5.5HF:4.5en (x=0 and y = 5 for VSB-1; x = 2.5 and y = 2.5 for CoVSB-1), similar to the previous reaction composition[19-20]. The reaction mixture was sealed and placed in a microwave oven (MDS-6 microwave digestion/extraction system, Shanghai SINEO , maximum power of 1 000 W). The precursor was preheated for 1 min at higher power of 1 000 W and then the microwave power was adjusted to 400 W. The predetermined temperature was 453 K and the crystallization time was from 3 min~360 min. The solid product was recovered with centrifugation carefully, washing with de-ionized water and drying. The synthesis of CoVSB-1 and VSB-1 by traditional electric heating was carried out at 453 K for 6 days and the product was named as CoVSB-1-6d and VSB-1-6d respectively. The yield of CoVSB-1-6d and VSB-1-6d was considered to be 100 % because the source of Ni and P to form the structure was almost consumed. Hence, the microwave reaction yield was determined by comparing the weight of the obtained solid under microwave heating with that of CoVSB-1-6d or VSB-1-6d.
1.2 Characterization of CoVSB-1
The structure was determined by X-ray powder diffraction (Bruker-AXS, D8 ADVANCE, Cu Kα radiation) over the range of 3°≤2θ≤45°. The morphology was analyzed with a scanning electron microscope (Philip XL-30). The fractions of mass of Ni, Co, P in CoVSB-1 were measured by an inductively coupled plasma spectrometer (Vista Axial CCD Simultaneous ICP-AES). The BET surface area of sample after being activated at 523 K for 18 h in vacuum was measured on a Micromeritics ASAP 2 020M porosimeter. UV-vis diffusing reflectance spectra were recorded on a Shimadzu UV-3 101 PC, equipped with an integrating sphere using BaSO4as the reference.
2 Results and discussion
Nanoporous metal phosphate CoVSB-1 and VSB-1 were prepared through continuous microwave irradiation. From Fig.1, it could be seen that the reaction mixture was heated up to 453 K in 3 min~4 min and maintained steadily during the next whole synthesis process. CoVSB-1 possessed a faster temperature rising rate than VSB-1 did. The crystallization kinetics of CoVSB-1 and VSB-1 were checked and the result was displayed in Fig. 2 CoVSB-1 product formed quickly within 3 min. There were just dark-red unreacted liquids when the crystallization time was shorter than 3 min. The yield of CoVSB-1 approached 89 % within 30 min and the crystallization time was shortened by about 8 times compared with the result (88 % in 4 h employing traditional hydrothermal synthesis) in our previous work[20]. VSB-1 crystal formed after microwave heating for at least 60 min. Otherwise the green amorphous dried gels appeared when the crystallization time was shorter than 60 min. It took 360 min for VSB-1 to obtain the yield of 83 %. It was indicated that the formation mechanisms of CoVSB-1 and VSB-1 might be different, since VSB-1 formed via amorphous solid phases, while CoVSB-1 formed directly from the liquid solution. It was inferred that the introduction of polar Co2+species enhanced the absorption of microwave energy which promoted the crystallization rate of microwave synthesis.
From Fig.3, it could be seen that the XRD pattern of CoVSB-1 and VSB-1 corresponded well with VSB-1 structure reported previously[19]. There was no crystal phase transformation due to isomorphous substitution of framework Ni2+with Co2+in VSB-1. The results of ICP chemical composition analysis showed that the fraction of mass of Ni, Co and P were 21.83 %, 21.43 % and 15.60 % respectively in CoVSB-1. Therefore, the amount of incorporated cobalt calculated by nCo/(nNi+nCo) was 50 mol%. It implied that microwave synthesis did not lead to amount decline of Co2+transition metal doped into the framework. SEM image was shown in Fig. 4 CoVSB-1 exhibited rod-like crystals with the sizes of (1-2)×(1-2)×(2-4) μm and the aspect ratios of ca. 2. Compared with CoVSB-1-4h obtained through traditional hydrothermal synthesis[20], the size of CoVSB-1 product here decreased by about 10 times with more homogeneous distribution. The aspect ratios were as much as 4 times smaller than that of CoVSB-1-4h and this would favor its catalytic property. The nitrogen adsorption on CoVSB-1 revealed the typicaltype I isotherm (Fig. 5) and the BET surface area is 161 m2/g.

Fig.1 The reaction temperature variety during the first 5min after microwave heating

Fig.2 Crystallization yield of CoVSB-1 and VSB-1 with crystallization time

Fig.3 XRD pattern of CoVSB-1 and VSB-1 prepared under microwave irradiation at 453 K for 30 min and 60 min respectively
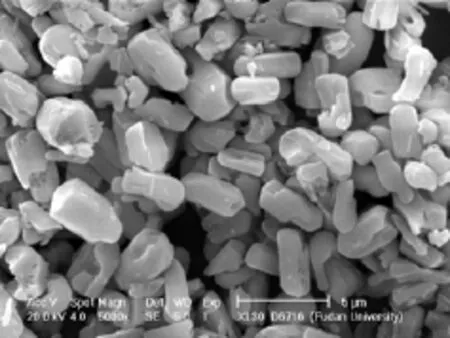
Fig.4 SEM image of the as-synthesized CoVSB-1 prepared under microwave irradiation at 453 K for 30 min

Fig.5 Nitrogen adsorption isotherm on CoVSB-1
In UV-Vis spectra of CoVSB-1 (Fig. 6), the absorption bands among 450 nm~700 nm caused by the d-d electronic transition of Co2+were obvious except for the peak at 417 nm belonging to octahedral Ni2+cations. Two peaks at 584 nm and 624 nm were attributed to the typical4A2(F) →4T1(P) triplet electronic absorption of tetrahedral Co2+cations[23]; the band at ca. 520 nm was associated with the overlap of two peaks: the4T1g(F) →4T1g(P) absorption peak of octahedral Co2+cations and the weak peak from the triplet absorption of tetrahedral Co2+cations[23]. The purple color of as-synthesized CoVSB-1 was mainly embodied by the octahedral framework Co2+cations.
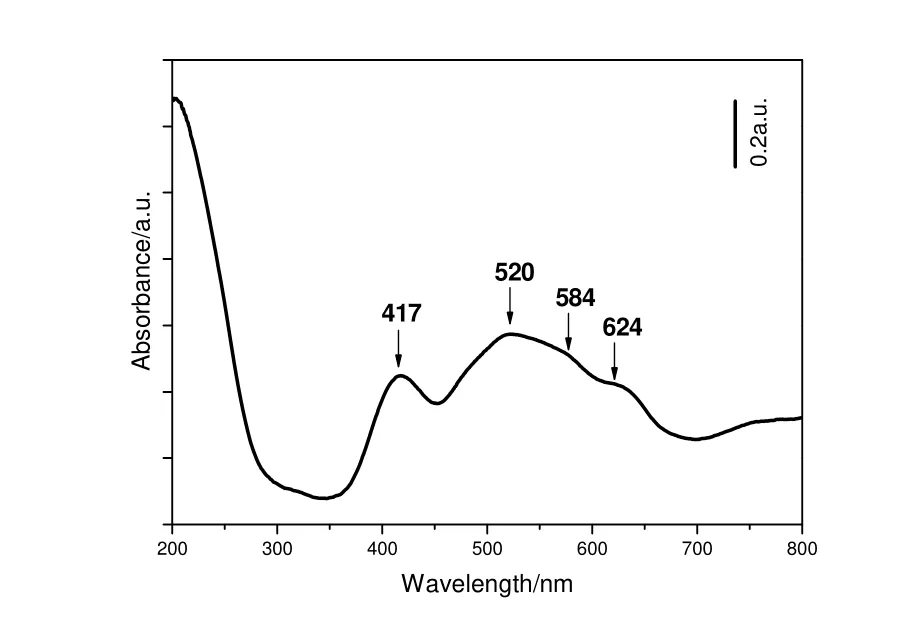
Fig.6 UV-vis spectra of CoVSB-1 prepared under microwave irradiation at 453 K for 30 min
3 Conclusion
The present work utilized microwave hydrothermal method to prepare nanoporous metal phosphate CoVSB-1. The yield of CoVSB-1 product was up to 89 % within 30 min. Compared with conventional synthesis, the crystallization rates increased by about 8 times and the size of CoVSB-1 crystals decreased by about 10 times through microwave irradiation. Meanwhile it manifested much more uniform crystal size distribution and smaller aspect ratios of ca. 2, which could promote its catalytic properties.
4 Acknowledgments
Financial support for this work is provided by the Natural Science Foundation (No. 50503011) and Shanghai Education Development Foundation (No.2007CG72). The authors also gratefully thank the financial support by Shanghai Key Construction Learning Subject P1701.
[1] ARAFAT A, JANSEN J C, EBAID A R, et al. Microwave preparation of zeolite Y and ZSM-5 [J]. Zeolites, 1993, 13(3): 162-165.
[2] PARK M, KOMARNENI S. Rapid synthesis of AlPO4-11 and cloverite by microwave-hydrothemal processing [J]. Micropor. Mesopor. Mater., 1998, 20 (1-3): 39-44.
[3] TOMPSETT G A, CONNER W C , YNGVESSON K S. Microwave synthesis of nanoporous materials [J]. Chem. Phys. Chem. , 2006, 7(2): 296-319 .
[4] XU X C, YANG W S, LIU J, et al. Synthesis of a high-permeance NaA zeolite membrane by microwave heating [J]. Adv. Mater., 2000, 12 (3): 195-198.
[5] JHUNG S H, CHANG J S, HWANG J S, et al. Selective formation of SAPO-5 and SAPO-34 molecular sieves with microwave irradiation and hydrothermal heating [J]. Micropor. Mesopor. Mater., 2003, 64 (1-3): 33-39.
[6] JHUNG S H, JIN T, HWANG Y K, et al. Microwave effect in the fast synthesis of microporous materials: which stage between nucleation and crystal growth is accelerated by microwave irradiation[J]. Chem. Eur. J., 2007, 13 (16): 4410-4417.
[7] JHUNG S H, LEE J H , CHANG J S. Crystal size control of transition metal ion-incorporated aluminophosphate molecular sieves: Effect of ramping rate in the syntheses [J]. Micropor. Mesopor. Mater., 2008, 112 (1-3): 178-186.
[8] JHUNG S H, JIN T, KIM Y H, et al. Phase-selective crystallization of cobalt-incorporated aluminophoshate molecular sieves with large pore by microwave irradiation[J]. Micropor. Mesopor. Mater., 2008, 109(1-9): 58-65.
[9] GUILLOU N, GAO Q, NOGUES M, et al. Zeolitic and magnetic properties of a 24-membered ring porous nickel(II) phosphate, VSB-1 [J]. C. R. Acad. Sci. Paris II C 2, 1999:387-392.
[10] JHUNG S H, YOON J W, HWANG J S, et al. Facile synthesis of nanoporous nickel phosphates without organic templates under microwave irradiation [J]. Chem. Mater., 2005, 17 (17): 4455-4460.
[11] COLMONT M, TERASAKI O. TEM investigation of the microporous compound VSB-1: Building units and crystal growth mechanisms [J]. J. Solid State Chem., 2007, 180 (3): 885-893.
[12] CHANG J S, PARK S E, GAO Q M, et al. Catalytic conversion of butadiene to ethylbenzene over the nanoporous nickel(II) phosphate, VSB-1 [J]. Chem.Commun., 2001 (9): 859-860.
[13] CHANG J S, HWANG J S, JHUNG S H, et al. Nanoporus metal-containing nickel phosphates: a class of shape-selective catalyst [J]. Angew. Chem. Int. Ed., 2004, 43(21): 2819-2822.
[14] FORSTER P M, ECKERT J, CHANG J S, et al. Hydrogen adsorption in nanoporous nickel(II) phosphates [J]. J. Am. Chem. Soc., 2003, 125 (5): 1309-1312.
[15] JHUNG S H, CHANG J S, YOON J W, et al. Synthesis of transition-metal-incorporated nickel phosphate molecular sieves TMI-VSB-1 [J]. Chem. Mater., 2004, 16 (26): 5552-5555.
[16] WANG X L, GAO Q M, WU C D, et al. Preparation, channel surface hydroxyl characterization and photoluminescence properties of nanoporous nickel phosphate VSB-1 [J]. Micropor. Mesopor. Mater., 2005, 85 (3): 355-364.
[17] CHEN Z, GAO Q M, WU C D, et al. Preparation and properties of an ordered, uniform 0.9 nm Ag array assembled in a nanoporous VSB-1 by a simple soft chemical method [J]. Chem. Commun., 2004, (17): 1998-1999.
[18] CHEN Z, GAO Q M , RUAN M L. Electronic coupling one-dimensional Ag/ZnS nanocomposites in a nanoporous nickel phosphate host [J]. Nanotechnology, 2007, 18 (25): 255607.
[19] XIE L L, GAO Q M, SU X L, et al. Synthesis and characterization of nanoporous nickel phosphates VSB-1 with systematic doping of cobalts in the frameworks [J]. Micropor. Mesopor. Mater., 2004, 75 (1-2): 135-141.
[20] XIE L L, GAO Q M, WU C D, et al. Rapid hydrothermal synthesis of bimetal cobalt nickel phosphate molecular sieve CoVSB-1 and its ammonia gas adsorption property [J]. Micropor. Mesopor. Mater., 2005, 86 (1-3): 323-328.
[21] XIE L L, HU J , WU C D. Adsorption properties of the nickel-phosphate-basis molecular sieves [J]. J. Inorg. Mater., 2007, 22 (1): 128-132 ( in Chinese).
[22] XIE L L, GAO Q M , LI Q H, Nanoporous metal phosphate CoVSB-1 catalyst for oxidation of styrene with H2O2[J]. Stud. Surf. Sci. Catal., 2007, 170B:1338-1343.
[23] VERBERCKMOES A A, WECKHUYSEN B M , SCHOONHEYDt R A. Spectroscopy and coordination chemistry of cobalt in molecular sieves [J]. Micropor. Mesopor. Mater., 1998, 22 (1-3): 165-178.
微波水热合成纳米孔金属磷酸盐CoVSB-1
解丽丽,王利军,袁昊,田震
(上海第二工业大学城市建设与环境工程学院,上海 201209)
微波水热合成了纳米孔金属磷酸盐CoVSB-1,453K微波辐照30 min产率可达89%。CoVSB-1的晶化时间缩短为传统水热晶化时间的1/8。快速结晶使CoVSB-1产物颗粒分布更均匀、长径比更小。VSB-1晶粒的生成至少需要微波水热晶化60 min。
CoVSB-1;VSB-1;微波水热合成
TB321
A
1001-4543(2010)01-0007-05
2009-12-10;
2010-01-18
解丽丽(1978-),女,山西人,副教授,博士,主要研究方向为环境友好功能材料,电子邮件:llxie@eed.sspu.cn。
国家自然科学基金(No. 50503011), 上海市教育发展基金会“晨光计划”基金(No.2007CG72)
