Methods of vascular control technique during liver resection: a comprehensive review
2010-06-29WanYeeLauEricLaiandStephanieLau
Wan-Yee Lau, Eric C. H. Lai and Stephanie H. Y. Lau
Hong Kong, China
Review Article
Methods of vascular control technique during liver resection: a comprehensive review
Wan-Yee Lau, Eric C. H. Lai and Stephanie H. Y. Lau
Hong Kong, China
BACKGROUND:Significant hemorrhage together with blood transfusion increases postoperative morbidity and mortality of hepatic resection. Hepatic vascular occlusion is effective in minimizing bleeding during hepatic parenchymal transection. This article aimed to review the current role and status of various techniques of hepatic vascular occlusion during hepatic resection.
DATA SOURCES:The relevant manuscripts were identified by searching MEDLINE, and PubMed for articles published between January 1980 and April 2010 using the keywords "vascular control", "vascular clamping", "vascular exclusion" and "hepatectomy". Additional papers were identified by a manual search of the references from the key articles.
RESULTS:One randomized controlled trial (RCT) and 5 RCTs showed intermittent Pringle maneuver and ischemic preconditioning followed by continuous Pringle maneuver were superior to continuous Pringle maneuver alone, respectively. Two RCTs compared the outcomes of hepatectomy with and without intermittent Pringle maneuver. One showed Pringle maneuver to be beneficial, while the other failed to show any benefit. One RCT showed that ischemic preconditioning had significantly less blood loss than using intermittent Pringle maneuver. Four RCTs evaluated the use of hemihepatic vascular occlusion. One RCT showed it had significantly less blood loss than Pringle maneuver, while the other 3 showed no significant difference. Only 1 RCT showed it had significantly less liver ischemic injury. No RCT had been carried out to assess segmental vascular occlusion. Two RCTs compared the outcomes of total hepatic vascular exclusion (THVE) and Pringle maneuver. One RCTshowed THVE resulted in similar blood loss, but a higher postoperative complication. The other RCT showed less blood loss using THVE but the postoperative complication rate was similar. Both studies showed similar degree of liver ischemic injury. Only one RCT showed that selective hepatic vascular exclusion (SHVE) had less blood loss and liver ischemic injury than Pringle maneuver.
CONCLUSION:Due to the great variations in these studies, it is difficult to draw a definitive conclusion on the best technique of hepatic vascular control.
(Hepatobiliary Pancreat Dis Int 2010; 9: 473-481)
vascular control; vascular exclusion; hepatectomy; liver neoplasm; Pringle maneuver
Introduction
Partial hepatectomy can be performed with low mortality, but intraoperative massive hemorrhage remains a potentially lethal problem, especially in patients with chronic hepatic fibrosis or cirrhosis.[1,2]Hemorrhage together with blood transfusion may increase postoperative morbidity and mortality.[3]Furthermore, blood transfusion has been found to enhance tumor recurrence in patients undergoing partial hepatectomy for liver malignancy.[4-6]The development of various hepatic vascular occlusion techniques is one of the major progresses in liver surgery in the past 2 to 3 decades. These hepatic vascular occlusion techniques can be categorized as:
I. Hepatic inflow vascular occlusion
A. Total inflow occlusion (Pringle maneuver)
1.Continuous Pringle maneuver;
2.Intermittent Pringle maneuver;
3.Ischemic preconditioning followed by continuous Pringle maneuver
B. Selective inflow occlusion
1.Hemihepatic vascular occlusion;
2.Segmental vascular occlusionII. Hepatic inflow and outflow vascular occlusion
A. Total hepatic vascular exclusion (THVE)
B. Selective hepatic vascular exclusion (SHVE)/ Hepatic vascular exclusion with preservation of the cavaflow (HVEPC)
Hepatic vascular occlusion is effective in minimizing bleeding during hepatic parenchymal transection. However, it has the potential drawback of causing hepatic ischemiareperfusion injury. This article aims at presenting the various techniques of hepatic vascular occlusion during liver resection using an evidence-based approach.
Methods
The relevant manuscripts were identified by searching MEDLINE, and PubMed for articles published between January 1980 and April 2010 using the keywords "vascular control", "vascular clamping", "vascular exclusion" and "hepatectomy". Additional papers were identified by a manual search of the references from the key articles.
Hepatic inflow vascular occlusion
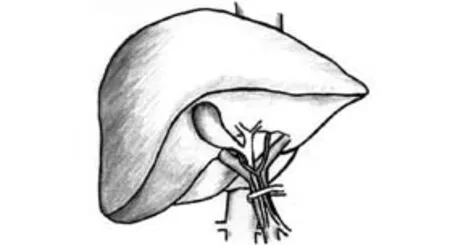
Fig. 1. Hepatic pedicle occlusion: Pringle maneuver.
Hepatic pedicle occlusion: Pringle maneuver (Fig. 1)Pringle first described the efficacy of hepatoduodenal ligament clamping in cases of liver trauma in 1908.[7]The Pringle maneuver is the simplest and oldest method of hepatic vascular control, and it is performed by encircling the hepatoduodenal ligament with a tape and then applying a tourniquet or a vascular clamp until the hepatic arterial pulse disappears distally. Prior to placing the occluding clamp or tourniquet, any adhesions around the hepatoduodenal ligament have to be freed. If a left hepatic artery which originates from the left gastric artery is found running separately, it requires separate clamping to totally occlude the arterial inflow. The hemodynamic response of Pringle maneuver is a 10% increase in mean arterial pressure, a 40% increase in systemic vascular resistance, a 5% decrease in pulmonary artery pressure and a 10% decrease in cardiac index.[8]Pringle maneuver is generally well tolerated, but it may induce ischemia-reperfusion injury to the remnant liver, the degree of which is accentuated in cirrhosis. Intermittent clamping of the portal triad has been used as an effective strategy to minimize ischemia-reperfusion injury in liver surgery. The major drawbacks inherent to intermittent clamping are the blood loss during the period of vascular unclamping and the increased operative time. One randomized controlled trial (RCT) showed intermittent Pringle maneuver to be superior to continuous Pringle maneuver. In a randomized study,[9]Belghiti et al compared 42 patients who underwent continuous Pringle maneuver with 44 patients who underwent intermittent Pringle maneuver with periods of 15 minutes of clamping and 5 minutes of unclamping. The duration of vascular clamping was comparable (mean 41 vs. 46 minutes). Intraoperative blood loss during liver transection was significantly higher in the intermitent Pringle group (mean 280 vs. 530 ml), although the proportion of patients requiring an intraoperative blood transfusion (28% vs. 32%) and the number of packed red blood cells transfused (3 vs. 2.3 units) were comparable in the two groups. In the continuous Pringle group, postoperative liver enzymes and serum bilirubin levels were significantly higher in the subgroup of patients with abnormal liver parenchyma. The incidence of postoperative complications was comparable in the two groups (31% vs. 26%), except for postoperative liver failure. Major postoperative deterioration of liver function occurred in 4 patients with abnormal liver parenchyma, with 2 postoperative deaths. All of these patients were in the continuous Pringle group. This study clearly demonstrated the better hepatic parenchymal tolerance to intermittent Pringle maneuver over continuous Pringle maneuver, especially in patients with abnormal liver parenchyma.
Two RCTs compared the perioperative outcomes of liver resection with and without intermittent Pringle maneuver. One showed the Pringle maneuver during liver transection to be beneficial,[10]while the other failed to show any benefit.[11]Man et al[10]randomized 100 consecutive patients who underwent hepatectomy for liver tumors. The patients were randomly assigned to liver transection under intermittent Pringle maneuver of clamp time of 20 minutes and a clamp-free intervals of 5-minute (n=50), or liver transection without Pringle maneuver (n=50). Thirteen patients and 16 patients had liver cirrhosis in the Pringle group and the controlgroup, respectively. The median vascular clamping time in the Pringle group was 88 minutes. The Pringle maneuver resulted in significantly less blood loss per cm2of transection area (median 12 vs. 22 ml/cm2), a shorter transection time per transection surface area (median 2 vs. 2.8 min/cm2), a significantly higher arterial ketone body ratio in the first 2 hours after hepatectomy, lower serum bilirubin levels in the early postoperative period, and, in cirrhotic patients, higher serum transferrin levels on postoperative days 1 and 8. The complication rates (26% vs. 30%), the hospital mortality rates (2% vs. 4%), and the indocyanine green retention at 15 minutes on postoperative day 8 (median 11.7% vs. 14.9%) were similar for the two groups. Capussotti et al[11]randomized 126 consecutive patients with resectable liver tumors into a group with resection with (n=63) intermittent Pringle maneuver of 15 minutes and a 5-minute clamp-free interval or a group without Pringle maneuver (n=63). Six patients and 13 patients had liver cirrhosis in the Pringle group and the control group, respectively. The mean vascular clamping time in the Pringle group was 49 minutes. In 2 patients in the control group, the hepatic pedicle was clamped because of excessive bleeding during liver transection: with a mean clamping time of 12.5 minutes. The transection time was significantly faster in the intermitted Pringle group (mean 49 vs. 72.7 minutes). The blood loss per transection surface area was similar in the two groups (mean 2.7 vs. 3.2 ml/cm2). In the subset of patients with abnormal livers, there were no differences in the blood loss per transection surface area (mean 3.1 vs. 2.9 ml/cm2). The rate of blood transfusion was not significantly higher in the control group. No differences were observed in the postoperative liver enzyme serum levels, the in-hospital mortality rate (1.6% vs. 1.6%) or the complication rate (33% vs. 25%).
An alternative to intermittent occlusion is ischemic preconditioning, which involves a brief period of ischemia and reperfusion to the liver prior to a prolonged period of portal triad occlusion. Ischemic preconditioning of the liver has been proposed as an effective hepatoprotective measure.[12]Its use avoids the repeated unclamping, which results in less intraoperative bleeding and a shortened liver parenchymal transection time. Ischemic preconditioning followed by continuous inflow occlusion is performed before starting the liver parenchymal transection by a 10-minute inflow occlusion followed by a 10-minute reperfusion period. Continuous Pringle maneuver is subsequently initiated at the beginning of liver transection and is maintained until the transection is completed. Although the exact mechanisms are not completely understood, the protective effects of ischemic preconditioning includes inhibition of apoptosis, activation of polymorphonuclear leukocytes, preservation of cellular adenosine triphosphate (ATP) content, and release of substances such as adenosine and nitric oxide by the ischemic tissue which protect the liver against the subsequent prolonged ischemia.[12-17]Four RCTs confirmed that ischemic preconditioning with continuous clamping is safe and effective for liver resection in non-cirrhotic liver and is better tolerated than continuous clamping alone for prolonged periods of ischemia.[18-21]There was only one small RCT which showed the potential benefit of ischemic preconditioning in cirrhosis.[22]Clavien et al[18]randomized 100 noncirrhotic patients undergoing major liver resection to either receive or not receive an ischemic preconditioning protocol followed by continuous inflow occlusion for at least 30 minutes. The mean vascular occlusion times were 35 minutes and 36 minutes, respectively. There was no difference between the ischemic preconditioning and control groups regarding the operative time (mean 225 vs. 240 minutes) and blood loss (mean 250 vs. 225 ml). Only 6 patients in the entire study received blood transfusion during surgery and were equally distributed with 3 in the preconditioning group and 3 in the control group, respectively. Postoperative serum transaminase levels were significantly lower in the preconditioning group than in the control group [median peak aspartate aminotransferase (AST), 364 vs. 520 U/L; alanine aminotransferase (ALT), 406 vs. 519 U/L]. Regression multivariate analysis revealed an increased benefit of ischemic preconditioning in younger patients, in patients with longer duration of inflow occlusion (up to 60 minutes), and in patients with lower resected liver volume (<50%). Patients with steatosis were also particularly protected by ischemic preconditioning. Nuzzo et al[19]randomized 42 noncirrhotic patients undergoing major or minor liver resection to either receive or not receive an ischemic preconditioning protocol followed by continuous inflow occlusion. The mean vascular occlusion time was 54 minutes and 36 minutes, respectively. Two patients in the ischemic preconditioning group (9.5%) and 3 in the control group (14.3%) received blood transfusions. In spite of the significantly longer duration of ischemia, patients with ischemic preconditioning followed by continuous occlusion had significantly lower AST and non-significantly lower ALT at postoperative day 1, with a similar trend at postoperative day 3. Choukèr et al[17,20]randomized 75 non-cirrhotic patients undergoing liver resection without Pringle maneuver, with Pringle maneuver, and with ischemic preconditioning using 10 minutes of ischemia and 10 minutes of reperfusion prior to Pringle maneuver forresection. The damage to the liver tissue due to the Pringle maneuver was completely prevented by ischemic preconditioning. Serum ALT levels at 48 hours after operation (mean 497 vs. 208.5 U/L) was significantly lower in the ischemic preconditioning group. These authors also found ischemic preconditioning to provide better hemodynamic stability intraoperatively. Heizmann et al[21]randomized 61 non-cirrhotic patients undergoing liver resection to either receive or not receive an ischemic preconditioning protocol followed by continuous inflow occlusion. The mean vascular occlusion time was 34 minutes and 33 minutes, respectively. The ischemic preconditioning group had significantly lower complications, which included death, severe liver dysfunction and biliary leakage (20% vs. 45%). Patients with ischemic preconditioning had significantly lower intraoperative blood loss (mean 1280 vs. 1940 ml), and significantly less patients required blood transfusion (n=5 vs.n=15). Li et al[22]randomized 29 cirrhotic patients undergoing liver resection for hepatocellular carcinoma to either receive or not receive an ischemic preconditioning protocol (5 minutes of ischemia followed by 5 minutes of reperfusion) followed by continuous inflow occlusion. The mean vascular occlusion time was 18 minutes and 17.4 minutes, respectively. There was no difference in operating time (mean 191.3 vs. 208.2 minutes) and blood loss (mean 469.2 vs. 602 minutes). On postoperative days 1, 3, 7, the AST and ALT levels in the ischemic preconditioning group were significantly lower than those in the group without ischemic preconditioning. On postoperative days 3, 7, the serum bilirubin levels in the ischemic preconditioning group were also significantly lower than those in the group without ischemic preconditioning.
As both intermittent Pringle maneuver and ischemic preconditioning followed by continuous inflow occlusion proved to be superior in RCTs to continuous inflow occlusion alone, Petrowsky et al[23]evaluated whether ischemic preconditioning with continuous inflow occlusion or intermittent inflow occlusion of the portal triad confers better protection during liver surgery. Non-cirrhotic patients undergoing major liver resection were randomized to receive ischemic preconditioning followed by continuous inflow occlusion (n=36) or intermittent portal triad occlusion (n=37). They demonstrated that ischemic preconditioning followed by continuous inflow occlusion was associated with significantly lower blood loss during transection (mean 146 vs. 250 ml), blood loss per transection area (mean 1.2 vs. 1.8 ml/cm2) and shorter transection time (mean 40.4 vs. 50.6 minutes). Overall (42% vs. 38%) and major (33% vs. 27%) postoperative complications were similar in the two groups. They concluded that ischemic preconditioning followed by both continuous inflow occlusion and intermittent portal triad occlusion were equally effective to minimize postoperative liver injury in non-cirrhotic patients undergoing major liver surgery.

Fig. 2. Hemihepatic vascular clamping.
Hemihepatic vascular clamping (Fig. 2)
In 1987, Makuuchi et al[24]proposed a hemihepatic vascular occlusion technique to reduce the severity of visceral congestion and total liver ischemia. The hemihepatic vascular occlusion technique selectively interrupts the arterial and venous inflow to the right or left hemiliver. The hemihepatic vascular occlusion technique has the potential advantages of preserved portal flow to the remnant liver, no splanchnic congestion, and no hemodynamic consequences. One of the concerns of the hemihepatic vascular occlusion technique is the risk of bleeding from the contralateral hemiliver. Three RCTs evaluated the clinical outcomes of the hemihepatic vascular occlusion technique by comparing it with intermittent Pringle maneuver,[25-27]while another RCT evaluated it in a three-arm study.[28]Wu et al[25]randomized 58 cirrhotic patients undergoing hepatic resection to either intermittent Pringle maneuver by a 15-minute inflow occlusion followed by a 5-minute reperfusion period (n=28) or intermittent hemihepatic vascular occlusion by a 30-minute inflow occlusion followed by a 5-minute reperfusion period (n=30). The mean duration of vascular occlusion time was 96 and 94.2 minutes, respectively. The amount of operative blood loss (mean 1685 vs. 1159 ml) and incidence of blood transfusion (n=12 vs.n=5) were significantly greater in the Pringle group because of greater blood loss during the period of reperfusion. The operative times (mean 6.82 vs. 6.65 hours), postoperative morbidity (28.6% vs. 33.3%), and postoperative changes in liver enzyme levels were not significantly different between the two groups. No in-hospital deaths occurred in either group.Figueras et al[26]randomized 80 patients undergoing minor hepatic resection to either intermittent Pringle maneuver (n=39) or intermittent hemihepatic vascular occlusion (n=41). Both groups had intermittent vascular occlusion of a 15-minute inflow occlusion followed by a 5-minute reperfusion. Eighteen patients and 21 patients had liver cirrhosis, respectively. The mean vascular occlusion time was 41 and 47 minutes, respectively. No significant differences were observed in the amount of blood loss (mean 671 vs. 735 ml) or number of patients that required blood transfusion (10% vs. 15%). There were no differences in postoperative morbidity between the two groups (38% vs. 29%). Cirrhotic patients with intermittent Pringle maneuver had significantly higher ALT (mean 7.7 vs. 4.5 μkat/L) and AST (mean 10.2 vs. 4.9 μkat/L) values on the first postoperative day than patients with intermittent hemihepatic vascular occlusion. Liang et al[27]randomized 80 patients undergoing hepatic resection to either intermittent Pringle (n=40) or continuous hemihepatic vascular occlusion (n=40). The mean duration of vascular occlusion time was 42.38 and 40.17 minutes, respectively. There were no significant differences in total blood loss during liver resection (mean 416.08 vs. 500.2 ml), blood loss per transection area (mean 6.40 vs. 7.59 ml/cm2), proportion of patients requiring blood transfusion (35% vs. 37.5%), postoperative morbidity rate (20% vs. 22.5%) and hospital stay (mean 9.85 vs. 10.12 days). The operative time of the hemihepatic vascular occlusion group was significantly longer than that of the Pringle group (mean 236.15 vs. 203.98 minutes). There was no significant difference in postoperative bilirubin, AST and ALT levels. Fu et al[28]randomized 180 patients into 3 groups according to the techniques used for inflow occlusion during hepatectomy: a hemihepatic vascular inflow occlusion group (n=60), a main portal vein inflow occlusion group (n=60), and a Pringle maneuver group (n=60). The vascular occlusion was continuous if the transection time was less than 30 minutes, or else intermittent vascular occlusion was performed with cycles of 15-minute inflow occlusion followed by 5 minutes of reperfusion. Thirty-five, 35 and 39 patients had liver cirrhosis, respectively. The mean vascular occlusion time was 14.9, 15.3 and 16.6 minutes, respectively. The Pringle maneuver group showed a significantly shorter operative time (mean 133.5 vs. 126.7 vs. 114.2 minutes). There were no significant differences between the 3 groups in intraoperative blood loss (mean 354.4 vs. 287.7 vs. 339.5 ml) and perioperative mortality (0% vs. 0% vs. 1.7%). The degree of postoperative liver ischemia-reperfusion injury and complication rates (20% vs. 21.7% vs. 31.7%) were significantly higher in the Pringle maneuver group, resulting in a significantly longer hospital stay (mean 10.2 vs. 9.8 vs. 13.7 days). Based on the currently available evidence, both of the techniques hemihepatic vascular occlusion and intermittent Pringle maneuver are equally effective and feasible for patients with normal liver undergoing hepatectomies. In cirrhotic patients, hemihepatic vascular occlusion induces less ischemia-reperfusion injury. Hemihepatic vascular occlusion is particularly indicated in dealing with a peripheral tumor in a cirrhotic liver.
Segmental vascular occlusion technique
The segmental vascular occlusion technique was first described by Shimamura et al[29]and was then developed by Castaing et al[30]to limit the ischemiareperfusion injury to the volume of the liver that is projected to be resected. This is done by accurately demarcating the anatomic territories to be resected by creating ischemic margins at the liver surface. The portal branch corresponding to the liver segment to be resected is identified by ultrasonography and is punctured in its longitudinal axis with a Chiba needle. Its position in the vein is confirmed by ultrasonography and by aspiration of venous blood on withdrawal of the stilette. A flexible guidewire is passed down to the portal vein. Following withdrawal of the cholangiography needle, a dilator, and an introducing catheter are placed in the portal venous branch. A balloon catheter is then passed through the seal of the introducer catheter. The balloon is inflated with up to 1 ml of isotonic saline and is positioned under ultrasound guidance to occlude the portal segmental vein. The inflated balloon is readily identified on ultrasound examination because of the presence of tiny microbubbles in the saline that appears as small hyperechoic areas. The corresponding side of the hepatic artery is dissected and occluded at the level of the hepatic pedicle to delineate the segment to be excised. The segment is further outlined by injectingmethylene blue dye in the side port of the introducing catheter. Segmental vascular occlusion technique allows a segment-based resection while minimizing the blood loss and ischemia-reperfusion injury.
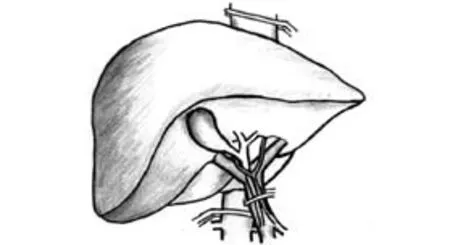
Fig. 3. Total hepatic vascular exclusion.
Inflow and outflow vascular occlusion
Total hepatic vascular exclusion (THVE) (Fig. 3)
Heaney and his colleagues first described hepatic resection with THVE.[31]THVE combines total inflow and outflow vascular occlusion of the liver, isolating it completely from the systemic circulation. It is achieved after complete liver mobilization, application of inflow occlusion as with the Pringle maneuver, and then placing a clamp across the infrahepatic inferior vena cava (IVC) above the renal veins and the right adrenal vein followed by placing a suprahepatic IVC clamp above the opening of the major hepatic veins. After completing the parenchymal transection and hemostasis, the clamps are removed in the reverse order in which they are placed.[32-34]THVE is a technically demanding technique that requires surgical and anesthetic expertise. THVE is mainly indicated for tumors approaching or infiltrating the major hepatic veins or the IVC. It is particularly useful when a tumor thrombus is present in the IVC, as application of THVE prevents intraoperative thrombus migration. It also allows major hepatic veins or IVC reconstruction. However, application of THVE is associated with significant hemodynamic changes and warrants close invasive monitoring and anesthetic expertise. Suppression of IVC flow causes marked (40%-60%) reduction of venous return and cardiac output, with a compensatory 80% increase in systemic vascular resistance and 50% increase in heart rate. Also, depending on the anesthesia technique, a 10%-17% decrease in mean arterial pressure and a 40%-50% decrease in cardiac index have been reported. A fall in cardiac output exceeding 50% or a decrease in mean arterial pressure exceeding 30% (i.e., less than 80 mmHg) in a euvolemic patient is defined as hemodynamic intolerance to THVE. It occurs in 10% to 20% of patients and is unpredictable preoperatively as it is caused by failure of the patient's adrenergic cardiovascular reflexes to increase cardiac output in the presence of decreased preload.[32-34]Adequate fluid expansion of the patient's blood volume before THVE is important to ensure that the procedure is well tolerated. Before placement of clamps, the patient is volume loaded with crystalloid to a central venous pressure (CVP) of 12 to 15 mmHg to prevent intolerance of the clamping. A trial exclusion for 5 minutes is performed to ensure stability. The large volume of fluids infused before and during THVE may lead to increased risks of postoperative liver, renal and pulmonary dysfunction, as well as abdominal fluid collection.
Two RCTs evaluated the clinical outcomes of THVE.[35,36]Belghiti et al[35]randomized 52 consecutive non-cirrhotic patients requiring a major liver resection to either THVE (n=28) or Pringle maneuver (n=24). The duration of vascular occlusion (mean 42 vs. 35 minutes) and the operative time (mean 366 vs. 301 minutes) were significantly longer in the THVE group. The longer operative period and ischemic period in the THVE group were related to caval dissection, vascular loading before clamping, exclusion trial, and the three-stage removal of the clamps with intermediate hemostasis. The amount of estimated intraoperative blood losses (mean 1195 vs. 989 ml) and the amount of intraoperative transfusions (mean 2.5 vs. 2.9 units) were not significantly different between the two groups. Postoperative parameters of hepatocyte damage and recovery were similar between the two groups. Both the rates of postoperative abdominal collections (15% vs. 35%) and pulmonary complications (10% vs. 25%) were 2.5-fold higher in the THVE group compared with the Pringle group but without statistical significance. However, the length of postoperative hospital stay was significantly longer in the THVE group (mean 22 vs. 14 days). The authors concluded that the two methods are equally effective in reducing blood loss, but THVE is associated with increased postoperative complications and longer hospital stay. Chen et al[36]modified the technique of THVE. In addition to Pringle maneuver, infra-hepatic IVC was dissected and taped with a vascular tape during the liver transection. It did not require the complete mobilization of the liver and encircling the supra-hepatic IVC. Chen et al[36]randomized 118 patients undergoing a liver resection to either the Pringle maneuver (n=24) and the modified technique of THVE. All patients with large tumors (>5 cm in diameter) which were located in the central portion of the liver but without direct invasion of the hepatic hilar plate were included in this study. The mean duration of vascular occlusion was 13.5 and 12.6 minutes, respectively. The blood loss during liver transection in the Pringle group was significantly greater than the modified technique of THVE group (mean 750 vs. 350 ml). The incidence of blood transfusion was significantly greater in the Pringle group (46.5% vs. 13.3%). There were no significant differences in liver enzyme changes, bilirubin, or morbidity (29.3% vs. 31.6%) in the postoperative period between the two groups.
Selective hepatic vascular exclusion (SHVE) (Fig. 4)
SHVE combines inflow vascular occlusion withdoes not improve liver tolerance to ischemia-reperfusion injury after hepatectomy under SHVE and does not affect morbidity or mortality rates. Arkadopoulos et al[43]also evaluated whether the ischemic preconditioning technique protects hepatocytes for liver resection performed under SHVE. They showed no improvement in blood loss and in hospital stay. They showed that for major hepatectomies performed under SHVE, ischemic preconditioning appears to attenuate apoptotic response of the liver remnant, possibly through alteration of the inflammatory and oxidative pathways.
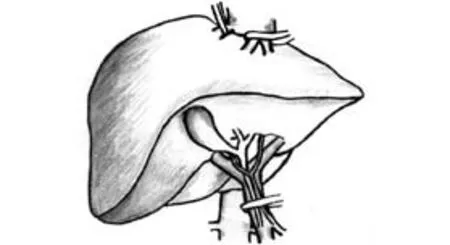
Fig. 4. Selective hepatic vascular exclusion.
Conclusions
Due to the great variation in the liver condition, pathology, experience of operating surgeons and surgical technique, it is difficult to make a valid conclusion based on these studies. The tumor extent, type of surgery, underlying liver disease, patient's cardiovascular status, and most importantly the experience of the surgical and anesthetic team should be taken into account to select the most appropriate technique for hepatic vascular control.
Funding:None.
Ethical approval:Not needed.
Contributors:LWY proposed the idea of the study. LECH arranged the structure and content of this article. LECH and LSHY did the literature search. LECH wrote the first draft. LWY also made the revision and final proof reading of the article. LWY is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Lau WY. A review on the operative techniques in liver resection. Chin Med J (Engl) 1997;110:567-570.
2 Muller MK, Petrowsky H, Clavien PA. Techniques of vascular control and protective strategies for parenchymal transection. In Hepatocellular Carcinoma, Lau WY (ed). World Scientific Publishing: Singapore;2008;507-528.
3 Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1 803 consecutive cases over the past decade. Ann Surg 2002;236:397-407.
4 Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg 2003;237:860-870.
5 Stephenson KR, Steinberg SM, Hughes KS, Vetto JT, Sugarbaker PH, Chang AE. Perioperative blood transfusions are associated with decreased time to recurrence and decreased survival after resection of colorectal liver metastases. Ann Surg1988;208:679-687.
6 van der Bilt JD, Kranenburg O, Verheem A, van Hillegersberg R, Borel Rinkes IH. Selective portal clamping to minimize hepatic ischaemia-reperfusion damage and avoid accelerated outgrowth of experimental colorectal liver metastases. Br J Surg 2006;93:1015-1022.
7 Pringle JH. Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg 1908;48:541-549.
8 Belghiti J, Marty J, Farges O. Techniques, hemodynamic monitoring, and indications for vascular clamping during liver resections. J Hepatobiliary Pancreat Surg 1998;5:69-76.
9 Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg 1999;229:369-375.
10 Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg 1997;226:704-713.
11 Capussotti L, Muratore A, Ferrero A, Massucco P, Ribero D, Polastri R. Randomized clinical trial of liver resection with and without hepatic pedicle clamping. Br J Surg 2006;93:685-689.
12 Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg 2000; 232:155-162.
13 Selzner M, Rüdiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology 2000;32:1280-1288.
14 Rüdiger HA, Kang KJ, Sindram D, Riehle HM, Clavien PA. Comparison of ischemic preconditioning and intermittent and continuous inflow occlusion in the murine liver. Ann Surg 2002;235:400-407.
15 Selzner N, Selzner M, Jochum W, Clavien PA. Ischemic preconditioning protects the steatotic mouse liver against reperfusion injury: an ATP dependent mechanism. J Hepatol 2003;39:55-61.
16 Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology 2003;125: 917-936.
17 Choukèr A, Martignoni A, Schauer R, Dugas M, Rau HG, Jauch KW, et al. Beneficial effects of ischemic preconditioning in patients undergoing hepatectomy: the role of neutrophils. Arch Surg 2005;140:129-136.
18 Clavien PA, Selzner M, Rüdiger HA, Graf R, Kadry Z, Rousson V, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg 2003;238: 843-852.
19 Nuzzo G, Giuliante F, Vellone M, De Cosmo G, Ardito F, Murazio M, et al. Pedicle clamping with ischemic preconditioning in liver resection. Liver Transpl 2004;10:S53-57.
20 Choukèr A, Schachtner T, Schauer R, Dugas M, Löhe F, Martignoni A, et al. Effects of Pringle manoeuvre and ischaemic preconditioning on haemodynamic stability in patients undergoing elective hepatectomy: a randomized trial. Br J Anaesth 2004;93:204-211.
21 Heizmann O, Loehe F, Volk A, Schauer RJ. Ischemic preconditioning improves postoperative outcome after liver resections: a randomized controlled study. Eur J Med Res 2008;13:79-86.
22 Li SQ, Liang LJ, Huang JF, Li Z. Ischemic preconditioning protects liver from hepatectomy under hepatic inflow occlusion for hepatocellular carcinoma patients with cirrhosis. World J Gastroenterol 2004;10:2580-2584.
23 Petrowsky H, McCormack L, Trujillo M, Selzner M, Jochum W, Clavien PA. A prospective, randomized, controlled trial comparing intermittent portal triad clamping versus ischemic preconditioning with continuous clamping for major liver resection. Ann Surg 2006;244:921-930.
24 Makuuchi M, Mori T, Gunvén P, Yamazaki S, Hasegawa H. Safety of hemihepatic vascular occlusion during resection of the liver. Surg Gynecol Obstet 1987;164:155-158.
25 Wu CC, Yeh DC, Ho WM, Yu CL, Cheng SB, Liu TJ, et al. Occlusion of hepatic blood inflow for complex central liver resections in cirrhotic patients: a randomized comparison of hemihepatic and total hepatic occlusion techniques. Arch Surg 2002;137:1369-1376.
26 Figueras J, Llado L, Ruiz D, Ramos E, Busquets J, Rafecas A, et al. Complete versus selective portal triad clamping for minor liver resections: a prospective randomized trial. Ann Surg 2005;241:582-590.
27 Liang G, Wen T, Yan L, Li BO, Wu G, Yang J, et al. A prospective randomized comparison of continuous hemihepatic with intermittent total hepatic inflow occlusion in hepatectomy for liver tumors. Hepatogastroenterology 2009;56:745-750.
28 Fu SY, Lau WY, Li GG, Tang QH, Li AJ, Pan ZY, et al. A prospective randomized controlled trial to compare Pringle maneuver, hemihepatic vascular inflow occlusion, and main portal vein inflow occlusion in partial hepatectomy. Am J Surg 2010 Apr 19. [Epub ahead of print]
29 Shimamura Y, Gunvén P, Takenaka Y, Shimizu H, Akimoto H, Shima Y, et al. Selective portal branch occlusion by balloon catheter during liver resection. Surgery 1986;100:938-941.
30 Castaing D, Garden OJ, Bismuth H. Segmental liver resection using ultrasound-guided selective portal venous occlusion. Ann Surg 1989;210:20-23.
31 Heaney JP, Stanton WK, Halbert DS, Seidel J, Vice T. An improved technic for vascular isolation of the liver: experimental study and case reports. Ann Surg 1966;163:237-241.
32 Bismuth H, Castaing D, Garden OJ. Major hepatic resection under total vascular exclusion. Ann Surg 1989;210:13-19.
33 Smyrniotis V, Farantos C, Kostopanagiotou G, Arkadopoulos N. Vascular control during hepatectomy: review of methods and results. World J Surg 2005;29:1384-1396.
34 Fu SY, Lau WY, Li AJ, Yang Y, Pan ZY, Sun YM, et al. Liver resection under total vascular exclusion with or without preceding Pringle manoeuvre. Br J Surg 2010;97:50-55.
35 Belghiti J, Noun R, Zante E, Ballet T, Sauvanet A. Portal triad clamping or hepatic vascular exclusion for major liver resection. A controlled study. Ann Surg 1996;224:155-161.
36 Chen XP, Zhang ZW, Zhang BX, Chen YF, Huang ZY, Zhang WG, et al. Modified technique of hepatic vascular exclusion: effect on blood loss during complex mesohepatectomy in hepatocellular carcinoma patients with cirrhosis. Langenbecks Arch Surg 2006;391:209-215.
37 Fu SY, Lai EC, Li AJ, Pan ZY, Yang Y, Sun YM, et al. Liver resection with selective hepatic vascular exclusion: a cohort study. Ann Surg 2009;249:624-627.
38 Li AJ, Pan ZY, Zhou WP, Fu SY, Yang Y, Huang G, Yin L, etal. Comparison of two methods of selective hepatic vascular exclusion for liver resection involving the roots of the hepatic veins. J Gastrointest Surg 2008;12:1383-1390.
39 Zhou W, Li A, Pan Z, Fu S, Yang Y, Tang L, et al. Selective hepatic vascular exclusion and Pringle maneuver: a comparative study in liver resection. Eur J Surg Oncol 2008; 34:49-54.
40 Leow CK, Leung KL, Lau WY, Li AK. Intermittent vascular exclusion of the liver (without vena cava clamping) during major hepatectomy. Br J Surg 1996;83:712.
41 Smyrniotis V, Theodoraki K, Arkadopoulos N, Fragulidis G, Condi-Pafiti A, Plemenou-Fragou M, et al. Ischemic preconditioning versus intermittent vascular occlusion in liver resections performed under selective vascular exclusion: a prospective randomized study. Am J Surg 2006;192:669-674.
42 Azoulay D, Lucidi V, Andreani P, Maggi U, Sebagh M, Ichai P, et al. Ischemic preconditioning for major liver resection under vascular exclusion of the liver preserving the caval flow: a randomized prospective study. J Am Coll Surg 2006; 202:203-211.
43 Arkadopoulos N, Kostopanagiotou G, Theodoraki K, Farantos C, Theodosopoulos T, Stafyla V, et al. Ischemic preconditioning confers antiapoptotic protection during major hepatectomies performed under combined inflow and outflow exclusion of the liver. A randomized clinical trial. World J Surg 2009;33:1909-1915.
Accepted after revision September 6, 2010
An ideal person is one who unites moral character, good health and great talent in one.
— Kimura Kuchi
August 20, 2010
Author Affiliations: Faculty of Medicine, the Chinese University of Hong Kong, Shatin, New Territories, Hong Kong SAR, China (Lau WY, Lai ECH and Lau SHY)
Wan-Yee Lau, Professor of Surgery, Faculty of Medicine, the Chinese University of Hong Kong, Shatin, New Territories, Hong Kong SAR, China (Tel: 852-26322626; Fax: 852-26325459; Email: josephlau@cuhk.edu.hk)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- News
- Resection of hepatic caudate lobe hemangioma: experience with 11 patients
- Hepatocellular carcinoma metastatic to the kidney mimicking renal oncocytoma
- Simultaneous breast and ovarian metastasis from gallbladder carcinoma
- An eight-year journey of Hepatobiliary & Pancreatic Diseases International
- Interferon and lamivudine combination therapy versus lamivudine monotherapy for hepatitis B e antigen-negative hepatitis B treatment: a meta-analysis of randomized controlled trials
