Interferon and lamivudine combination therapy versus lamivudine monotherapy for hepatitis B e antigen-negative hepatitis B treatment: a meta-analysis of randomized controlled trials
2010-06-29YuShiYiHuaWuZheYueShuWanJunZhangJunYangandZhiChen
Yu Shi, Yi-Hua Wu, Zhe-Yue Shu, Wan-Jun Zhang, Jun Yang and Zhi Chen
Hangzhou, China
Meta-analysis
Interferon and lamivudine combination therapy versus lamivudine monotherapy for hepatitis B e antigen-negative hepatitis B treatment: a meta-analysis of randomized controlled trials
Yu Shi, Yi-Hua Wu, Zhe-Yue Shu, Wan-Jun Zhang, Jun Yang and Zhi Chen
Hangzhou, China
BACKGROUND:It has been demonstrated that only a minority of patients with hepatitis B e antigen (HBeAg)-negative chronic hepatitis B (CHB) obtain a sustained response after either interferon (IFN) or nucleos(t)ide analogue monotherapy. Therefore, combination therapy of drugs with synergistic antiviral effects was proposed to have a sustained response in these patients. We compared the effect and safety of lamivudine monotherapy and its combination with IFN including conventional interferon (CON-IFN) and pegylated interferon (PEG-IFN) for HBeAg-negative CHB patients.
DATA SOURCES:A group of three independent reviewers identified 9 eligible randomized controlled trials through electronic searches (MEDLINE, OVID, EMBASE, the Cochrane Library Clinical Trials Registry, and the Chinese Medical Database), manual searches, and contact with experts. Sustained virological and biochemical responses were defined as primary efficacy measures. We performed quantitative meta-analyses to assess differences between CON-IFN plus lamivudine combination and lamivudine monotherapy groups.
RESULTS:No greater sustained virological and biochemical rates were found in patients receiving CON-IFN/lamivudine combination therapy [29.1% vs. 26.7%, odds ratio (OR)=0.98, 95% confidence interval (CI) 0.65-1.50,P=0.94, and 41.8% vs. 40.3%, OR=1.13, 95% CI 0.78-1.65,P=0.51, respectively],though a reduced YMDD mutation rate was achieved in the combination group [8.39% vs. 30.0%, OR=0.16, 95% CI 0.076-0.33,P<0.001]. However, data from one PEG-IFN trial showed greater sustained virological and biochemical rates in patients receiving combination therapy [response rate 19.5% vs. 6.6%, OR=3.42, 95% CI 1.71-6.84,P<0.001 and 60.0% vs. 44.2%, OR=1.88, 95% CI 1.23-2.85,P=0.003, respectively].
CONCLUSIONS:Addition of CON-IFN to lamivudine did not improve treatment efficacy but suppressed YMDD mutation by lamivudine. Combination of PEG-IFN and lamivudine might increase the sustained response, and further clinical trials are needed for confirmation.
(Hepatobiliary Pancreat Dis Int 2010; 9: 462-472)
lamivudine; interferon-alpha; combination therapy; monotherapy; HBeAg-negative; chronic hepatitis B
Introduction
Hepatitis B is a major health burden with more than 400 million people chronically infected worldwide.[1,2]Based on the status of hepatitis B e antigen (HBeAg), chronic hepatitis B (CHB) can be categorized into two clinically distinctive patterns: HBeAg-positive and HBeAg-negative.[3,4]HBeAg-positive CHB is common among patients acquiring infection perinatally and is characterized by high levels of HBV DNA replication.[5,6]Seroconversion of HBeAg, which is frequently accompanied by a durable response of viral suppression and clinical improvement,[7-9]marks a treatment end-point for HBeAg-positive CHB.[10-12]HBeAg-negative CHB, with frequent mutation in the precore or core promoter region of HBV, precluding the expression of HBeAg,[13,14]is associated withprogressive liver damage and a lower level of HBV replication than HBeAg-positive CHB.[15-17]Though the majority of patients with HBeAg-negative CHB initially respond well to both nucleos(t)ide analogues and interferon-alpha (IFN-α),[3,18]there is no definite indication for discontinuation of antiviral therapy like the seroconversion in HBeAg-positive CHB[19]and most patients undergo a relapse after treatment cessation.[20-23]In recent years, the notion of combination therapy has been proposed and several randomized controlled trials have evaluated the therapeutic effect and safety of such therapies for HBeAg-negative CHB.[24-26]However, the results from different trials are controversial.[25-27]In the present study, we performed a systematic review and meta-analysis of eligible clinical trials to compare the effect of lamivudine monotherapy with lamivudine plus IFN combination therapy in HBeAg-negative patients.
Methods
Literature search and eligibility criteria
A group of three independent researchers conducted the literature search; trial selection and data extraction and disagreements were resolved by consensus. We identified eligible trials by searching the electronic databases MEDLINE, OVID, EMBASE, the Cochrane Library Clinical Trials Registry, and the Chinese Medical Database. Included terms were "chronic hepatitis B", "lamivudine", "interferon", "drug combination", "combination therapy", and "sequential therapy". Searching with both MeSH terms and free keywords was conducted. We also performed manual searches of the bibliographies of relevant articles and conference proceedings. We included randomized controlled trials comparing lamivudine monotherapy with IFN plus lamivudine combination therapy in adult HBeAg-negative CHB patients, irrespective of publication status or language. In addition, if multiple trials were derived from the same or partly overlapping study populations, only the largest or most recent eligible trial was included. The searches of the entire databases were conducted by September 2009.
Data extraction and efficacy measure definitions
For each trial, we gathered data on the following characteristics: location where trials were conducted, inclusion and exclusion criteria, regimen design (including type and dose of IFN, drug dose, and administration method), efficacy measures, duration of treatment and follow-up, losses to follow-up, and trial quality. Disagreements were resolved through discussion among reviewers. Incomplete data were supplemented by contact with primary investigators.
We used end-of-follow-up (sustained) virological and biochemical response rates as primary efficacy measures. End-of-treatment virological and biochemical response rates, histological response, incidence of YMDD (tyrosine, methionine, and aspartate) motif mutations, liver-related mortality, and treatment safety were used as secondary efficacy measures. Virological response was defined as suppression of HBV DNA below the lower detection limit as determined by polymerase chain reaction (PCR). Biochemical response was defined as alanine aminotransferase (ALT) normalization. Histological response was defined as at least a two-point reduction in the Knodell score[28]for pre- and post-treatment liver histopathology studies. HBsAg seroconversion was defined by the loss of HBsAg and the presence of anti-HBsAg antibody. Treatment safety was defined as the occurrence rate of adverse effects causing withdrawal from therapy.
Assessment of methodological quality and statistical analysis
We assessed trial quality using the Jadad quality scale.[29]Each study was evaluated by examining the allocation sequence generation, allocation concealment, blinding of outcome assessors, and reporting of patient withdrawal and dropout. Studies with scores more than 4 were defined as high-quality.
Quantitative meta-analysis was conducted using STATA version 10.0 (STATA Corp., College Station, Texas, USA). We pooled conventional interferon (CON-IFN) plus lamivudine combination therapy and lamivudine monotherapy as an overall effect and performed separate meta-analyses examining the defined efficacy measures. Subgroup analysis based on treatment duration (1 year and 2 years) or sensitivity analysis excluding trials with a treatment duration of 2 years was performed. The effect measures of differences between the two groups were odds ratios (OR) and the corresponding 95% confidence intervals (CI). APvalue of less than 0.05 was considered to indicate a statistically significant difference. Heterogeneity was assessed for each analysis by means of Cochrane'sQtest. APvalue less than 0.10 indicated heterogeneity. The fixed effect model was used if no heterogeneity existed and the random effect model was used if heterogeneity was detected. The potential risk of publication bias was examined by the Egger test. Publication bias was indicated if thePvalue was less than 0.10. Intention to treat analysis was used in the study except for histological response rate analysis because the reporting rate was low.
Results
Patient selection and characteristics
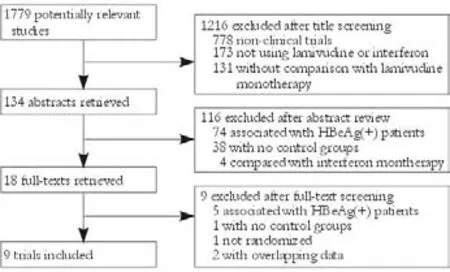
Fig. 1. Flow chart of article selection.
We identified 3131 references and 1779 duplicates were deleted. Then after title, abstract and full-text screening, we finally included nine remaining trials involving 942 patients[24-27,30-34](Fig. 1). CON-IFN was used in eight trials (n=579)[24-27,30-33]and one used pegylated (PEG) IFN-α (n=360).[34]Two trials (n=240) included only antiviral treatment-naive patients,[26,30]three studies (n=162) exclusively studied IFN non-responders,[24,25,33]and the others (n=540) included both IFN treatmentnaive and previously treated patients.[27,31,32,34]Only one study used sequential therapy (n=162)[26]and the others (n=780)[24,25,27,30-34]used simultaneous therapy. Three trials (n=535) comprised 48 weeks of treatment followed by 24 weeks of follow-up,[26,33,34]whereas patients in three trials (n=162) were treated for 96 weeks,[25,27,32]3 had a longer follow-up (n=187)[24,30,31]and one (n=58) had no follow-up data (the trial was on-going when published).[27]Three trials (n=492) were of high methodological quality (Jadad scores ≥3)[30,32,34]and the others (n=450) were not (Jadad scores <3);[24-27,31,33]however, none of the included studies were doubleblinded. All studies were published in English as full publications (Tables 1 and 2).
End-of-treatment virological response
Eight trials reported the end-of-treatment virological response rate.[24-27,30-33]No significant difference in this rate was found between patients in combination and monotherapy groups [78.0% vs. 70.3%, OR=1.37, 95% CI 0.92-2.05,P=0.12]. The fixed effects model was used because no substantial heterogeneity existed (χ2=8.15,df=7,P=0.32). Subgroup analysis showed no greater response in patients receiving either 1-year [77.9% vs. 70.2%, OR=1.33, 95% CI 0.82-2.16,P=0.25] or 2-year treatment [78.2% vs. 70.7%, OR=1.47, 95% CI 0.73-2.98,P=0.28]. No publication bias was detected (P=0.60, Egger test) (Fig. 2).
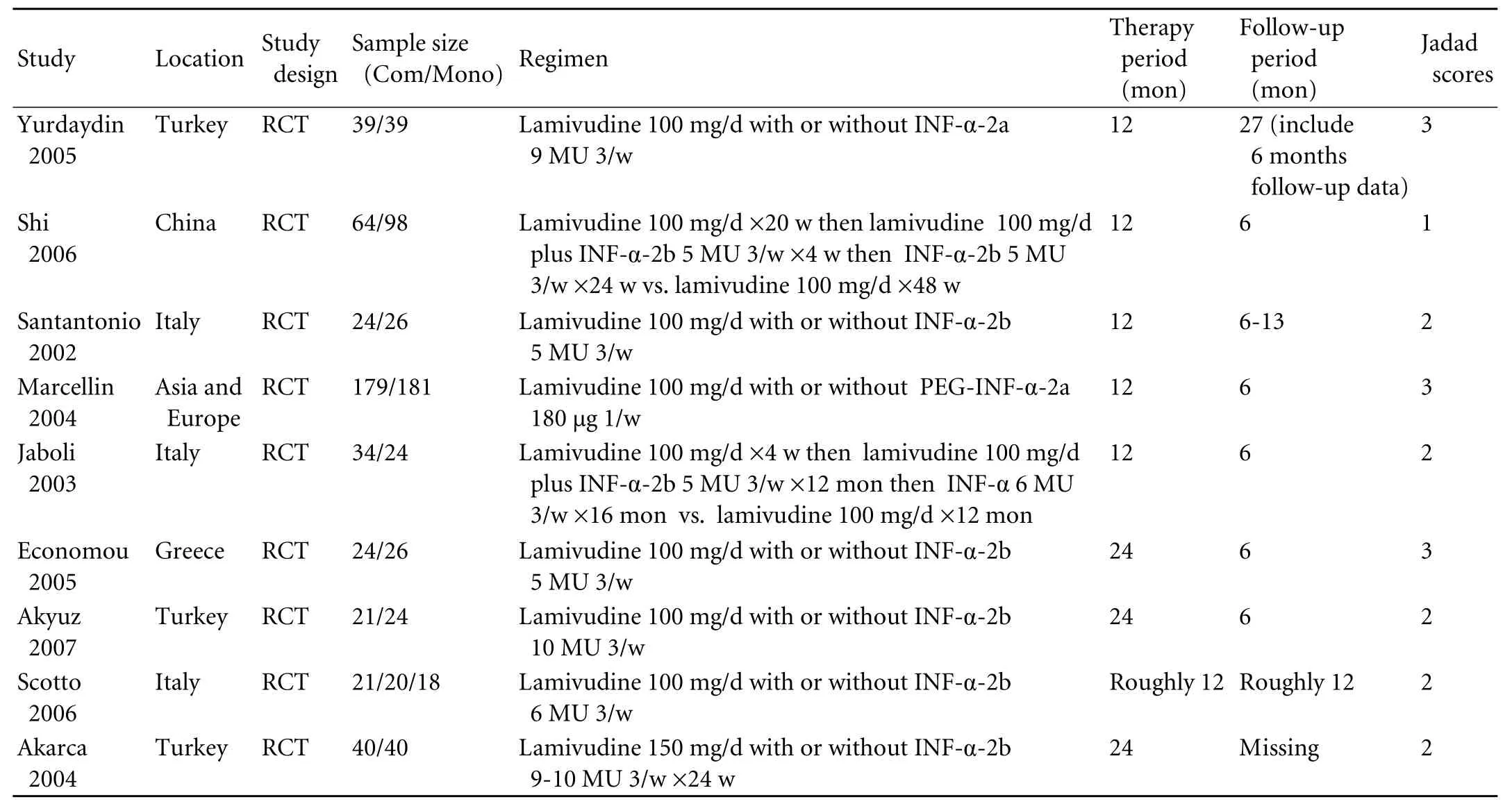
Table 1. Characteristics of included randomized controlled trials

Table 2. Selection criteria of included trials in the study

Fig. 2. End-of-treatment virological response. Com: conventional interferon with lamivudine combination therapy; Mono: lamivudine monotherapy.
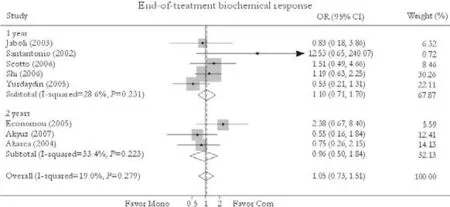
Fig. 3. End-of-treatment biochemical response. Com: conventional interferon with lamivudine combination therapy; Mono: lamivudine monotherapy.
End-of-treatment biochemical response
Eight trials reported the end-of-treatment biochemical response rate.[24-27,30-33]No significant difference in this rate was found between combination and monotherapy groups [69.9% vs. 67.0%, OR=1.05, 95% CI 0.73-1.51,P=0.77]. The fixed effects model was used because of substantial heterogeneity (χ2=8.65,df=7,P=0.28). Subgroup analysis showed no greater response in patients receiving either 1-year [69.3% vs. 65.0%, OR=1.10, 95% CI 0.71-1.70,P=0.66] or 2-year treatment [71.3% vs. 71.7%, OR=0.96, 95% CI 0.50-1.84,P=0.89]. No publication bias was detected (P=0.40, Egger test) (Fig. 3).
Sustained virological response
Seven trials reported the sustained virological response rate.[24-26,30-33]No significant difference in this was found between combination and monotherapy groups [29.1% vs. 26.7%, OR=0.98, 95% CI 0.65-1.50,P=0.94]. No substantial heterogeneity was found (χ2=3.07,df=6,P=0.80) and the fixed effects model was used. Subgroup analysis showed no greater response in patients receiving either 1-year [31.1% vs. 28.4%, OR=0.98, 95% CI 0.62-1.55,P=0.93] or 2-year treatment [20.0% vs. 20.0%, OR=1.01, 95% CI 0.37-2.75,P=0.99]. No publication bias was detected (P=0.92, Egger test) (Fig. 4).
Sustained biochemical response
Seven trials reported the sustained biochemicalresponse rate.[24-26,30-33]Compared with patients in the monotherapy group, a greater rate was found in patients receiving combination therapy [41.8% vs. 40.3%, OR=1.13, 95% CI 0.78-1.65,P=0.51]. No statistically significant heterogeneity was found (χ2=3.62,df=6,P=0.73) and the fixed effects model was used. Subgroup analysis showed no greater response in patients receiving either 1-year [46.2% vs. 44.2%, OR=1.18, 95% CI 0.79-1.78,P=0.42] or 2-year treatment [22.2% vs. 24.0%, OR=0.91, 95% CI 0.35-2.35,P=0.84]. No publication bias was detected (P=0.96, Egger test) (Fig. 5).
Incidence of YMDD mutation during treatment
Seven trials reported the incidence of YMDD mutation at the end of treatment;[24-26,30-33]however, three were excluded because only patients who did not respond to treatment were tested for YMDD variants.[24,25,31]Compared with patients in the monotherapy group, a lower YMDD mutation emergence rate was found in patients receiving combination treatment [8.39% vs. 30.0%, OR=0.16, 95% CI 0.076-0.33,P<0.001]. No statistically significant heterogeneity was found (χ2=1.74,df=3,P=0.63) and the fixed effects model was used. Sensitivity excluding trials with a 2-year treatment duration did not change the trend [7.63% vs. 27.3%, OR=0.17, 95% CI 0.077-0.39,P<0.001]. Substantial publication bias was detected (P=0.23, Egger test) (Fig. 6).
Histological response
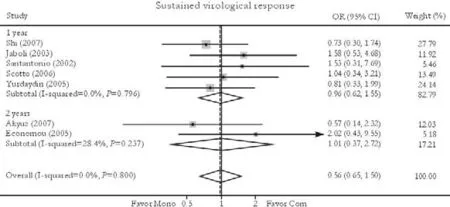
Fig. 4. Sustained virological response. Com: conventional interferon with lamivudine combination therapy; Mono: lamivudine monotherapy.
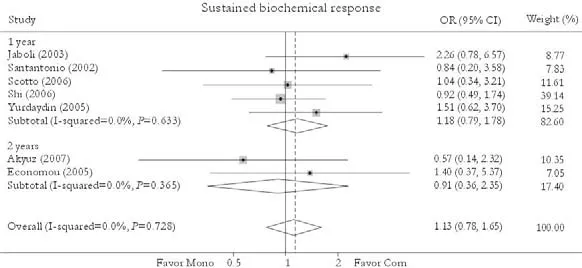
Fig. 5. Sustained biochemical response. Com: conventional interferon with lamivudine combination therapy; Mono: lamivudine monotherapy.

Fig. 6. YMDD mutation rate. Com: conventional interferon with lamivudine combination therapy; Mono: lamivudine monotherapy.
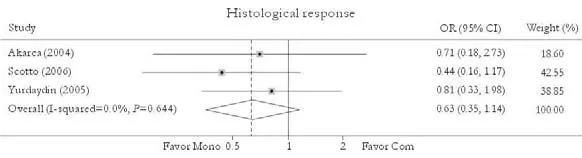
Fig. 7. Histological response. Com: conventional interferon with lamivudine combination therapy; Mono: lamivudine monotherapy.
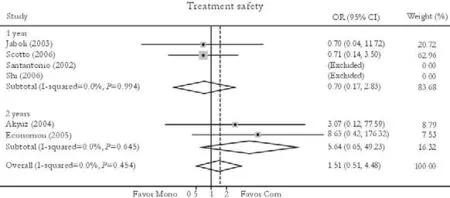
Fig. 8. Treatment safety. Com: conventional interferon with lamivudine combination therapy; Mono: lamivudine monotherapy.
Three studies reported the histological response rate.[24,27,30]No significant difference in this rate was found between patients in combination and monotherapy group [47.7% vs. 56.0%, OR=0.63, 95% CI 0.35-1.14,P=0.13]. No substantial heterogeneity was found (χ2=0.88,df=2,P=0.64) and the fixed effects model was used. Sensitivity excluding trials with 2-year treatment duration did not change the trend [36.4% vs. 48.6%, OR=0.61, 95% CI 0.32-1.19,P=0.15]. No publication bias was detected (P=0.95, Egger test) (Fig. 7).
HBsAg loss or seroconversion
Four trials reported the HBsAg loss or seroconversion rate[24,26,27]and no such cases were found.
Treatment safety
Six trials reported the treatment safety rate.[24,26,27,31-33]No significant difference in this rate was found between patients in the combination and monotherapy groups [3.98% vs. 1.69%, OR=1.51, 95% CI 0.51-4.48,P=0.46]. No substantial heterogeneity was found (χ2=2.62,df=3,P=0.45) and the fixed effects model was used. Subgroup analysis also showed no statistically significant difference in the two subgroups [3.12% vs. 2.37%, OR=0.70, 95% CI 0.17-2.83,P=0.62 (1 year); 6.06% vs. 0.00%, OR=5.64, 95% CI 0.64-49.23,P=0.12 (2 years)]. No publication bias was detected (P=0.28, Egger test) (Fig. 8).
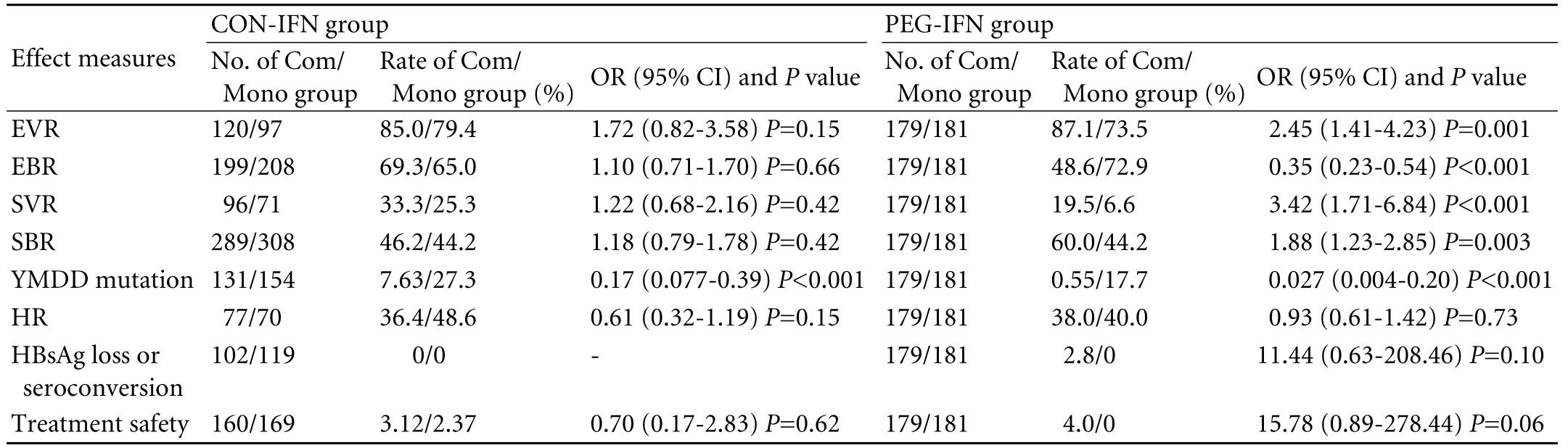
Table 3. Comparison of CON-IFN and PEG-IFN combination therapy (1-year results)
Liver-related mortality
No liver-related death was reported in the included trials.
CON-IFN combination therapy versus PEG-IFN combination therapy
Only one trial used PEG-IFN, and the treatment duration was one year. We listed the 1-year pooled results of CON-IFN combination therapy with those of PEG-IFN combination therapy (Table 3). Our findings showed significantly higher end-of-treatment virological response, sustained virological response, and sustained biochemical response in patients receiving combination therapy than in those who received monotherapy in the PEG-IFN group, which was different from the results of the CON-IFN group. The differences in histological response, HBsAg loss or seroconversion rate, and treatment safety remained of no statistical significance in the PEG-IFN group. And the reduced YMDD mutation was also consistent with the results of the CON-IFN group.
Discussion
HBeAg-negative CHB is associated with higher risk of cirrhosis, hepatocyte failure, and HCC than the HBeAg-positive pattern, which prompted long-term viral suppression treatment.[16,35,36]However, patients receiving monotherapy with either nucleos(t)ide analogues or IFN-α frequently failed to achieve sustained remission.[37,38]Therefore, the notion of combination therapy was proposed, aiming to decrease mutagenicity and obtain a synergistic effect.[39-41]This review, comprised 10 randomized controlled trials, compared the effect and safety of lamivudine plus IFN-α combination therapy with lamivudine monotherapy for patients with HBeAg-negative CHB. Our findings demonstrated that though addition of CON-IFN reduced the YMDD mutation emergence rate, it improved neither end-of-treatment nor sustained response rates, and this conclusion was supported by both the 1-year and 2-year results. There were also no statistically significant differences in histological response rate, HBsAg loss or seroconversion rate, and the occurrence of severe adverse events between patients receiving the two therapies.
Based on the data from one multicenter and randomized trial, our analysis suggested that addition of PEG-IFN is superior to lamivudine monotherapy in maintaining the sustained response. However, it was noted that the sustained virologic response in this trial was significantly lower than in the CON-IFN trials. This discrepancy in response rate may be due to the stricter definition of virological response applied in the PEG-IFN trial, defined as suppression of HBV DNA to below 400 copies/ml, while most of the CON-IFN trials used a threshold level of 5 pg/ml. Nonetheless, further randomized controlled trials with large simple sizes are needed to draw a definite conclusion.
We found a YMDD-prevention effect of both CON- and PEG-IFN combination therapies. First, the two drugs inhibit different targets in the HBV DNA replication pathway and therefore provide synergistic antiviral activity.[31,42,43]Lamivudine acts primarily as a DNA polymerase inhibitor,[44]whereas IFN suppresses HBV DNA by inducing posttranscriptional degradation of HBV RNA and the expression of antiviral proteins.[45-47]Furthermore, several studies demonstrated that lamivudine treatment reconstitutesthe cytotoxic T lymphocyte-mediated immune response against HBV[48-50]while the immunomodulatory effect of IFN has long been confirmed.[51-53]Second, due to this synergistic antiviral effect, patients with combination therapy tend to achieve a sharper reduction of HBV DNA level than those with monotherapy. Accumulating evidence indicates an inverse correlation between the rapidity and profundity of HBV DNA suppression and the emergence of resistance.[54-57]This may explain the low occurrence rate of YMDD motif mutation in patients receiving IFN-α and lamivudine combination therapy. Considering that the duration of interferon therapy was predefined; however, the cessation of interferon treatment might negate this YMDD-suppressing effect.
We also found that PEG-IFN/lamivudine but not CON-IFN/lamivudine achieved a sustained virological and biochemical response. Peg-IFN-α, produced by covalently attaching a 40-kDa branchedchain polyethylene glycol moiety to IFN-α,[58]has pharmacokinetics superior to conventional IFN-α.[59,60]Therefore PEG-IFN may elicit a more pronounced immune response in the host against HBV replication and elimination of reservoirs of infected cells compared with conventional IFN. A randomized controlled trial comparing the effect of PEG-IFN and CON-IFN monotherapy in HBeAg-positive patients showed that PEG-IFN-treated patients achieved a greater magnitude of HBV DNA reduction.[61]In addition, three trials in the CON-IFN subgroup exclusively studied IFN nonresponders, whereas the only trial included in the PEGIFN subgroup selected a mixed population of treatmentnaive and previously treated patients. This may explain why the PEG-IFN/lamivudine combination is superior to CON-IFN/lamivudine in obtaining a sustained response.
This review is limited in several aspects. First, the heterogeneity of studied population characteristics, quality score, regimen design, and follow-up protocols among the included studies may have led to certain biases in our meta-analysis. However, these concerns may be alleviated by the lack of substantial heterogeneity and publication bias and the low loss rate of subjects included in the trials. Second, 1-year antiviral monotherapy with lamivudine in several studies, which is not consistent with current guidelines, would contribute to the high relapse rate after treatment discontinuation. However, this concern was alleviated by the confirmation of 2-year results. Third, only one trial using PEG-IFN was included and the patients in the monotherapy arm received only 1-year treatment with lamivudine, which would reduce the validity of the evaluation of PEG-IFN. In addition, there was methodological heterogeneity between the PEG-IFN and CON-IFN groups. In particular, the trial evaluating PEG-IFN was of high quality, whereas the CON-IFN subgroup included several studies with small sample sizes and low quality. And we used the 1-year treatment results when we compared the efficacy between the CON-IFN and PEG-IFN groups, which would undermine the conclusion.
In conclusion, combination of CON-IFN and lamivudine added no benefit but reduced the YMDD mutation rate. PEG-IFN combined with lamivudine, however, might improve sustained therapeutic efficacy, which needs further clinical trials with long-term therapy to be confirmed.
Funding:The work was supported by grants from the Major State Basic Research Development Program (973) (No. 2007CB512905), the National Natural Science Foundation of China (No. 30771918), and the Major State S&T Projects of China (11th Five-Year) (2008ZX10002-007).
Ethical approval:Not needed.
Contributors:SY and CZ proposed the study. SY, WYH and SZY wrote the first draft. SY and ZWJ analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. YJ and CZ are the guarantors.
Competing interest:No bene fits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Pungpapong S, Kim WR, Poterucha JJ. Natural history of hepatitis B virus infection: an update for clinicians. Mayo Clin Proc 2007;82:967-975.
2 Lee WM. Hepatitis B virus infection. N Engl J Med 1997;337: 1733-1745.
3 Dienstag JL. Hepatitis B virus infection. N Engl J Med 2008; 359:1486-1500.
4 Taylor BC, Yuan JM, Shamliyan TA, Shaukat A, Kane RL, et al. Clinical outcomes in adults with chronic hepatitis B in association with patient and viral characteristics: A systematic review of evidence. Hepatology 2009;49:S85-95.
5 Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol 2008;6:1315-1341.
6 Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507-539.
7 Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med 2004; 116:829-834.
8 Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology 2002;35:1522-1527.
9 Hui CK, Leung N, Shek TW, Yao H, Lee WK, Lai JY, et al. Sustained disease remission after spontaneous HBeAg seroconversion is associated with reduction in fibrosis progression in chronic hepatitis B Chinese patients. Hepatology 2007;46:690-698.
10 Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009;50:661-662.
11 Liaw YF. HBeAg seroconversion as an important end point in the treatment of chronic hepatitis B. Hepatol Int 2009 Jun 24. [Epud ahead of print]
12 Keeffe EB. Hepatitis B: explosion of new knowledge. Gastroenterology 2007;133:1718-1721.
13 Ahmad N, Alam S, Mustafa G, Adnan AB, Baig RH, Khan M. e-antigen-negative chronic hepatitis B in Bangladesh. Hepatobiliary Pancreat Dis Int 2008;7:379-382.
14 Shi YH, Shi CH. Molecular characteristics and stages of chronic hepatitis B virus infection. World J Gastroenterol 2009;15:3099-3105.
15 Yang CG, Yu YC, Chen JJ, Sun J, Guo YB, Luo KX, et al. A comparison of clinical and virological characteristics of 1686 cases of HBeAg-negative and HBeAg-positive chronic hepatitis B. Zhonghua Nei Ke Za Zhi 2005;44:648-651.
16 Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigennegative chronic hepatitis B. Hepatology 2001;34:617-624.
17 Zhao DY, Qin YQ, Tang XM, Liu GZ, Zheng W, Nong HR, et al. Relationship among pathological changes in Liver tissues and level of serum HBV DNA, HBeAg and ALT of 194 patients with chronic hepatitis B. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2007;21:35-37.
18 Zhang FK. Interferon-alfa in the treatment of chronic hepatitis B. Hepatobiliary Pancreat Dis Int 2004;3:337-340.
19 Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology 2007;45:1056-1075.
20 Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med 1999; 341:1256-1263.
21 Kaymakoglu S, Danalioglu A, Demir K, Karaca C, Akyuz F, Onel D, et al. Long-term results of interferon alpha monotherapy in patients with HBeAg-negative chronic hepatitis B. Dig Dis Sci 2007;52:727-731.
22 Vassiliadis TG, Giouleme O, Koumerkeridis G, Koumaras H, Tziomalos K, Patsiaoura K, et al. Adefovir plus lamivudine are more effective than adefovir alone in lamivudineresistant HBeAg- chronic hepatitis B patients: a 4-year study. J Gastroenterol Hepatol 2010;25:54-60.
23 Hou J, Yin YK, Xu D, Tan D, Niu J, Zhou X, et al. Telbivudine versus lamivudine in Chinese patients with chronic hepatitis B: Results at 1 year of a randomized, double-blind trial. Hepatology 2008;47:447-454.
24 Scotto G, Palumbo E, Fazio V, Cibelli DC, Saracino A, Angarano G. Efficacy and tolerability of lamivudine alone versus lamivudine plus alpha-interferon for treatment of chronic active hepatitis B in patients with a precore-mutant variant. Infez Med 2006;14:145-151.
25 Akyuz F, Kaymakoglu S, Demir K, Aksoy N, Karaca C, Danalioglu A, et al. Lamivudine monotherapy and lamivudine plus interferon alpha combination therapy in HBeAg negative chronic hepatitis B not responding to previous interferon alpha monotherapy. Acta Gastroenterol Belg 2007;70:20-24.
26 Shi M, Wang RS, Zhang H, Zhu YF, Han B, Zhang Y, et al. Sequential treatment with lamivudine and interferonalpha monotherapies in hepatitis B e antigen-negative Chinese patients and its suppression of lamivudine-resistant mutations. J Antimicrob Chemother 2006;58:1031-1035.
27 Akarca US, Ersoz G, Gunsar F, Karasu Z, Saritas E, Yuce G, et al. Interferon-lamivudine combination is no better than lamivudine alone in anti-HBe-positive chronic hepatitis B. Antivir Ther 2004;9:325-334.
28 Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981;1: 431-435.
29 Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12.
30 Yurdaydin C, Bozkaya H, Cetinkaya H, Sahin T, Karaoğuz D, Törüner M, et al. Lamivudine vs lamivudine and interferon combination treatment of HBeAg(-) chronic hepatitis B. J Viral Hepat 2005;12:262-268.
31 Santantonio T, Niro GA, Sinisi E, Leandro G, Insalata M, Guastadisegni A, et al. Lamivudine/interferon combination therapy in anti-HBe positive chronic hepatitis B patients: a controlled pilot study. J Hepatol 2002;36:799-804.
32 Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206-1217.
33 Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, et al. Evaluation of alanine transaminase and hepatitis B virus DNA to predict liver cirrhosis in hepatitis B e antigennegative chronic hepatitis B using transient elastography. Am J Gastroenterol 2008;103:3071-3081.
34 Economou M, Manolakopoulos S, Trikalinos TA, Filis S, Bethanis S, Tzourmakliotis D, et al. Interferon-alpha plus lamivudine vs lamivudine reduces breakthroughs, but does not affect sustained response in HBeAg negative chronic hepatitis B. World J Gastroenterol 2005;11:5882-5887.
35 Jaboli MF, Fabbri C, Liva S, Azzaroli F, Nigro G, Giovanelli S, et al. Long-term alpha interferon and lamivudine combination therapy in non-responder patients with anti-HBe-positive chronic hepatitis B: results of an open, controlled trial. World J Gastroenterol 2003;9:1491-1495.
36 Zarski JP, Marcellin P, Leroy V, Trepo C, Samuel D, Ganne-Carrie N, et al. Characteristics of patients with chronic hepatitis B in France: predominant frequency of HBe antigen negative cases. J Hepatol 2006;45:355-360.
37 Serfaty L, Thabut D, Zoulim F, Andreani T, Chazouillères O, Carbonell N, et al. Sequential treatment with lamivudine and interferon monotherapies in patients with chronic hepatitis B not responding to interferon alone: results of a pilot study. Hepatology 2001;34:573-577.
38 Tillmann HL. Antiviral therapy and resistance with hepatitis B virus infection. World J Gastroenterol 2007;13:125-140.
39 Terrault NA. Benefits and risks of combination therapy for hepatitis B. Hepatology 2009;49:S122-128.
40 Sarin SK, Kumar M, Hissar S, Sharma BC. Combination of pegylated interferon and lamivudine for patients withchronic hepatitis B who have failed treatment. Hepatobiliary Pancreat Dis Int 2006;5:374-380.
41 Sarin SK, Sood A, Kumar M, Arora A, Amrapurkar D, Sharma BC, et al. Effect of lowering HBV DNA levels by initial antiviral therapy before adding immunomodulator on treatment of chronic hepatitis B. Am J Gastroenterol 2007; 102:96-104.
42 Papadopoulos V, Protopapas A, Tsianos E, Mimidis K. Threeyear follow-up of pegylated interferon alfa-2b as monotherapy or in combination with lamivudine in patients with HBeAgnegative chronic hepatitis B. Scand J Gastroenterol 2009;44: 1021-1022.
43 Bowden S, Shaw T. Hepatitis B: the case for combination therapy. Curr Opin Investig Drugs 2009;10:795-803.
44 Doong SL, Tsai CH, Schinazi RF, Liotta DC, Cheng YC. Inhibition of the replication of hepatitis B virus in vitro by 2',3'-dideoxy-3'-thiacytidine and related analogues. Proc Natl Acad Sci U S A 1991;88:8495-8499.
45 Rang A, Günther S, Will H. Effect of interferon alpha on hepatitis B virus replication and gene expression in transiently transfected human hepatoma cells. J Hepatol 1999;31:791-799.
46 Gordien E, Rosmorduc O, Peltekian C, Garreau F, Bréchot C, Kremsdorf D. Inhibition of hepatitis B virus replication by the interferon-inducible MxA protein. J Virol 2001;75:2684-2691.
47 Peltekian C, Gordien E, Garreau F, Meas-Yedid V, Soussan P, Willams V, et al. Human MxA protein participates to the interferon-related inhibition of hepatitis B virus replication in female transgenic mice. J Hepatol 2005;43:965-972.
48 Boni C, Bertoletti A, Penna A, Cavalli A, Pilli M, Urbani S, et al. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J Clin Invest 1998;102:968-975.
49 Boni C, Penna A, Ogg GS, Bertoletti A, Pilli M, Cavallo C, et al. Lamivudine treatment can overcome cytotoxic T-cell hyporesponsiveness in chronic hepatitis B: new perspectives for immune therapy. Hepatology 2001;33:963-971.
50 Boni C, Penna A, Bertoletti A, Lamonaca V, Rapti I, Missale G, et al. Transient restoration of anti-viral T cell responses induced by lamivudine therapy in chronic hepatitis B. J Hepatol 2003;39:595-605.
51 García Buey L, González Mateos F, Moreno Otero R. Interferon in hepatitis B. Enferm Infecc Microbiol Clin 2008; 26:19-31.
52 Perrillo R. Benefits and risks of interferon therapy for hepatitis B. Hepatology 2009;49:S103-111.
53 Buster EH, Schalm SW, Janssen HL. Peginterferon for the treatment of chronic hepatitis B in the era of nucleos(t)ide analogues. Best Pract Res Clin Gastroenterol 2008;22:1093-1108.
54 Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med 2007;357:2576-2788.
55 Gauthier J, Bourne EJ, Lutz MW, Crowther LM, Dienstag JL, Brown NA, et al. Quantitation of hepatitis B viremia and emergence of YMDD variants in patients with chronic hepatitis B treated with lamivudine. J Infect Dis 1999;180: 1757-1762.
56 Gauthier J, Bourne EJ, Lutz MW, Crowther LM, Dienstag JL, Brown NA, et al. Baseline characteristics and early ontreatment response predict the outcomes of 2 years of telbivudine treatment of chronic hepatitis B. J Hepatol 2009; 51:11-20.
57 Reijnders JG, Leemans WF, Hansen BE, Pas SD, de Man RA, Schutten M, et al. On-treatment monitoring of adefovir therapy in chronic hepatitis B: virologic response can be assessed at 24 weeks. J Viral Hepat 2009;16:113-120.
58 Bailon P, Palleroni A, Schaffer CA, Spence CL, Fung WJ, Porter JE, et al. Rational design of a potent, long-lasting form of interferon: a 40 kDa branched polyethylene glycolconjugated interferon alpha-2a for the treatment of hepatitis C. Bioconjug Chem 2001;12:195-202.
59 Keam SJ, Cvetković RS. Spotlight on peginterferon-alpha-2a (40 kD) plus ribavirin in the management of chronic hepatitis C mono-infection. BioDrugs 2009;23:63-68.
60 Keam SJ, Cvetković RS. Peginterferon-alpha-2a (40 kD) plus ribavirin: a review of its use in the management of chronic hepatitis C mono-infection. Drugs 2008;68:1273-1317.
61 Zhao H, Si CW, Wei L, Wan MB, Ying YK, Hou JL, et al. A multicenter, randomized, open-label study of the safety and effectiveness of pegylated interferon alpha 2b and interferon alpha 2b in treating HBeAg positive chronic hepatitis B patients. Zhonghua Gan Zang Bing Za Zhi 2006;14:323-326.
March 10, 2010
Accepted after revision May 26, 2010
Author Affiliations: State Key Laboratory for Diagnosis and Treatment of Infectious Diseases (Shi Y, Wu YH, Yang J and Chen Z) and Key Laboratory of Combined Multi-Organ Transplantation, Ministry of Public Health (Shu ZY), First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China; Department of Gastroenterology, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310009, China (Zhang WJ)
Zhi Chen, MD, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel: 86-571-87236579; Fax: 86-571-87036035, Email: zju.zhichen@gmail.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- News
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Life made easy: simplifying reconstruction for dual portal veins in adult right lobe live donor liver transplantation
- Effect of Danshen on apoptosis and NF-кB protein expression of the intestinal mucosa of rats with severe acute pancreatitisor obstructive jaundice
- Roles of Smad3 and Smad7 in rat pancreatic stellate cells activated by transforming growth factor-beta 1
- Salmonella typhi and gallbladder cancer: report from an endemic region
