FABRICATION AND PROPERTIES OF MICROENCAPSULATED PHASE CHANGE MATERIAL CONTAINING PARAFFIN BY USING DIFFERENT TYPES OF SMA AS EMULSIFIER
2010-02-23XUMengyiLINShulePIPihuiWENXiufangCHENGJiangYANGZhuoru
XU Meng-yi, LIN Shu-le, PI Pi-hui, WEN Xiu-fang, CHENG Jiang, YANG Zhuo-ru
(School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510641,China)
0 Introduction
Phase change materials(PCMs), i.e., eutectic salts, paraffin[1], aliphatic polyether etc., can absorb, store, and release large amounts of latent heat over a defined temperature range while undergoing phase changes, and their application in thermal energy storage has been well known in many fields[2,3]. However, due to their inherent characteristics, many PCMs are difficult to handle in practical application. Microcapsules are tiny particles that the core materials are surrounded by a coating or shell[4]. Microencapsulation of PCMs have provided an interesting alternative for using PCMs in manufacture of thermo-regulated fibers[5,6], foams[7], building materials, and solar and nuclear energy storage systems etc. in recent years.
MicroPCMs have been synthesized with melamine-formaldehyde(MF)[8], urea-melamine-formaldehyde polymer(UMF)[9], polyurethane polymer(PU)[10,11], and polymethylmethacrylate(PMMA)[12]etc. as shell materials. As the MF has good seal tightness and endurance, acid and alkaline resistance, fire resistance and microencapsulation of MF is easy to handle compared to other polymer, MF is more often used as shell material in the field of microPCMs. In this study, a series of micro-PCMs were prepared byinsitupolymerization using melamine-formaldehyde(MF) as shell and paraffin as core. The effects of different alcohols as etherifying agents adding into MF prepolymer on the surface morphology and phase change behaviors were systematically studied.
1 Experimental
1.1 Materials
Melamine (A.R.) and formaldehyde (37wt% aqueous, A.R.) were used as monomers; paraffin wax with a phase change temperature range 56~58 ℃ was used as core material. SMA (three tyes, showned in Tab.1) was employed as an emulsifier. Triethylamine (A.R.) and glacial acetic acid (A.R.) were used to adjust the pH value.
1.2 Fabrication of microcapsules
1.2.1 Preparation of copolymer emulsifier
SMA powder(10.0 g) was dissolved in 90 g of 3wt% sodium hydroxide solution under stirring at 80 ℃ for 3 h, deriving a transparent solution about 10wt%, denoted as HSMA.
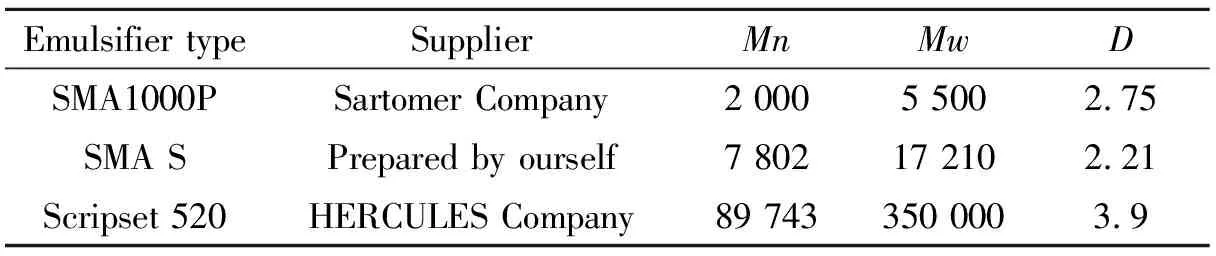
Tab.1 Molecular weight of different types SMA emulsifier
1.2.2 Synthesis of precursor
The precursor was prepared by mixing 6.3 g of melamine, 11.5 mL of formaldehyde and 20 mL of distilled water. The mixture was adjusted to pH 7.5 with triethylamine and stirred at 60 ℃ till the suspension became clearly transparent, then the reaction mixture was cooled down to ambient temperature.
1.2.3 Synthesis of capsules
The encapsulation of the paraffin wax was carried out by aninsitupolymerization. After a given amount of HSMA solution was dissolved in 50 mL of water, the solution was heated to 80 ℃. Paraffin melt (10.8 g) was dispersed into the aqueous solution with a homomixer at a stirring speed of 1 200 r/min for 30 min. Then the pH of the emulsion was adjusted to 5.5 with glacial acetic acid and cooled down to 60 ℃. After all of the prepolymer was added into the emulsion, the mixture was continued to stir for 120 min. Then the pH of the emulsion was adjusted to 9.0 with triethylamine. The resultant dispersion was cooled down to ambient temperature. After a sequential treatment including filtration, wash with water and drying at 60 ℃ in a vacuum oven, the PCM capsules were obtained.
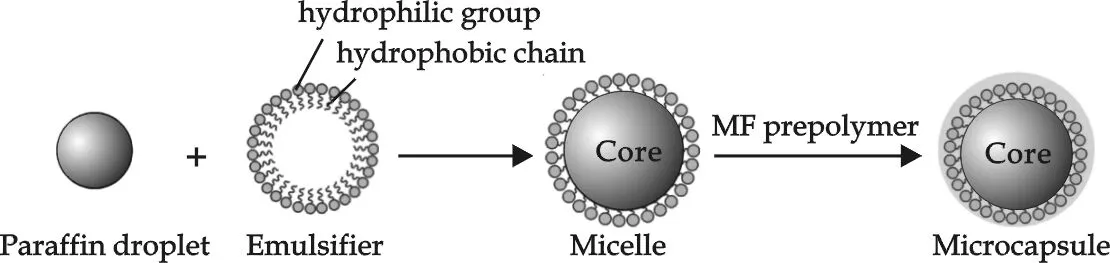
Fig.1 Schematic formation of microcapsules based on paraffin core and MF shell through in situ polymerization
1.3 Characterization
FT-IR transmittance of paraffin and microcapsules were obtained by using a Bruker Vector 33 spectrometer at room temperature. The morphology and average particle size of the capsules were characterized using a Hitachi S-3700N scanning electronic microscope (SEM), operated at an accelerating voltage of 15 kV. The particle size distribution was obtained by using a Mettler Toledo FBRM®D600. The phase change properties of the dried microcapsules were obtained by using a TA Q200 differential scanning calorimeter (DSC) at a heating rate of 5 ℃·min-1in a nitrogen atmosphere.
2 Results and Discussion
2.1 Fabrication of microcapsules
The synthesis scheme of the microcapsules is shown in Fig.1.
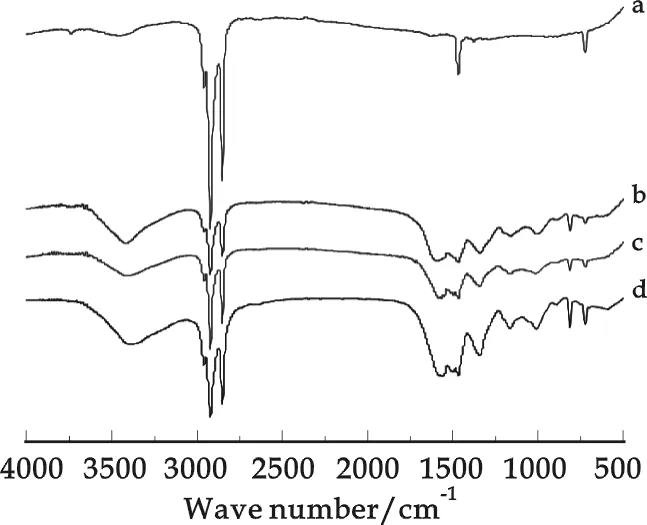
Fig.2 FTIR spectra of bulk paraffin and the microcapsules:(a)paraffin; (b)with HSMA 1000P; (c)HSMA S; and (d)HSMA 520
2.2 FTIR spectra
The composition of the microcapsules was characterized as shown in Fig.2. The broad peak around 3 700~3 200 cm-1is associated with hydroxyl, imino and amino stretching. The multiple absorption peaks around 3 000~2 800 cm-1are attributed to the aliphatic C-H stretching vibration and the C=N multiple stretching one in the triazine ring, which is also associated to the peak at 1 560 cm-1. At 1 490 cm-1and 1 360 cm-1, there are two absorption peaks shown due to the bending vibration of the group of CH2. The absorption peak at 810 cm-1is attributed to the stretching vibration of triazine ring, and the one at 717 cm-1is assigned to the in-plane rocking vibration of the methylene group. The FTIR spectrum of paraffin is also displayed as a reference in Fig.2(a). And all of the characteristic peaks for paraffin can be easily figure out in the spectra of the microcapsules, meaning the success of encapsulation by MF copolymer.
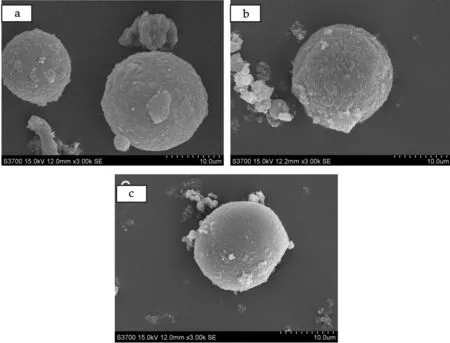
Fig.3 SEM images of the microcapsules by using (a)HSMA 1000P; (b)HSMA S; and (c)HSMA 520
2.3 Morphology
Fig.3 shows the images of the micsrocapsules prepared by using different HSMA as emulsiun. It can be seen from Fig.3(a) that the microcapsules prepared with low molecular weight are loose and irregular. Fig.3(b) shows that the surfaces of microcapsules are smoother as shown in Fig.3(a). As is seen from Fig.3(c), by using the highes molecular weight HSMA emulsion resulted in a smoothest surface. It can be deduced that the entire MF prepolymer were nearly deposited on the surface of core materials with the help of charge effect of anionic SMA emulsifier, and the higher molecular weight the HSMA emulsion has, the easier MF prepolymer will deposite.
As a result, by using HSMA with high molecular weight can conduct a smoother surface.
The particle size is very important in practical application, and herein it is mainly controlled by adjusting the feeding type of HSMA at a given amont. Fig.4 shows the effect of HSMA type on particle size and disteribution of the PCM capsule. With increasing the molecular weight of HSMA, the capsule average diameter decreased and their size distribution became narrower. Ther size analysis is consistent with SEM results.
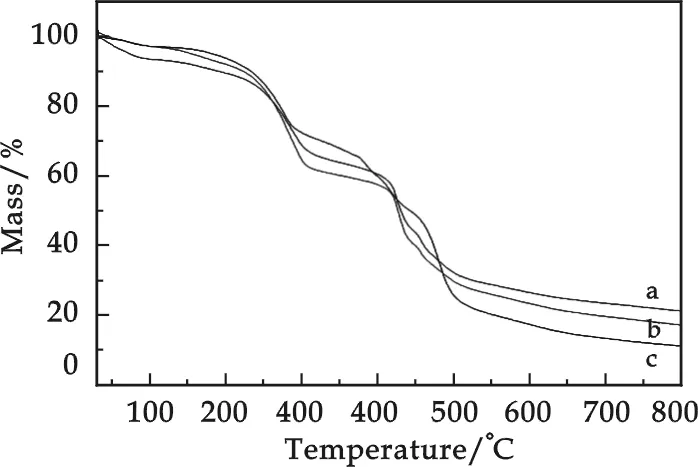
Fig.4 Particle size distribution plots when the reaction finished with different SMA emulsifiers

Fig.5 TG curves of the microcapsules by using (a)HSMA 520;(b)HSMA S; (c)HSMA 1000P
2.4 Phase change behaviors of microcapsules
Fig.5 shows the TG curves of microcapsules by using different HSMA emulsions. In Fig.5 shows that there are two steps of significant mass loss starting from 170 to 500 ℃, respectively. The first step can be atttibuted to the evaporation of the core material paraffin and some reagent. And the mass loss of the second step can be attributed to the decomposition of MF resin. It can be seen from Fig.5(a) that the two extrapolated onset temperature are 253.7 ℃ and 416.8 ℃. In Fig.5(b) that are 239.7 ℃ and 414.9 ℃, which are slightly lower than that of Fig.5(a), and slightly higher than that of Fig.5(a) with 234.4 ℃ and 407.2 ℃. According to the analysis of TG curves, the thermal stability of microPCMs increases slightly with the increasing of the molecular weight of HSMA emulsion.
3 Conclusion
In conclusion, the micro-PCMs based on paraffin core and MF shell with different HSMA emulsion have been synthesized byinsitupolymerization method. Microcapsules have an average diameter about 10 μm and with regularly spherical shape. The morphology investigation suggested that the low molecular weight of HSMA made the globular surface becomes rougher, while a higher molecular weight resulted in a smoother and more compact surface. The thermal stability of microPCMs increases slightly with the increasing of the molecular weight of HSMA emulsion.
[1] M. Hadjieva, St. Kanev, J. Argirov. Thermophysical properties of some paraffins applicable to thermal energy storage[J]. Solar Energy Materials and Solar Cells, 1992, 27(2):181-187.
[2] Macit Toksoy, Zafer IIken. The effects of phase change during the stand-by period in latent heat energy storage systems[J]. Solar Energy, 1991, 47(2):69-73.
[3]A. Abhat. Low temperature latent heat thermal energy storage: heat storage materials[J]. Solar Energy, 1983, 30(4):313-332.
[4]S. Benita. Microencapsulation: Methods and Industrial Applications[M]. Marcel Dekker, Inc., New York, 1996:1-3.
[5]Gordon Nelson. Application of microencapsulation in textiles[J]. International Journal of Pharmaceutics, 2002, 242(1-2):55-62.
[6]S. Mondal. Phase change materials for smart textiles——An overview[J]. Applied Thermal Engineering, 2008, 28(11-12):1 536-1 550.
[7]M. You, X.X. Zhang, W. Li, X.C. Wang. Effects of microPCMs on the fabrication of microPCMs/polyurethane composite foams[J].ThermochimicaActa, 2008, 472(1-2):20-24.
[8] LI Wei, ZHANG Xing-xiang, WANG Xue-chen,etal. Preparation and characterization of microencapsulated phase change material with low remnant formaldehyde content[J]. Materials Chemistry and Physics, 2007, 106(2-3):437-442.
[9]ZHANG Xing-xiang, FAN Yao-feng , TAO Xiao-ming,etal. Crystallization and prevention of supercooling of microencapsulatedn-alkanes[J]. Journal of Colloid and Interface Science, 2005, 281(2):299-306.
[10]SU Jun-feng, WANG Li-xin,REN Li. Synthesis of polyurethane microPCMs containing n-octadecane by interfacial polycondensation: Influence of styrene-maleic anhydride as a surfactant[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2007, 299(1-3):268-275.
[11]ZHANG Huan-zhi , WANG Xiao-dong. Synthesis and properties of microencapsulatedn-octadecane with polyurea shells containing different soft segments for heat energy storage and thermal regulation[J]. Solar Energy Materials and Solar Cells, 2009, 93(8):1 366-1 376.
[12]Cemil Alkan, Ahmet Sart, Ali Karaipekli,etal. Preparation, characterization, and thermal properties of microencapsulated phase change material for thermal energy storage[J]. Solar Energy Materials and Solar Cells, 2009, 93(1):143-147.
