Molecular Dynamics Simulation of Effect of Salt on the Compromise of Hydrophilic and Hydrophobic Interactions in Sodium Dodecyl Sulfate Micelle Solutions*
2009-05-15GAOJian高健RENYing任瑛andGEWei葛蔚
GAO Jian (高健), REN Ying (任瑛),2 and GE Wei (葛蔚),**
Molecular Dynamics Simulation of Effect of Salt on the Compromise of Hydrophilic and Hydrophobic Interactions in Sodium Dodecyl Sulfate Micelle Solutions*
GAO Jian (高健)1, REN Ying (任瑛)1,2and GE Wei (葛蔚)1,**
1State Key Lab of Multi-phase Complex System, Institute of Process Engineering, CAS, Beijing 100190, China2Graduate University of the Chinese Academy of Sciences, Beijing 100039, China
The presence of salt has a profound effect on the size, shape and structure of sodium dodecyl sulfate (SDS) micelles. There have been a great number of experiments on SDS micelles in the presence and absence of salt to study this complex problem. Unfortunately, it is not clear yet how electrolyte ions influence the structure of micelles. By describing the compromise between dominant mechanisms, a simplified atomic model of SDS in presence of salt has been developed and the molecular dynamics (MD) simulations of two series of systems with different concentrations of salt and charges of ion have been performed. Polydispersity of micelle size is founded at relatively high concentration of SDS and low charge of cation. Although the counter-ion pairs with head groups are formed, the transition of micelle shape is not observed because the charge of cation is not enough to neutralize the polar of micelle surface.
molecular dynamics simulation, compromise, sodium dodecyl sulfate, salt, micelle
1 INTRODUCTION
Two surfactant molecules in aqueous solution above a critical micelle concentration (CMC) tend to self-aggregate to form micelles of various shapes, spherical, ellipsoidal, rodlike or disk-like,[1]. In a sense, the micelles are formed due to compromise between hydrophilic and hydrophobic interactions [2]. Different composition of the solution will lead to different balance points presenting this compromise. Among which, the transition in size and shape from spherical to rodlike micelles with increasing concentration or addition of salt in the ionic surfactant systems is a well-known phenomenon [3]. Furthermore, salt ions play a significant role in biomolecular systems [4]. For an anionic surfactant, the repulsion between head groups determines the shape and size of micelles formed by different amphiphiles [5]. This interaction is considerably affected by the counter-ions present in the head group region of the micelle, because an increase in surfactant concentration or adding salt leads to a screening of electrostatic repulsions between the charges on the micellar surface. However, how the salt affects the properties of micelles is not well understood. It is a topic of great interest, and Rharbi. [6] have provided a good summarization.
Sodium dodecyl sulfate (SDS) is one of the most important surfactants in common use. In the absence of salt, SDS has a critical micelle concentration of 8.3 mmol·L-1and aggregation number (agg) of 50 [7]. Properties of aqueous sodium dodecyl sulfate micelles with and without salt have received extensive study [8-18]. It is widely assumed that the shape of SDS micelles at low ionic strength is spherical and changes to more ellipsoidal with salt addition.
Small-angle neutron diffraction (SANS) experiments [8] suggested that, in the absence of added electrolyte, SDS micelles grow as
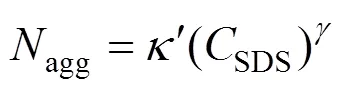
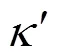
At higher salt and surfactant concentrations, SDS micelles undergo a transition from spherical to rodlike or wormlike shape [10]. Almgren. [11] used a combination of SANS and time-resolved fluorescence quenching (TRFQ) to examine the properties of SDS in concentration ranging from 10 to 80 mmol·L-1with NaCl concentration of 800 mmol·L-1. The short rodlike structures were found at the lowest temperature (25°C) and the highest surfactant concentration. Deeper insights into the shape of these aggregates were provided by the SANS measurements of Bergström and Pedersen [12]. Moreover, Ikeda. [10] studied the rodlike micelles of SDS in sodium halide solutions through light scattering measurements, and concluded that the sphere-rod transition of the SDS micelle is not influenced by the halide ion species of added salt. It means that the transition is mainly caused by the electrostatic effect of counter-ion binding on the micelle.
Molecular dynamics (MD) simulation has become a unique tool for investigating surfactant systems. In particular, MD simulations can shed light on the atomistic details of the structure and dynamics of surfactant systems. The experimental studies were completed and, more importantly, the access to various static and dynamic properties was provided, which is not accessible by current experimental methods. But MD simulation studies on effect of salt on micelles are insufficient so far. In this work the dependence of the micelle properties, such as size, shape and structure of SDS micelles, on the cation charge and concentration in SDS/NaCl/water systems is investigated. As far as know, our simulation systems are the largest system on scale in the reported MD simulation researches.
2 MODEL AND SIMULATION METHOD
Currently, it is technologically not feasible to perform all-atom simulations with explicit solvent since at practical concentrations of micelles the size of the simulation cell is on the order of 10-20 nm, which would imply introduction of 105-106water molecules [19]. We have simplified atomistic model and simulated the formation of SDS micelles in absence of salt in a previous work [20]. Based on this model, the salt ions with a certain quantity of static electricity are added. The model has been detailed previously [20] and a concise introduction is followed.
The structure of the SDS molecule is showed in Fig. 1. Sulfur and oxygen atoms are explicitly represented by five particles, however, each CHradical in SDS is modeled as a single particle C. Each water molecule or salt ion is also simulated as a single particle. The total potential energy of the system can be expressed as
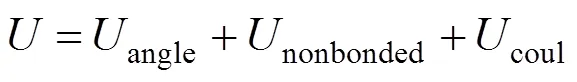
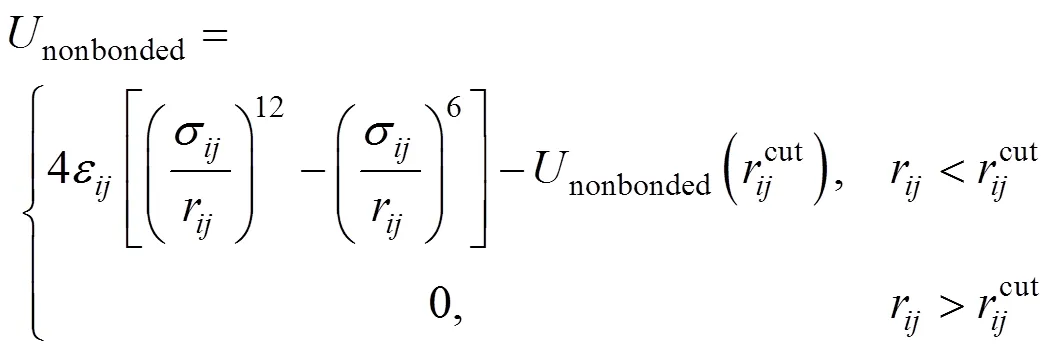
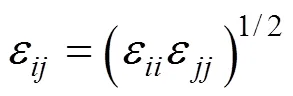
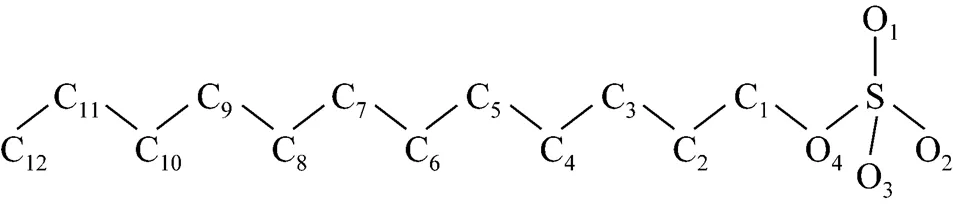
Figure 1 Simplified molecular structure of SDS
The computation cost of long-range electrostatic forces is tremendous, which is the primary reason for the lack of simulations for larger systems and longer time. To cope with this problem, truncation method that neglects the long-range Coulombic tail and truncates the interactions at some suitable distance can save computational cost considerably. In the present work, the truncation method considering computational cost is chosen. In order to investigate the relationship between the charge of the sodium ion and the properties of SDS micelles separately, the electrostatics of water and the chloride ion are neglected, and only those of sodium ion and SDS head groups are considered. The truncation distance is chosen as 2.5for convenience. For the sake of running smoothly on the parallel computers, theNain different systems is divided by 2.5, 3.0, 3.5 and 4.0, respectively. The charge of particles [25] is showed in Table 1.
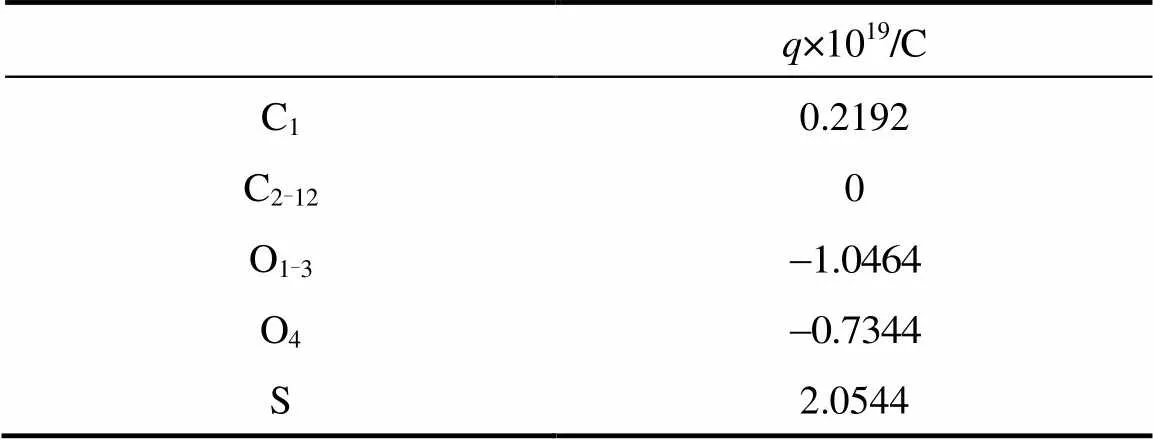
Table 1 Partial charges of the various atom types used in our MD simulations
3 SIMULATION RESULTS AND DISCUSSIONS
To understand the effect of charge and concentration of cation on micelles, a series of classical molecular dynamics simulations are performed. Two series of systems are treated in the simulations. Series A includes four systems of different concentrations with equal charge of cation, while Series B contains four systems of equal concentration of cation with different charges. The number of salt ion in the two series of systems is shown in Table 2.
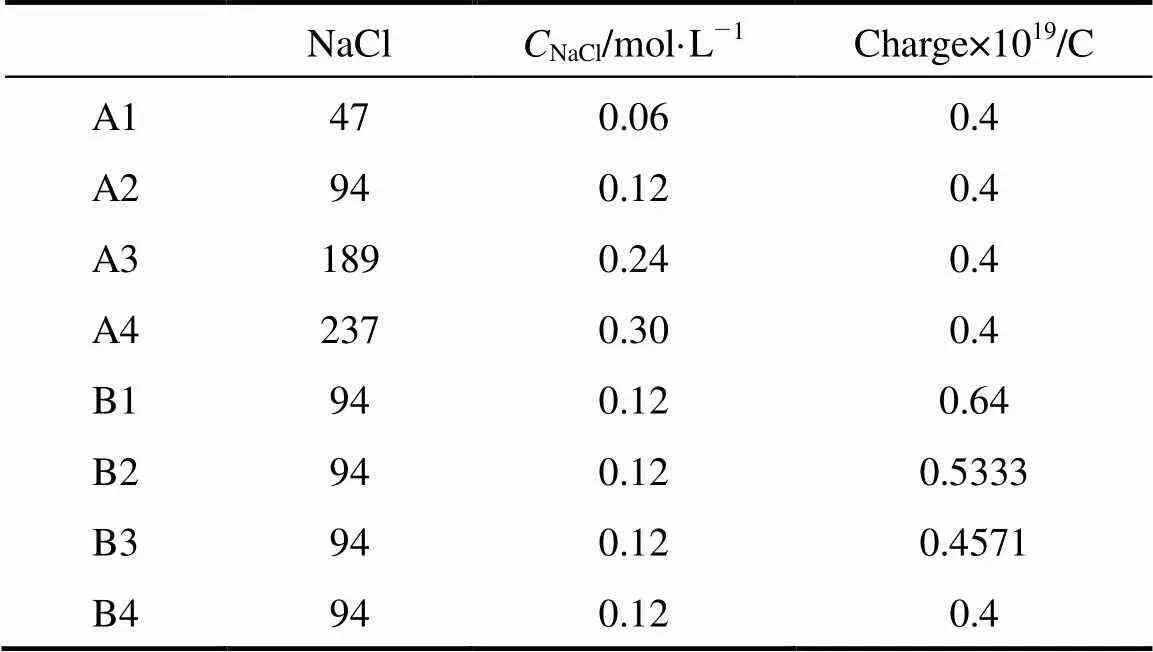
Table 2 The numbers of particles and charge of cation in systems
The magnitude of this reduced charge (0.4-0.64 C) is merely a parameter. In physical terms, the reduced charge mimics the implicit screening of the first hydration shell,.., the particle represents a hydrated ion. The number of water and SDS molecule are 37650 and 343 respectively, resulting in a SDS concentration of about 0.44 mol·L-1. Among these systems, A2 is same as B4. The SDS molecules are evenly distributed in a uniform matrix at the beginning of the simulations. For each system, the simulations are performed for four nanoseconds on 12 PIII 1.13G CPUs. Figs. 2 and 3 show the variations of the total potential energy of the systems in Series A and the interaction potential energy between the head group and the sodium ions of the systems in Series B, respectively. As illustrated in Fig. 2, the total potential energy reaches a stationary state in about 1 ns, thus the data after 1 ns are collected to analyze its statistical properties. In Fig. 3 the curves fluctuate along with the formation of micelles. The fluctuation is larger after 2 ns for B1 system. The reason is likely that the error induced by truncation is relatively larger because the charge of sodium ion here is the maximum among all systems.
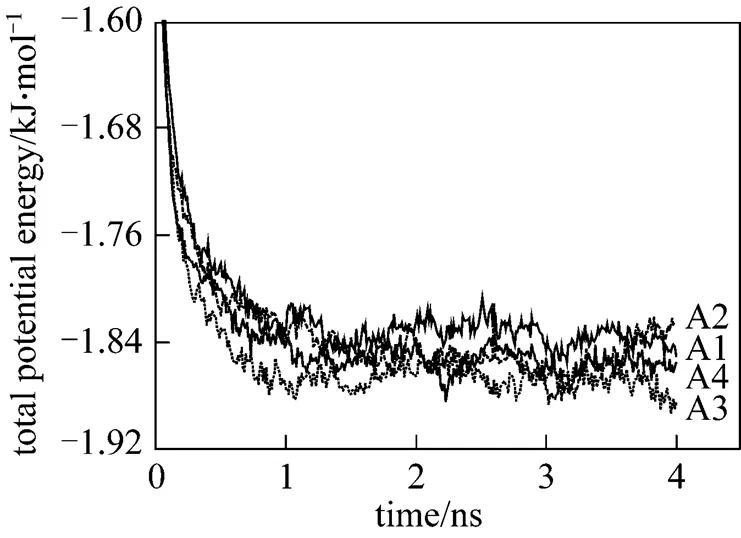
Figure 2 Time evolution of the total potential energy of systems in the Series A
Figure 3 Time evolution of the interaction potential energy between head group and Na ion of systems in the Series B
3.1 Monodispersity or polydispersity
A matter of some controversy is the influence of the salt concentration on the distribution of micelle sizes. The size distribution is finite but narrow at low ionic strength. The width of the distribution has been obtained as a fitting parameter in micelle relaxation kinetics experiments and from scattering experiments. Several authors [17] have concluded from SANS measurements that, even at low salt concentrations, there is a significant polydispersity in micelle size. More extensive time-resolved fluorescence quenching (TRFQ) experiments has supported a narrow SDS micelle size distribution [28]. Hall responded to these results with a theoretical analysis that supports the possibility of larger micelles maintaining a narrow size distribution [29]. He showed that a narrow size distribution can be maintained if the “effective degree of micellar dissociation”decreases with increasing aggregation number. In our simulations, the numbers of aggregates formed in systems and the numbers of molecules in these aggregates are shown in Table 3. The results show polydispersity when the concentration of salt is low with less charge of Na ion. The size distribution becomes narrow as the concentration of salt and the charge of Na ion increase.
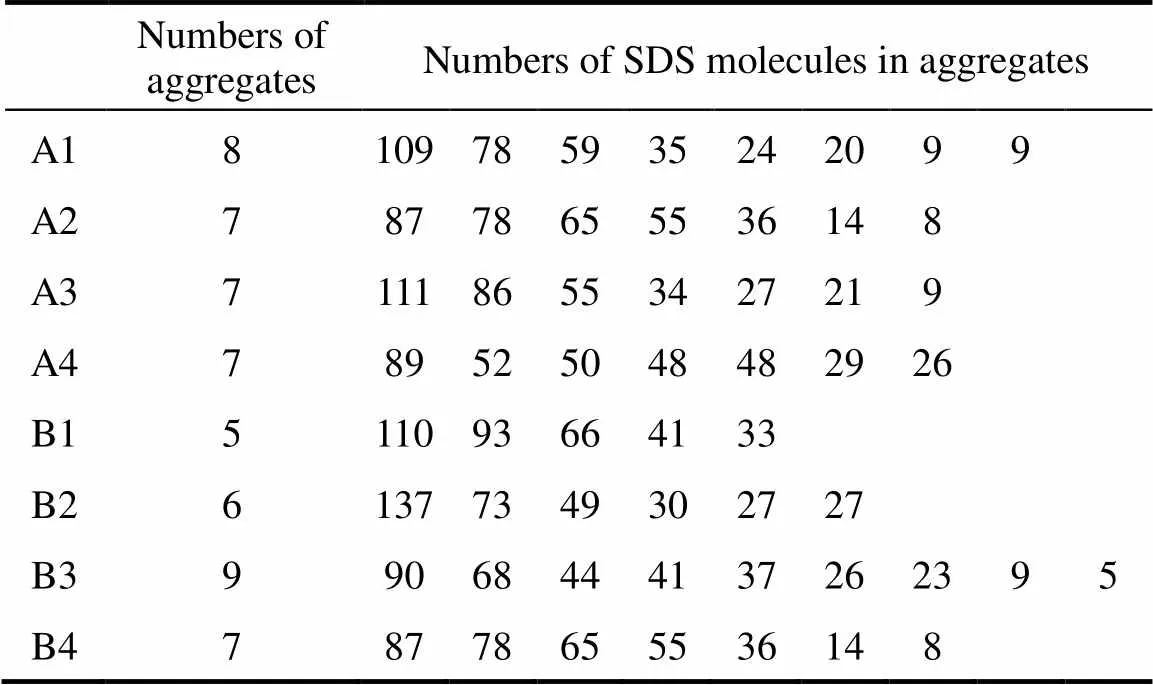
Table 3 The numbers of aggregates and SDS molecules in these aggregates of systems by the end of simulations
3.2 Degree of counter-ion dissociation
The effect of electrolyte on the degree of counter-ion dissociationis a very complex problem. Some researchers considered that the degree of dissociation of micelles does not depend on the concentration of electrolyte [30], whereas others report its increase [31] or decrease [32] with increasing concentration of electrolyte. We calculatedthrough taking count of all Na ions within 0.35 nm of the head groups in the systems of Series A. As showed in Table 4, the degree of counter-ion dissociation decreases with increasing concentration of Na ion. In aqueous solutions with high ionic strengths, a greater number of counter-ions bind to the micelle surface, causing a decrease in the degree of micelle dissociation.
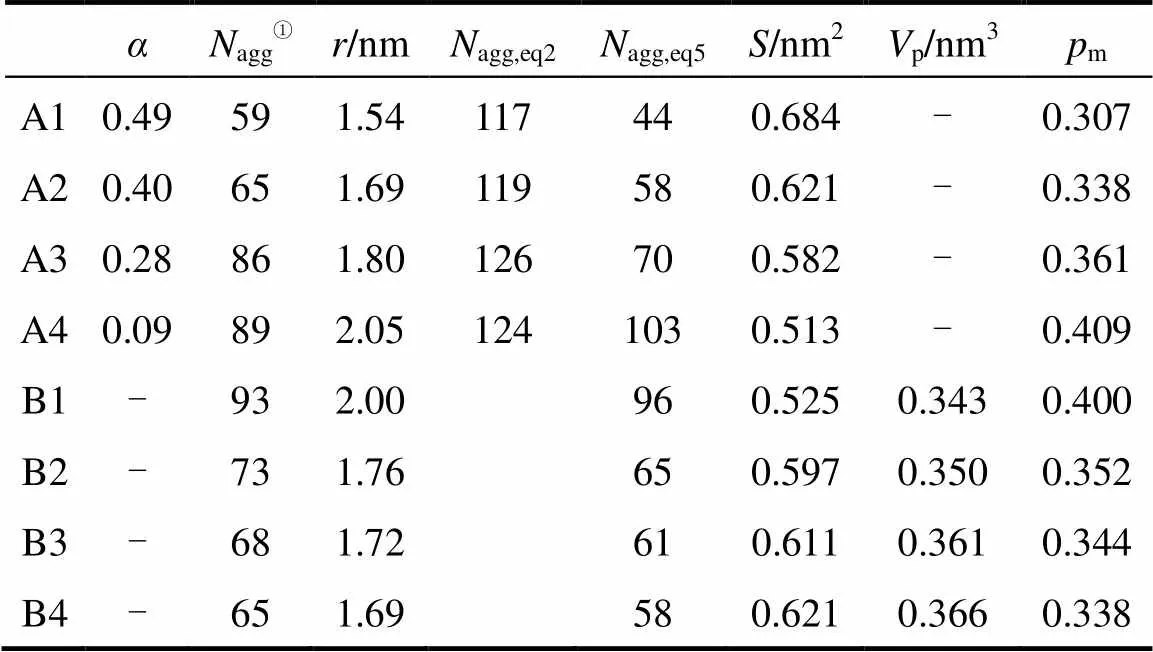
Table 4 Values of α, Nagg, r, Nagg,eq2, Nagg,eq5, S, Vp, pm for different concentrations of SDS or NaCl at 298 K
① The number of SDS molecules in micelle selected.
3.3 Micelle structure and size
The effect of salt on the structure and size of micelles is investigated in this work. Anézo. [33] performed simulations of lipid bilayer, and concluded that with the right combination of methodology and force field, lipid bilayers with areas close to the experimentally determined one can be obtained irrespective of the way long-range electrostatics is treated. We assume it holds for SDS molecules too. However, the aggregation number of micelle cannot be determined, because it in all systems is not enough in a statistical sense. One micelle in each system of the two series is selected for the calculation of area per molecule. We select a larger micelle in the higher SDS or cation concentration system based on the knowledge that aggregation number will increase with SDS or cation concentration.
The aggregation numberaggand the radius of gyrationof eight micelles selected are shown in Table 4. The aggregation number (agg,eq2) of each system is calculated by Eq. (2). It is found that the values ofagg,eq2are larger than those of micelles obtained from the simulated systems. The possible reason is that the charges of Na ion in those systems are much smaller than in reality. Moreover, presuming the micelle to be spherical in shape,aggis computed from the following relation (agg,eq5) [31].
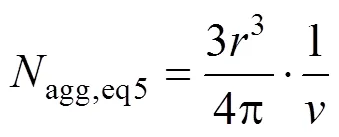
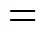
The area per head group,, in the micelle can be calculated from [35]
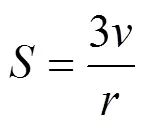
As shown in Table 4, the values ofdecrease as the aggregation number increases. Jakubowska measured the area per SDS molecule at different NaCl concentration at 25°C. He obtained values from 0.66 to 0.47 nm2in a NaCl concentration range from 0.0 to 0.4 mol·L-1[36]. The value we obtained are a little larger than that of the experiment, but is still reasonable.
For Series B, the volume per SDS molecule in the polar shell of the spherical micelle,p, is computed from [36]
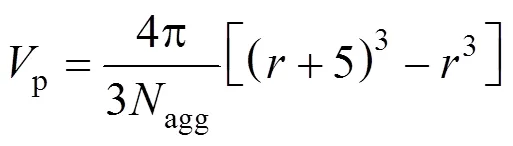
The values calculated are shown in Table 4. The SDS molecules arrange more closely, as a result of decreasing the repulsive interactions between the head groups in the micelles (the electrostatic screening effect). The values ofp, therefore, decrease with increasing concentration of the electrolyte added.
3.4 Headgroup-ion radial distribution functions
The shape, size, fractional charge of the micelle and the intermicellar interaction depend on the distribution of counterions. It is well known that salts induce pronounced growth of micelles due to charge neutralization on the micellar surface by these salts [37]. It must be pointed out that several studies encompassing only a few hundred picoseconds suggested that the ionic atmosphere was not yet in equilibrium within the micelle. It is manifested that at least 1 ns of dynamical evolution is required to reach a stable counter-ion distribution from work by Bruce[25]. Our simulations last for 4 ns, the equilibrium of ionic atmosphere can be assumed.
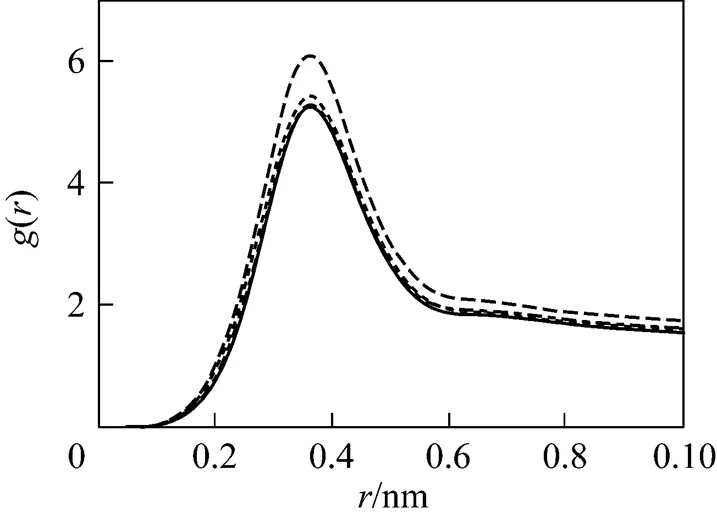
In the majority of studies involving ionic micelles, counter-ions were found to form contact pairs with headgroups as evidenced by the maxima in the head group-ion radial distribution functions (RDFs) observed at positions corresponding to the radius of the counter-ion. As showed in Figs. 4 and 5, the maxima are located at about 0.35 nm, indicating that counter-ion pairs formed between head groups and Na ion are in contact. Except for B1, there is only one peak in these rdfs. For system B1, the second peak of rdfs appears at about 0.7 nm, which is larger than about 0.5 nm reported by Rakitin[38]. The charge of Na ion in system B1 is 0.64 C which is the closest to reality among all simulating systems. These RDFs in Fig. 5 show that the electrostatic quantity of the electrolyte ions changes the distribution of ion around micelles evidently. The second peak will be vanished gradually with reducing charge of Na ion. The locations of peaks increase with decreasing charges of Na ion in B series. At the same time, the density of Na ion around head group decreases, showing that the influence of Na ion on micelles becomes weak gradually. The less charge neutralization on micellar surface finally induces growth of micelles.
The number of counter-ion pars is also calculated for system B1. The value is 0.7 per head group which is a little larger than the typical value (0.3-0.6 per head group) [38].


3.5 Transition of micelle shape
Addition of salt screens the electrostatic repulsion between head groups in a micelle, leading to reduced area per SDS molecule. This gives rise to a new packing condition for the micelles with a lower surface to volume ratio. Therefore, as the salt concentration increases, these micelles will grow in size and undergo a sphere-to-rod transition in shape. The transition will take place at 0.4 mol·L-1Na ions [39].
The critical packing parameterm(Table 4) is calculated by Eq. (8), which controls the micellar shape [34].
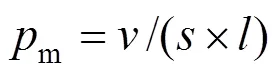
4 CONCLUSIONS
The influence of salt on micelle structure is a very complex problem. In this paper, molecular dynamics simulations of spherical SDS micelles in the presence of salt have characterized the shape, dispersity, and degree of neutralization by counter-ions.
The values ofaggincrease with increasing electrolyte concentration because the repulsive interactions between the head groups in the micelles decrease (the electrostatic screening effect). It is seen that the fractional charge on the micelle decreases when the salt concentration in the micellar solution is increased. The neutralization of the charge at the surface of the micelle by the increase in the counter-ion condensation decreases the area of the effective head group and increases the volume for the surfactant monomer to occupy in the micelle. The degree of counter-ion dissociation decreases gradually with increasing salt concentration. The transition of micelle shape is not observed in the systems simulated, but polydispersity of micelle size is found.
1 Jönsson, B., Lindman, B., Holmberg, K., Kronberg, B., Surfactants and Polymers in Aqueous Solution, John Wiley, Chichester (1998).
2 Li, J., Kwauk, M., “Exploring complex system in chemical engineering—The multi-scale methodology”,..., 58, 521-535 (2003).
3 von Berlepsch, H., Stähler, K., Zana, R., “Micellar properties of sodium sulfopropyl alkyl maleates in aqueous solution. A time-resolved fluorescence quenching study”,, 12, 5033-5041 (1996).
4 Gurtovenko, A.A., Miettinen, M., Karttunen, M., Vattulainen, I., “Effect of monovalent salt on cationic lipid membranes as revealed by molecular dynamics simulations”,..., 109, 21126-21134 (2005).
5 Tanford, C., “Theory of micelle formation in aqueous solutions”,..., 78, 2469-2479 (1974).
6 Rharbi, Y., Chen, L., Winnik, M.A., “Exchange mechanisms for sodium dodecyl sulfate micelles: high salt concentration”,...., 126, 6025-6034 (2004).
7 Bales, B.L., Messina, L., Vidal, A., Peric, M., Nascimento, O.R., “Precision relative aggregation number determinations of SDS micelles using a spin probe. A model of micelle surface hydration”,..., 102, 10347-10358 (1998).
8 Bezzobotnov, V.Y., Borbely, S., Cser, L., Farago, B., Gladkih, I.A., Ostanevich, Y.M., “Temperature and concentration dependence of properties of sodium dodecyl sulfate micelles determined from small angle neutron scattering experiments”,..., 92, 5738-5743 (1988).
9 Quina, F.H., Nassar, P.M., Bonilha, J.B.S., Bales, B.L., “Growth of sodium dodecyl sulfate micelles with detergent concentration”,..., 99, 17028-17031 (1995).
10 Ikeda, S., Hayashi, S., Imae, T., “Rodlike micelles of sodium dodecyl sulfate in concentrated sodium halide solutions”,..., 85, 106-112 (1981).
11 Almgren, M., Gimel, J.C., Wang, K., Karlsson, G., Edwards, K., Brown, W., Mortensen, K., “SDS micelles at high ionic strength. A light scattering, neutron scattering, fluorescence quenching, and CryoTEM investigation”,.., 202, 222-231 (1998).
12 Bergström, M., Pedersen, J.S., “Structure of pure SDS and DTAB micelles in brine determined by small-angle neutron scattering (SANS)”,...., 1, 4437-4446 (1999).
13 Warr, G.G., Grieser, F., “Determination of micelle size and polydispersity by fluorescence quenching. Theory and numerical results”,...,., 82, 1813-1828 (1986).
14 Siemiarczuk, A., Ware, W.R., Liu, Y.S., “A novel method for determining size distributions in polydisperse micelle systems based on the recovery of fluorescence lifetime distributions”,..., 97, 8082-8091 (1993).
15 Reekmans, S., Bernik, D., Gehlen, M., van Stam, J., Van der Auweraer, M., de Schryver, F.C., “Change in the micellar aggregation number or in the size distribution? A dynamic fluorescence quenching study of aqueous cetyltrimethylammonium chloride”,, 9, 2289-2296 (1993).
16 Mazer, N.A., Benedek, G.B., Carey, M.C., “An investigation of the micellar phase of sodium dodecyl sulfate in aqueous sodium chloride solutions using quasielastic light scattering spectroscopy”,..., 80, 1075-1085 (1976).
17 Cabane, B., Duplessix, R., Zemb, T., “High-resolution neutron-scattering on ionic surfactant micelles - SDS in water”,.., 46, 2161-2178 (1985).
18 Bhaskar, D.G., van Stam, J., de Schryver, F.C., “Are aqueous sodium dodecyl sulfate micelles in the presence of added salt polydisperse? A time-resolved fluorescence quenching study with global analysis”,, 13, 1957-1963 (1997).
19 Keiper, J., Romsted, L.S., Yao, J., Soldi, V., “Interfacial compositions of cationic and mixed non-ionic micelles by chemical trapping: A new method for characterizing the properties of amphiphilic aggregates”,.., 176, 53-67 (2001).
20 Gao, J., Ge, W., Hu, G.H., Li, J.H., “From homogeneous dispersion to micelles. A molecular dynamics simulation on the compromise of the hydrophilic and hydrophobic effects of sodium dodecyl sulfate in aqueous solution”,, 21, 5223-5229 (2005).
21 Salaniwal, S., Cui, S.T., Cochran, H.D., Cummings, P.T., “Molecular simulation of a dichain surfactant/water/carbon dioxide system (I) structural properties of aggregates”,, 17, 1773-1783 (2001).
22 Brown, D., Clarke, J.H.R., “A molecular dynamics study of chain configurations in-alkane-like liquids”,..., 100, 1684-1692 (1994).
23 Salaniwal, S., Cui, S.T., Cummings, P., Cochran, H.D., “Self-assembly of reverse micelles in water/surfactant/carbon dioxide systems by molecular simulation”,, 15, 5188-5192 (1999).
24 Allen, M.P., Tildesley, D.J., Computer Simulation of Liquids, Clarendon Press, Oxford (1987).
25 Bruce, C.D., Berkowitz, M.L., Perera, L., Forbes, M.D.E., “Molecular dynamics simulation of sodium dodecyl sulfate micelle in water: micellar structural characteristics and counterion distribution”,..., 106, 3788-3793 (2002).
26 Rapaport, D.C., The Art of Molecular Dynamics Simulation, Cambridge University Press, Cambridge (1995).
27 Maiti, P.K., Lansac, Y., Glaser, M.A., Clark, N.A., “Self-assembly in surfactant oligomers: A coarse-grained description through molecular dynamics simulations”,, 18, 1908-1918 (2002).
28 Almgren, M., Lofroth, J.E., “Determination of micelle aggregation numbers and micelle fluidities from time-resolved fluorescence quenching studies”,.., 81, 486-499 (1981).
29 Hall, D.G., “Polydispersity of sodium dodecyl sulfate (SDS) micelles”,, 15, 3483-3485 (1999).
30 Ranganathan, R., Peric, M., Bales, B.L., “Time-resolved fluorescence quenching measurements of the aggregation numbers of normal sodium alkyl sulfate micelles well above the critical micelle concentrations”,..., 102, 8436-8439 (1998).
31 Paul, B.C., Islam, S.S., Ismail, K., “Effect of acetate and propionate co-ions on the micellization of sodium dodecyl sulfate in water”,..., 102, 7807-7812 (1998).
32 Ikeda, S., “Stability of spherical and rod-like micelles of ionic surfactants, in relation to their counterion binding and modes of hydration”,.., 269, 49-61 (1991).
33 Anézo, C., de Vries, A.H., Höltje, H., Tieleman, D.P., Marrink, S., “Methodological issues in lipid bilayer simulations”,..., 107, 9424-9433 (2003).
34 Turner, D., Gracie, K., Taylor, T., Palepu, R., “Micellar and thermodynamic properties of sodium dodecyl sulfate in binary aqueous solutions of di-, tri-, and tetraethylene glycols”,.., 202, 359-368 (1998).
35 Briganti, P., Puvvada, S., Blankschtein, D., “Effect of urea on micellar properties of aqueous solutions of nonionic surfactants”,..., 95, 8989-8995 (1991).
36 Dutkiewicz, E., Jakubowska, A., “Effect of electrolytes on the physicochemical behaviour of sodium dodecyl sulphate micelles”,.., 280, 1009-1014 (2002).
37 Aswal, V.K., Goyal, P.S., Thiyagarajan, P., “Small-angle neutron-scattering and viscosity studies of CTAB/NaSal viscoelastic micellar solutions”,..., 102, 2469-2473 (1998).
38 Rakitin, A.R., Pack, G.R., “Molecular dynamics simulations of ionic interactions with dodecyl sulfate micelles”,..., 108, 2712-2716 (2004).
39 Dutkiewicz, E., Jakubowska, A., “Water activity in aqueous solutions of inhomogeneous electrolytes”,..., 103, 9898-9902 (1999).
2008-11-12,
2009-05-25.
the Outstanding Overseas Research Team Project of the Chinese Academy of Sciences, the National Natural ScienceFoundation of China (20221603), and the Research Fund of Key Lab for Nanomaterials, Ministry of Education, China (2006-1).
** To whom correspondence should be addressed. E-mail: wge@home.ipe.ac.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Removal of Uranium (VI) by Fixed Bed Ion-exchange Column Using Natural Zeolite Coated with Manganese Oxide*
- Phase Equilibrium of Isobutanol in Supercritical CO2
- Conversion of Methane by Steam Reforming Using Dielectric-barrier Discharge*
- Permeability and Selectivity of Sulfur Dioxide and Carbon Dioxide in Supported Ionic Liquid Membranes*
- Hydroxyapatite Coatings on Titanium Prepared by Electrodeposition in a Modified Simulated Body Fluid*
- Model Study on a Submerged Catalysis/Membrane Filtration System for Phenol Hydroxylation Catalyzed by TS-1*
