Model Study on a Submerged Catalysis/Membrane Filtration System for Phenol Hydroxylation Catalyzed by TS-1*
2009-05-15CHENRizhi陈日志JIANGHong姜红JINWanqin金万勤andXUNanping徐南平
CHEN Rizhi (陈日志), JIANG Hong (姜红), JIN Wanqin (金万勤) and XU Nanping (徐南平)
Model Study on a Submerged Catalysis/Membrane Filtration System for Phenol Hydroxylation Catalyzed by TS-1*
CHEN Rizhi (陈日志), JIANG Hong (姜红), JIN Wanqin (金万勤)**and XU Nanping (徐南平)
State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemistry and Chemical Engineering, Nanjing University of Technology, Nanjing 210009, China
This research is focused on the development of a simple design model of the submerged catalysis/ membrane filtration (catalysis/MF) system for phenol hydroxylation over TS-1 based on the material balance of the phenol under steady state and the reported kinetic studies. Based on the developed model, the theoretical phenol conversions at steady state could be calculated using the kinetic parameters obtained from the previous batch experiments. The theoretical conversions are in good agreement with the experimental data obtained in the submerged catalysis/MF system within relative error of ±5%. The model can be used to determine the optimal experimental conditions to carry out the phenol hydroxylation over TS-1 in the submerged catalysis/MF system.
submerged catalysis/membrane filtration system, phenol hydroxylation, TS-1
1 INTRODUCTION
In 1983, Taramasso. first synthesized titanium silicalites [1]. As a new catalytic material, titanium silicalites-1 (TS-1) has been investigated extensively for their high catalytic activity and selectivity in the selective oxidation of organic compounds [2-4]. The hydroxylation of phenol over TS-1 with H2O2as the oxidant is a promising process for the preparation of dihydroxybenzene (DHB) including hydroquinone (HQ) and catechol (CA), which is an important chemical intermediate that is widely used in petrochemical industry, manufacture of medicine, pesticide, dyestuff, perfume, rubber,. One of the challenges in further development of the technology is an effective means of separating the catalyst particles from the reaction mixture. A major difficulty in the separation of TS-1 particles arises because TS-1 particles are too fine to be removed by gravity settling or porous tube filtration [5]. The separation problem can be overcome by attaching the catalysts to a suitable substrate, but with a drawback of decreasing the effective surface areaof the catalysts particles. In fact, catalysts in suspension have a better efficiency than those immobilized [6-8].
A very promising method for solving the above-mentioned problem is coupling the heterogeneous catalytic reaction of phenol hydroxylation with the membrane filtration to structure a catalysis/MF system [9]. The concept of a catalysis/MF system is based on the potential of semipermeable membranes to retain the catalyst but not the reactants or products formed during the reaction [10, 11]. There are two configurations either as a side-stream catalysis/MF system or as a submerged catalysis/MF system [11]. A side-stream catalysis/MF system is more flexible and easier to scale up due to the segregation of reaction zone and separation zone, but the potential loss of fine catalysts could be greater in the pipe and pumping systems [11] and the use of recirculation loops leads to increased energy costs [12]. Recently, the submerged catalysis/MF system received more attentions, although most works focused on the biochemical processes and wastewater treatment with polymeric membranes [11-13]. In our pervious studies, a submerged catalysis/MF system has been developed for the continuous hydroxylation of phenol to HQ and CA over ultrafine TS-1, and the effects of operating conditions on the properties of the coupling process were investigated experimentally in detail [14].
A mathematical model is a practical, reliable, and useful package for the analysis, design, scale-up, and optimization of the process systems under given conditions. The important attributes of a model are its robustness and reliability in providing the best design features for a new or modernized system, besides determining optimal operating conditions for existing systems. The reactor performance can be modeled by formulating equations based on the reactant consumption or product formation [10, 15-18].
In order to better understand the coupling process, a design model of the submerged catalysis/MF system for phenol hydroxylation catalyzed by TS-1 was developed in the present study based on the material balance and the reported kinetic studies. The reliability of the model was verified by the experimental results, and the optimal experimental conditions were determined using the simulation model for certain conditions.
2 EXPERIMENTAL
2.1 Materials
TS-1 catalyst (average particle size, 200 nm; specific surface area, 95 m2·g-1; the Si/Ti mass ratio, 9) was provided by Baling Petrochemical Company, SINOPEC. Methanol (>99.9% Chromatography Grade) was supplied by Yuwang Group and pure water by Hangzhou Wahaha Group (China). Deionized water (electrical conductivity <12ms·cm-1) was homemade. Other materials (phenol provided by Shanghai Experiment Reagent Co., China; 30% (by mass) hydrogen peroxide by Sinopharm Chemical Reagent Co., China; CA by Shanghai Sansi Reagent Co., China and HQ by Guangdong Xilong Chemical Co., China) are all of reagent grade and used without further treatment.
2.2 Apparatus
The submerged catalysis/MF system used in this work has been described previously [14] and is shown in Fig. 1, which consists mainly of a slurry stirred reactor, a ceramic membrane module, a feeding system, a products collecting system and a heating system. The reactor was made of glass with a working volume of 300 ml. The membrane employed was 12 mm in outer diameter, 8 mm in inner diameter with a filtration area of 38 cm2. It was made of a fine layer of α-Al2O3with a nominal pore size of 0.2 µm on the outer wall of a tubular α-Al2O3porous support, and was provided by Jiusi High-Tech Co., Nanjing, China. The membrane tube was connected with the liquid outlet valve at one end. The other end was sealed with glaze. Two controlled volume pumps (Qinpu Huxi Instrument Factory, Shanghai, China) were used to feed hydrogen peroxide solution and phenol solution to the system separately while a peristaltic pump (Lange Constant-flow Pump Co., Baoding, China) was used to take out the mixture from the reactor. The reactor was immersed in a water bath whose temperature was controlled by a temperature controller (Huachen Medical Instruments Co., Shanghai, China).
2.3 Hydroxylation experiment
For the sake of verifying the model, some hydroxylation experiments were carried out in the submerged catalysis/MF system at different operation modes from the previous studies [14]. In previous studies, the reactor was operated as a semi-batch reactor initially for 60 min and as a continuous reactor thereafter. In the present study, the phenol hydroxylation was carried out continuously, a desired mode for the stable operation.
The experimental procedure was as follows. After adding 290 ml phenol aqueous solution and 5 g TS-1 catalyst into the reactor, the system was heated to a desired temperature under stirring (380 r·min-1). When the temperature reached the set value of 80°C, the phenol aqueous solution and hydrogen peroxide solution were feed continuously into the reactor. Meanwhile, the products were taken out with a peristaltic pump at a certain flow rate, equal to the total feeding flow rate. Thus, the liquid level in the reactor was kept constant and the system was operated at a constant membrane flux. At the initial operation stage, TS-1 catalyst particles would easily adsorb on the clean membrane to form a cake layer, making the filtration resistance and the operation pressure increase. In order to keep the liquid level in the reactor constant, the discharge flow rate (.. the membrane flux) would be adjusted by changing the rotating speed of the peristaltic pump. As the equilibrium of deposition and leaving of catalyst particles happened, the operation pressure tended to stabilize and the system was operated at a steady state. The catalyst particles were completely retained in the reactor by the ceramic membrane. The products were collected in a 1000 ml graduated cylinder. The operation pressure of the membrane separation was monitored by a vacuum gauge. Each test run was in operation until steady state was reached with samples taken regularly for analysis. After the reaction was completed, the membrane was rinsed with deionized water for use in the next test.
The products were regularly taken from the outlet of the reactor and analyzed by HPLC system (Agilent 1100 Series, USA) equipped with a diode array detector (DAD) and an auto-sampler [19]. Chromatographic separations were performed at 303 K using a ZORBAX Eclipse XDB-C18, 5 μm, 4.6 mm×250 mm column. A mobile phase composed of 40% methanol and 60% water at a flow rate of 1 ml·min-1was used. Phenol conversion and DHB selectivity were calculated based on the starting amount of phenol [20-22], according to Eqs. (1) and (2), respectively.
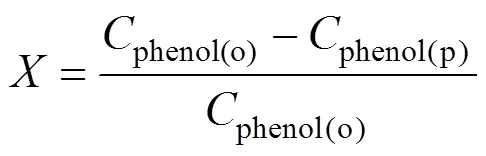
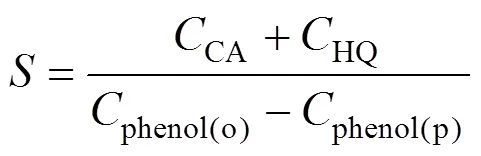
whereis the phenol conversion,phenol(o)the initial phenol concentration in the feed (mol·L-1),phenol(p)the phenol concentration in the outlet of the reactor (mol·L-1),CAandHQthe concentration of CA and HQ in the outlet of the reactor (mol·L-1).
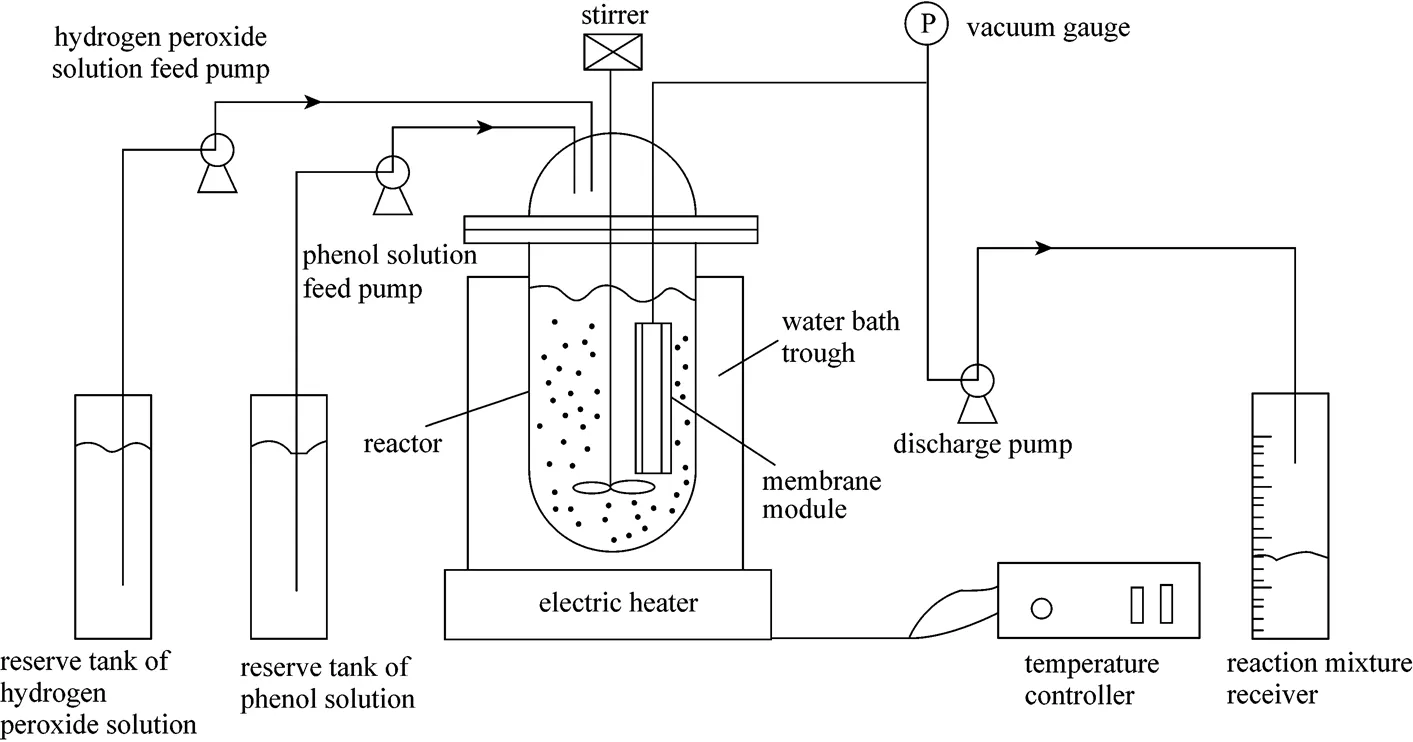
Figure 1 Diagram of the submerged catalysis/membrane filtration system for phenol hydroxylation to HQ and CA
3 MATHEMATICAL MODEL
In order to optimize the design of the catalysis/MF system, it is necessary to develop a predictive model describing the reactor behaviors. The model should adequately represent the phenomenological aspects of the actual process or effectively simulate the system. Therefore, reasonable assumptions are made to account for all the physicochemical and transport sub- processes relevant to the process as follows:
(1) The submerged catalysis/MF system can be considered as a completely mixed flow reactor because of the high stirring rate. Tracer tests performed under these conditions in the catalysis/MF system manifested results pertaining to a completely mixed flow system.
(2) TS-1 catalyst particles can be completely retained in the reactor by the ceramic membrane used. Leakage of the catalyst particles through the membrane was not observed.
(3) The reactants or products can not be retained by the ceramic membrane used, namely the concentrations of reactants or products in bulk liquid phase are the same as those in membrane permeate.
In this work, the yield is considered as the objective function. For a continuous catalysis/MF system, the yield is defined as moles of DHB produced in a minute per reaction volume, and is calculated as
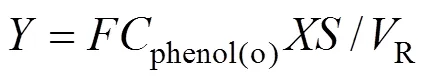
whereis the feeding rate (ml·min-1),phenol(o)the initial concentration of phenol in the feed (mol·L-1),the phenol conversion,the DHB selectivity,Rthe reaction volume (ml).
Under steady state the system is described by the following material balance:
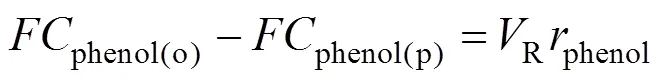
wherephenolis the reaction rate,the rate of the feed and the permeate, since the liquid level in the reactor is constant. The other parameters have been described above.
According to Eq. (1), Eq. (4) can be simplified to
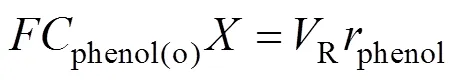
In an earlier work [23], the kinetic model of phenol hydroxylation over TS-1 was developed based on the phenol concentration measurements. The reaction rate can be expressed as
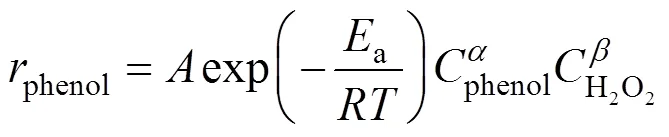
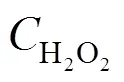
According to assumption (3),

The concentration of H2O2in the outlet of the reactor can be expressed as a function of the phenol conversion.

According to Eqs.(5) to (8), the phenol conversion can be expressed as

Then the expression describing the relation between the yield and the kinetics of phenol hydroxylation is obtained:
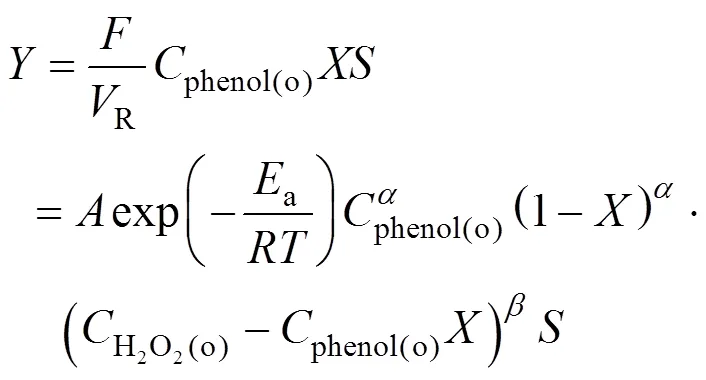
4 RESULTS AND DISCUSSION
4.1 Phenol hydroxylation in the catalysis/MF system
For a given continuous catalysis/MF system, the feeding flow rate and phenol concentration in the feed are two important controlling parameters affecting the yield according to Eq. (3). Therefore, the effects of the two parameters were investigated experimentally first.
The effect of feeding rate on the conversion and selectivity of phenol hydroxylation was examined from 1.7 to 3 ml·min-1with phenol concentration of 4.1 mol·L-1and H2O2of 1.4 mol·L-1in the feed. The results are shown in Fig. 2. The conversion increases with time until a constant value is reached corresponding to the steady state, irrespective of the feeding rate. The same trend was also reported by Bódalo[10]. Because the initial content with the conversion being zero, the hydroxylation took some time to approach the steady state. With the decreasing feeding rate, namely the increase in the space time, the conversion increases gradually from 21.9% to 26.2%. In addition, the higher the feeding rate, the shorter the time required for the establishment of the steady state as shown in Fig. 2 (a). It is found from Fig. 2 (b) that the feeding rate has little influence on the selectivity in the studied experimental range, which was roughly constant at about 83%.
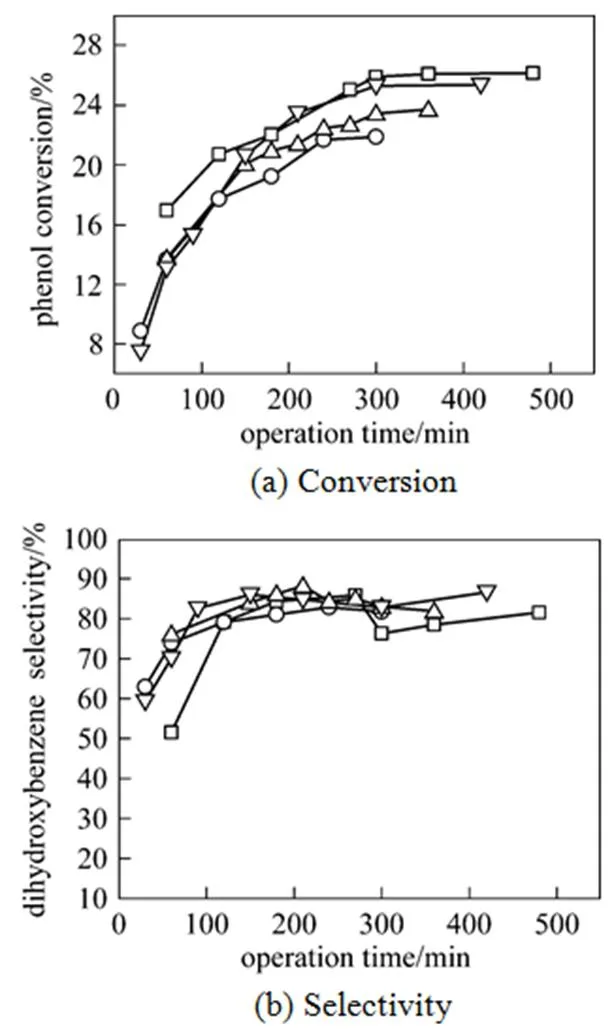
Figure 2 Effect of feeding rate on the conversion and selectivity of phenol hydroxylation over TS-1 feeding rate/ml·min-1:□ 1.7;▽ 2.0;△ 2.4;○ 3.0
Figure 3 Effect of phenol concentration in the feed on the conversion and selectivity of phenol hydroxylation over TS-1phenol concentration/mol·L-1:□ 4.1;▽ 4.7;△ 5.3;○ 6.0
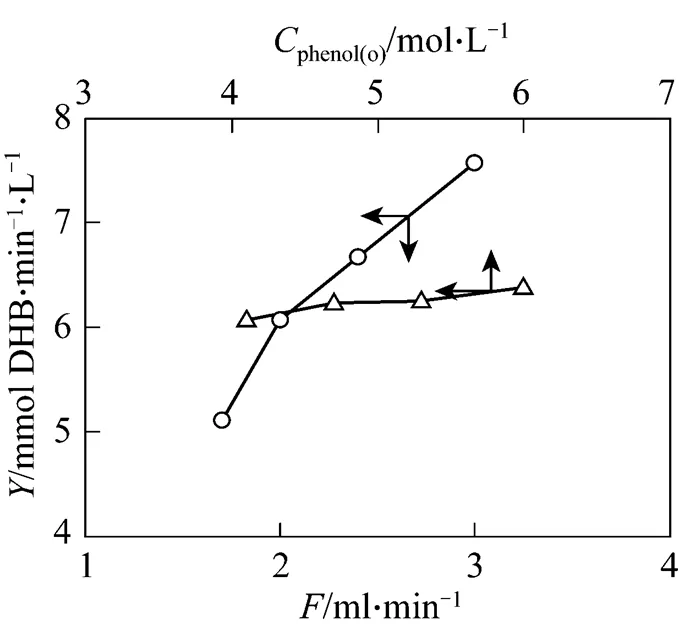
Figure 4 Experimental yields at steady state (Data from Figs. 2 and 3 respectively)
Figure 3 presents the effect of phenol concentration in the feed on the conversion and selectivity of hydroxylation reaction at the phenol feeding rate of 1.7 ml·min-1and H2O2(1.4 mol·L-1) feeding rate of 0.3 ml·min-1. With respect to a given phenol concentration in the feed, the conversion increases with time until a constant value is reached corresponding to the steady state, like the trend as shown in Fig. 2 (a). With increasing the phenol concentration in the feed, the conversion decreases gradually while the selectivity changes insignificantly. In these experiments, the phenol is excessive compared to H2O2, the extent of phenol transformation is limited by the feed of H2O2. As a result,when the concentration of phenol increases, the calculated conversion according to Eq. (1) becomes lower.
According to Eq. (3), the yield was calculated for each case considering the conversion and selectivity at steady state. The yield shown in Fig. 4 increases with the feeding rate or phenol concentration in the feed, although the phenol conversion decreases as shown in Figs. 2 (a) and 3 (a), indicating that the both parameters have significant influence on the yield in a given submerged catalysis/MF system.
4.2 Simulation and optimization
The reliability of the above-mentioned model is verified in this section by comparing the simulated results with the experimental data. According to Eq. (9), theoretical phenol conversions at steady state under different feeding rates and phenol concentrations in the feed were calculated using the kinetic parameters in Table 1. These data were obtained from a previous study in our lab with experiments carried out in a batch reactor [23]. The comparison between the calculated phenol conversions with experimental data is shown in Fig. 5, with the relative error below 5%. This good agreement indicates that the kinetic model and the values of the kinetic parameters are adequate to describe the studied reaction system. Thus, the design equation of the catalysis/MF system (Eq. (4)) can be used as an optimization tool.
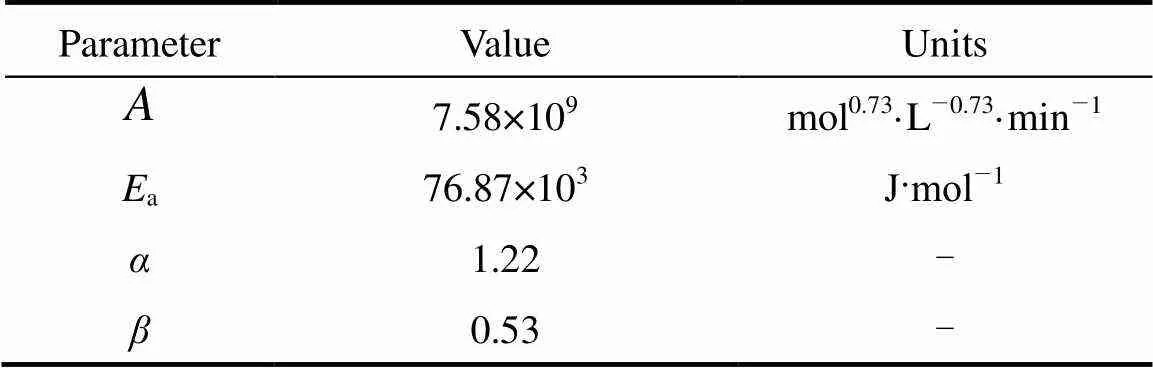
Table 1 Kinetic parameters of phenol hydroxylation catalyzed by TS-1 [23]
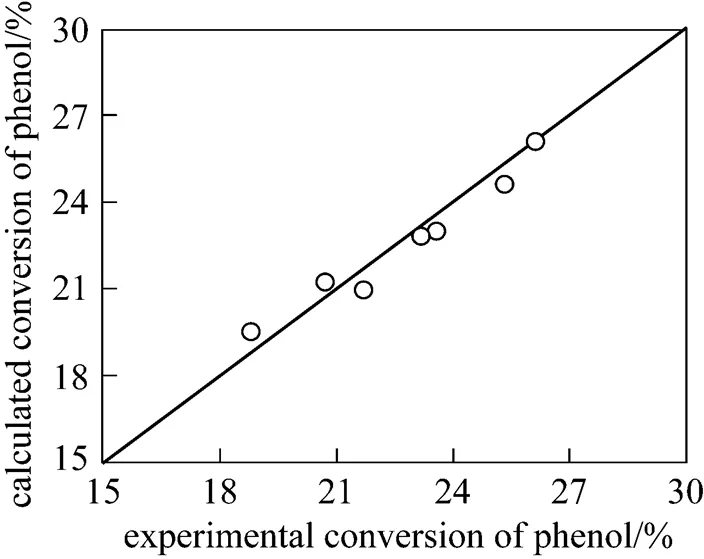
Figure 5 Comparison of calculated and experimental phenol conversion
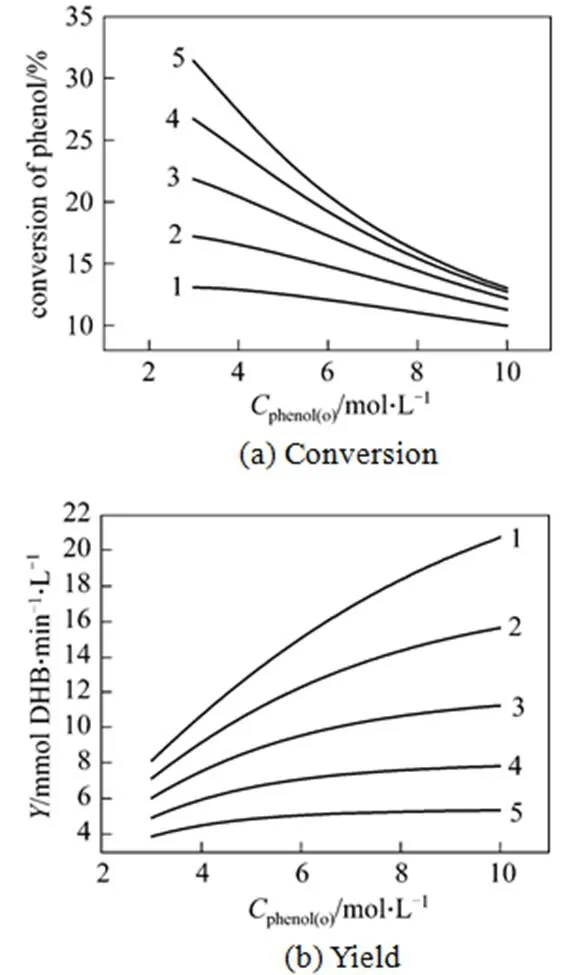
Figure 6 The calculated conversion and yield against phenol concentration in the feed for different values of space time/min: 1—40; 2—60; 3—90; 4—135; 5—202.5
It is seen from Fig. 6 (b), for a constant space time the yield increases gradually as the phenol concentration in the feed increases although the conversion at the reactor outlet decreases as presented in Fig. 6 (a). The same effect is also observed for the decreasing space time at constant phenol concentration in the feed. In other words, the yield of the catalysis/MF system can be increased by increasing the phenol concentration in the feed or decreasing the space time. However, it is noted by comparing the data that the increased degree of yield decreases with increasing the phenol concentration in the feed or decreasing the space time. For example, as the space time decreases from 202.5 min to 135 min the yield increases by a factor of 1.47 and as the space time decreases from 90 min to 60 min the yield increases by a factor of 1.39 at a phenol concentration in the feed of 10 mol·L-1. In addition, the shorter space time results in lower conversion as presented above, which would make the separation of phenol from the reaction mixture difficult. At the same time, increasing the phenol concentration in the feed to improve the yield is limited by the solubility of phenol in the used solvent. Therefore, in a real situation, the experimental conditions are chosen for reaching a high yield but not the limit yield, when taking into account the separation and solubility of phenol.
5 CONCLUSIONS
In this work, a submerged catalysis/MF system was constructed for the continuous phenol hydroxylation to CA and HQ over ultrafine TS-1 catalyst. A model of the submerged catalysis/MF system for phenol hydroxylation over TS-1 has been developed based on the material balance of the phenol at steady state and the previous kinetic studies. The model has been validated by comparing the model calculation with the experimental data on phenol conversion.
In summary, the hybrid system is found to be a feasible route for the continuous heterogeneous catalytic reaction. The developed model is capable of describing the behavior of the coupling system and valuable for the process optimization.
1 Taramasso, M., Perego, G., Notari, B., “Preparation of porous crystalline synthetic material comprised of silicon and titanium oxides”, US Pat., 4410501 (1983).
2 Pozzo, D.L., Fornasari, G., Monti, T., “TS-1, catalytic mechanism in cyclohexanone oxime yield”,.., 3, 369-375 (2002).
3 Liang, X.H., Mi, Z.T., Wang, Y.Q., Wang, L., Zhang, X.W., “Synthesis of acetone oxime through acetone ammoximation over TS-1”,...., 82, 333-337 (2004).
4 Zhong, Z.X., Xing, W.H., Liu, X., Jin, W.Q., Xu, N.P., “Fouling and regeneration of ceramic membranes used in recovering titanium silicalite-1 catalysts”,..., 301, 67-75 (2007).
5 Zhong, Z.X., Xing, W.H., Jin, W.Q., Xu, N.P., “Adhesion of nanosized nickel catalysts in the nanocatalysis/UF system”,., 53, 1204-1210 (2007).
6 Lee, S., Choo, K., Lee, C., Lee, H., Hyeon, T., Choi, W., Kwon, H., “Use of ultrafiltration membranes for the separation of TiO2photocatalysts in drinking water treatment”,...., 40, 1712-1719 (2001).
7 Meng, Y.B., Huang, X., Yang, Q.H., Qian, Y., Kubota, N., Fukunaga, S., “Treatment of polluted fiver water with a photocatalytic slurry reactor using low-pressure mercury lamps coupled with a membrane”,, 181, 121-133 (2005).
8 Sopajaree, K., Qasimi, S.A., Basak, S., Rajeshwar, K., “An integrated flow reactor-membrane filtration system for heterogeneous photocatalysis (I) Experiments and modeling of a batch-recirculated photoreactor”,..., 29, 533-539 (1999).
9 Fu, J.F., Ji, M., Wang, Z., Jin, L.N., An, D.N., “A new submerged membrane photocatalysis reactor (SMPR) for fulvic acid removal using a nano-structured photocatalyst”,..., B131, 238-242 (2006).
10 Bódalo, A., Gómez, J.L., Gómez, E, Bastida, J., Máximo, M.F., Montiel, M.C., “Ultrafiltration membrane reactors for enzymatic resolution of amino acids: Design model and optimization”,.., 28, 355-361 (2001).
11 Gan, Q., Allen, S.J., Taylor, G., “Design and operation of an integrated membrane reactor for enzymatic cellulose hydrolysis”,..., 12, 223-229 (2002).
12 Shim, J.K., Yoo, I.K., Lee, Y.M., “Design and operation considerations for wastewater treatment using a flat submerged membrane bioreactor”,.., 38, 279-285 (2002).
13 Yang, W., Cicek, N., Ilg, J., “State-of-the-art of membrane bioreactors: Worldwide research and commercial applications in North America”,..., 270, 201-211 (2006).
14 Lu, C.J., Chen, R.Z., Xing, W.H., Jin, W.Q., Xu, N.P., “A submerged membrane reactor for continuous phenol hydroxylation over TS-1”,., 54, 1842-1849 (2008).
15 Bhatia, S., Long, W.S., Kamaruddin, A.H., “Enzymatic membrane reactor for the kinetic resolution of racemic ibuprofen ester: Modeling and experimental studies”,..., 59, 5061-5068 (2004).
16 Bódalo, A., Gómez, J.L., Gómez, E, Máximo, M.F., Montiel, M.C., “Development and experimental checking of an unsteady-state model for ultrafiltration continuous tank reactors”,..., 60, 4225-4232 (2005).
17 Tsai, H.H., Ravindran, V., Pirbazari, M., “Model for predicting the performance of membrane bioadsorber reactor process in water treatment applications”,..., 60, 5620-5636 (2005).
18 Abo-Ghandera, N.S., Gracea, J.R., Elnashaieb, S.S.E.H., Lim, C.J., “Modeling of a novel membrane reactor to integrate dehydrogenation of ethylbenzene to styrene with hydrogenation of nitrobenzene to aniline”,..., 63, 1817-1826 (2008).
19 Chen, R.Z., Du, Y., Xing, W.H., Xu, N.P., “The effect of titania structure on Ni/TiO2catalysts for-nitrophenol hydrogenation”,...., 14, 665-669 (2006).
20 Du, Y.C., Yang, Y., Liu, S., Xiao, N., Zhang, Y.L., Xiao, F.S., “Mesoporous aluminophosphates and Fe-aluminophosphates with highly thermal stability and large surface area templated fromsemi-fluorinated surfactant”,..., 114, 250-256 (2008).
21 Wu, C.,Kong, Y., Gao, F., Wu, Y., Lu, Y.N., Wang, J., Li, D., “Synthesis, characterization and catalytic performance for phenol hydroxylation of Fe-MCM41 with high iron content”,..., 113, 163-170 (2008).
22 Villa, A.L., Caro, C.A., Correa, C.M.D., “Cu- and Fe-ZSM-5 as catalysts for phenol hydroxylation”,...., 228, 233-240 (2005).
23 Lu, C.J., Chen, R.Z., Jin, W.Q., Xu, N.P., “Intrinsic kinetics of hydroxylation of phenol with hydrogen peroxide catalyzed by titanium silicalite-1”,.. (), 36, 38-41 (2008). (in Chinese)
2008-09-10,
2009-06-21.
the National Basic Research Program of China (2009CB623406), the National High Technology Research and Development Program of China (2007AA06A402) and the National Natural Science Foundation of China (20636020).
** To whom correspondence should be addressed. E-mail: wqjin@njut.edu.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Removal of Uranium (VI) by Fixed Bed Ion-exchange Column Using Natural Zeolite Coated with Manganese Oxide*
- Phase Equilibrium of Isobutanol in Supercritical CO2
- Conversion of Methane by Steam Reforming Using Dielectric-barrier Discharge*
- Permeability and Selectivity of Sulfur Dioxide and Carbon Dioxide in Supported Ionic Liquid Membranes*
- Hydroxyapatite Coatings on Titanium Prepared by Electrodeposition in a Modified Simulated Body Fluid*
- Molecular Dynamics Simulation of Effect of Salt on the Compromise of Hydrophilic and Hydrophobic Interactions in Sodium Dodecyl Sulfate Micelle Solutions*
