Conversion of Methane by Steam Reforming Using Dielectric-barrier Discharge*
2009-05-15ZhangXu张旭WangBaowei王保伟LiuYongwei刘永卫andXuGenhui许根慧
Zhang Xu (张旭), Wang Baowei (王保伟), Liu Yongwei (刘永卫) and Xu Genhui (许根慧)
Conversion of Methane by Steam Reforming Using Dielectric-barrier Discharge*
Zhang Xu (张旭), Wang Baowei (王保伟)**, Liu Yongwei (刘永卫) and Xu Genhui (许根慧)
Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering Technology, Tianjin University, Tianjin 300072, China
Conversion of methane by steam reforming was carried out by means of dielectric-barrier discharge. A systemic procedure was employed to determine the suitable experimental conditions. It was found that one of the plasma generators can match the system best. A higher power input can always bring a higher conversion, but the selectivity to C2H6decreased from 52.48% to 39.43% as the power increased from 20W to 49W. When discharge distance was 4 mm, selectivities to almost all main products reached the max. The inner electrode made of stainless steel and the outer electrode with aluminum foil were one of the best options which can obviously enhance the conversion of methane. A larger flow rate always resulted in a lower conversion of methane. In the most time, 19.93% steam promoted conversion of methane.
methane, steam, dielectric-barrier discharge, plasma
1 Introduction
Methane or natural gas is widely applied in industry to obtain hydrogen or synthesis gas as source materials for the production of raw chemicals [1-6]. Although the steam reforming process has been industrially used to produce synthesis gas from methane recently, there are still problems waiting for being solved [7]. Efforts had been devoted on the catalysts applied in the methane steam reforming [8-12]. Considering the advantages of plasma, many articles also emerged in the field of conversion of methane by using plasma [13-15]. Patiño. [7] firstly used radio frequency glow discharge to carry out the non-oxidative coupling of CH4/H2mixtures and studied the reforming of methane with CO2, O2, and steam plasma with the same system to produce higher hydrocarbons. Bo. [16] investigated the influence of feed gases proportion on the performance of gliding arc discharge plasma assisted methane reforming with carbon dioxide process, which can effectively convert the reagents into synthesis gas with C2H4and C2H2as the main hydrocarbon compounds. Li. [17] compared the performance of the non-thermal plasma generated by four different electric discharge techniques in the conversion of methane and found that ethane is the major C2product in the dielectric-barrier discharge (DBD) processes. Considerable studies had also been done by our team [18-21]. On the basis of these works, effects of more detail factors, including the match of reactor with plasma generators, discharge frequency, input power, discharge distance, different electrode and source gas were studied in this paper.
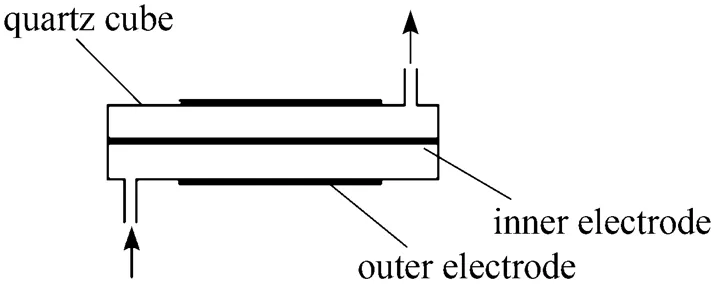
Figure 1 Schematic diagram of DBD reactor
2 Experimental
Figure 1 shows the plasma rector which consists of a quartz tube (with a outer diameter of 10mm and a inner diameter of 6 mm, if not mentioned specially), a metal stick (with a diameter of 3 mm, if not mentioned specially) inside it as the inner electrode and a metal foil or net (with a length of 90 mm) around it as the outer electrode. Fig. 2 shows the schematic diagram of the reaction and analysis system. Methane controlled by mass flow controllers (D07-7A/ZMM) was introduced and passed a bottle which was immersed in a water bath and filled with water to carry and mix with steam. The plasma was generated by a high-voltage generator. After reaction and condensation to remove water, the gases from the reactor were analyzed by gas chromatogram (FULI9790II) online. All experiments were carried out at room temperature and atmospheric pressure.
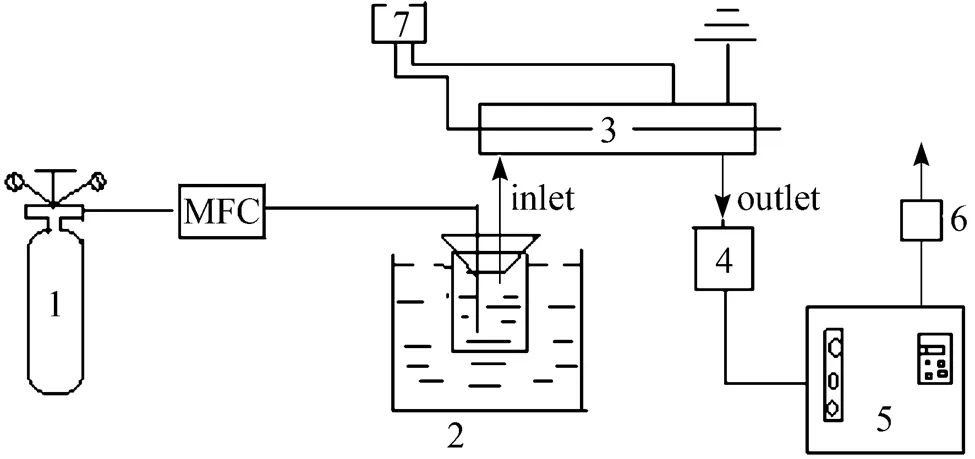
Figure 2 Flow chart of the experiment 1—CH4; 2—water bath; 3—reactor; 4—condensator; 5—gas chromatogram; 6—flowmeter; 7—plasma generator
Methane conversion and product selectivity are defined as follows:

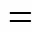
3 Results and Discussion
3.1 Effect of plasma generators
Four plasma generators were examined in the same conditions in the experiments. The parameters and the results are listed in Table 1 and Fig. 3, respectively. It is shown that when plasma generator 4 was used, not only the conversion of CH4but also the selectivities to H2and C2H4were highest. The difference of other selectivities was not very obvious and the yields reached the max for all the products. It was declared that plasma generator 4 can match to the reactor best. The reason is that the reactor of DBD is a capacitor in fact and the plasma generator is a circuitry, which includes adjustable capacitor and inductance. The reactor and the plasma generator can produce acceptor resonance or current resonance when the characteristic of the electrical apparatus of reactor matches with the plasma generator, resulting in the highest energy efficiency. At the same time, the appropriate discharge voltage and frequency are needed for conversion of methane and steam. The plasma generator 4 was chosen in the following works.
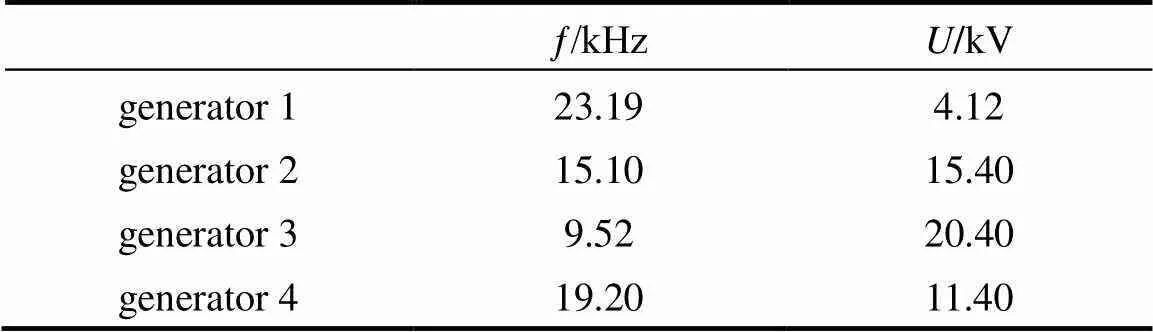
Table 1 Parameter of plasma generator
3.2 Effect of input power
The input power, which can influence the conversion and product distribution, was tested as one important factor. From Fig. 4 (a), it is shown that as the power increased from 20 W to 49 W, CH4conversion gradually increased when others conditions were constant. The density of free radicals, which is responsible to the reaction rate, increased with the power of plasma. The increasing power leads to an increase in the temperature and density of electrons. Thus, the activity of plasma is enhanced, which accelerates the breakage of the bond of CH4, and finally improves the conversion of CH4.
The distribution variation shown in Fig. 4 (b) suggests that as the input power increased, the selectivities to H2and C2H4rise from 31.12% to 41.29% and from 4.24% to 7.18%, respectively, but the selectivity to C2H6decreased from 52.62% to 41.29%. The selectivity to CO was less than 4% in all ranges. The change could be attributed to the increasing of the conversion. When more CH4was active in this process, more H∙ and CH3∙ were emerged to form more H2and abundant C2H6. However, the larger power also made it likely to transform CH3∙ to CH2∙ and H∙, which further led to the change of the selectivities to C2H4and H2. After calculation, it is found that a larger power brought a higher yield for every product.
3.3 Effect of discharge distance
In order to investigate the effect of discharge distance, the experiments were conducted by changing the diameters of inner electrodes and quartz tubes. The diameters, thickness of tube and discharge distances are shown in Table 2.

Table 2 Diameters of electrodes and discharge distance
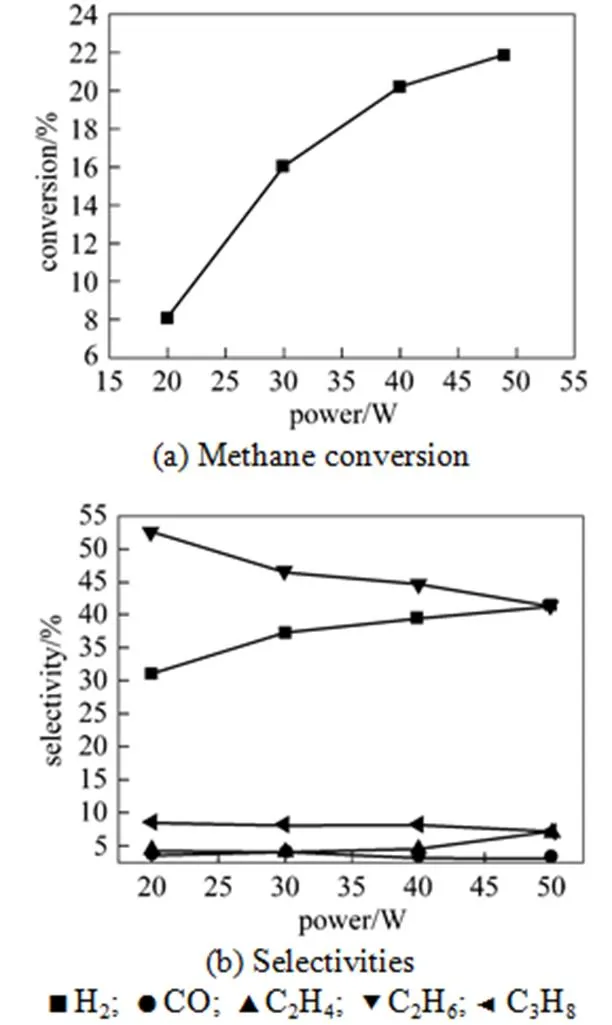
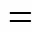
Figure 5 suggests that the conversion of CH4was reduced from 16.42% to 7.13% by increasing the diameter of the reactor, which directly decreased the average energy carried by elections and the intensity of electric field in the discharge area. All selectivities came through the process of increasing and then decreasing and most of them reached the max when the distance was 4 mm. But as the range of conversion was more obvious, the trends of the yields were as the same as the conversion.
3.4 Effect of different electrode
Inner electrodes made of red copper, brass, aluminum and stainless steel were examined with the outer and inner diameters of quartz tube changed to 12 mm and 10 mm, respectively. The results are shown in Table 3. The conversion of CH4varied with the material of electrodes in the following order: stainless steel>red copper>brass>aluminum. Also, the lowest selectivity to H2and highest selectivity to C2H6were obtained when aluminum inner electrode was used and the variety of selectivities was reverse for red copper inner electrode. The differences may be attributed to the catalytic effect of the different metals. In the reaction, the temperature of electrode directly contacted with CH4and steam could reach 200°C and play an important role beside of transferring electron.
Table 3 Effect of inner electrode on methane conversion and the selectivities of the products (P40 W, steam concentration19.93%, 40 ml·min-1)
Two kinds of outer electrodes,.., aluminum foil and iron net were examined in the experiment. The results are listed in Table 4. It clearly shows that the former one was better in the activity and selectivity to H2but worse in the selectivities to C2H6and C3H8. Because both of these electrodes did not contact with the gases, there was no catalytic influence. Perhaps compared with the iron net with a lot of meshes, the larger area helped the foil to make a uniform and stable electric field, which led to the higher conversion. However, the net is likely more propitious to focus the energy and form longer hydrocarbons.
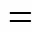
Table 4 Effect of outer electrode on methane conversion and the selectivities of products (P30 W, 40 ml·min-1)
3.5 Effect of flow rate and steam proportion
The flow rate and steam proportion were also studied. The influence of steam proportion was not well-regulated. As a whole, when the proportion was 19.93%, the highest conversion was obtained. Fig. 6 shows that the conversion of CH4decreased with the flow rate. It can be explained by the fact that the increase in flow rate leads to the decrease of the residence time. As the power is constant, the number of high-energy electron remains in a stable level, which leads to the average energy of CH4receives declines and results in less chance for the molecule to be excited and the reduction of the conversion.

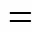
The influences of the two factors in the selectivities were listed in Tables 5 (a) and (b) which took the situations of steam proportion were 0 and 19.93% as examples. It is not surprising that when steam was not introduced into the reaction system, there was no CO formation because of absence of O element. The results shown in Table 4 indicate that the variation of selectivities to C2H4and C2H6was as same as discussed above.
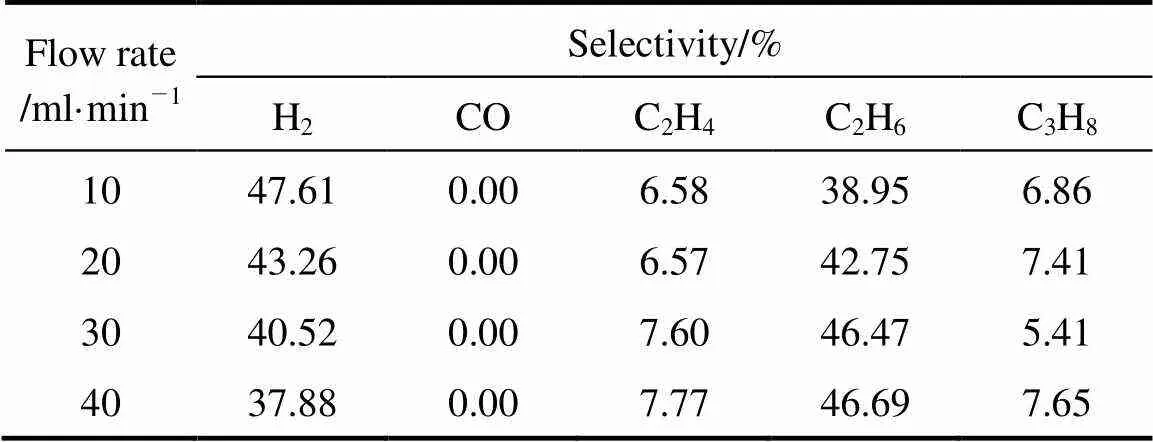
Table 5 (a) Effect of flow rate on product selectivities (pure CH4)
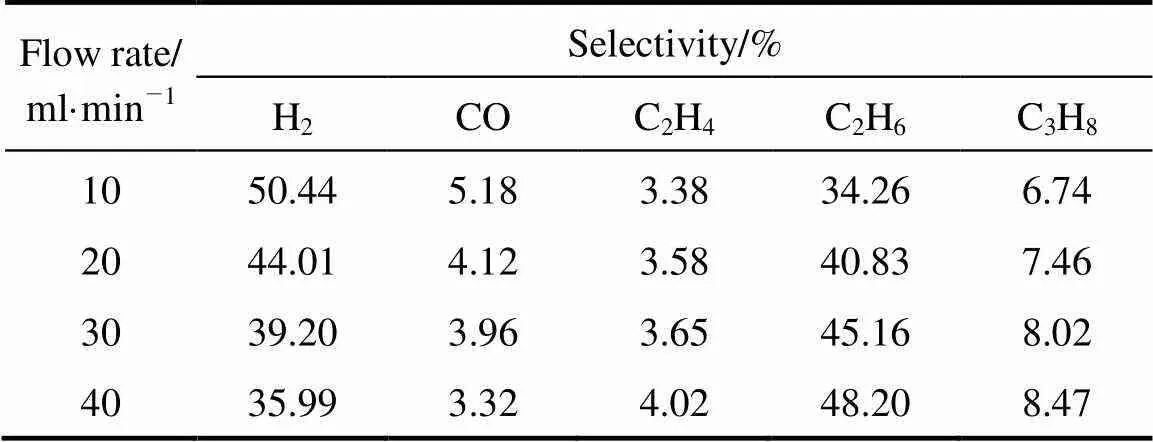
Table 5 (b) Effect of flow rate on product selectivities (with 19.93% steam concentration)
4 Conclusions
Conversion of methane by steam reforming was accomplished by means of DBD and the effects of several factors were discussed. By using plasma generator which can match the reactor, higher yields could be obtained. Higher conversion of methane could be obtained by using higher input power and smaller discharge distance. The electrodes made of stainless steel and aluminum foil with 19.93% steam proportion were favorable for the reaction.
1 Jasiński, M., Dors, M., Mizeraczyk, J., “Production of hydrogenmethane reforming using atmospheric pressure microwave plasma”,., 181 (1), 41-45 (2008)
2 Dong, X.F., Zhang, H., Lin, W. M., “Preparation and characterization of a perovskite-type mixed conducting SrFe0.6Cu0.3Ti0.1O3-δmembrane for partial oxidation of methane to syngas”,...., 16 (3), 411-415 (2008).
3 Wei, W.S., Xu, J., Fang, D.W., Bao, X.J., “Catalytic partial oxidation of methane with air to syngas in a pilot-plant-scale spouted bed reactor”,...., 11 (6), 643-648 (2003).
4 Xu, J., Wei, W.S., Bao, X.J., “Thermodynamic study on the catalytic partial oxidation of methane to syngas”,...., 10 (1), 56-62 (2002).
5 Quincoses, C.E., Gonzalez, M.G., “Kinetic study on CO2reforming of methane”,...., 9 (2), 190-195 (2001).
6 Wu, S.F., Beum, T.H., Yang, J.I., Kim, J.N., “The characteristics of a sorption-enhanced steam-methane reaction for the production of hydrogen using CO2sorbent”,...., 13 (1), 43-47 (2005).
7 Patiño, P., Pérez, Y., Caetano, M., “Coupling and reforming of methane by means of low pressure radio-frequency plasma”,, 84 (16), 2008-2014 (2005).
8 Xu, J.H., Yeung, C.M.Y., Ni, J., Meunier, F., Acerbi, N., Fowles, M., Tsang, S.C., “Methane steam reforming for hydrogen production using low water-ratios without carbon formation over ceria coated Ni catalysts”,.., 345 (2), 119-127 (2008).
9 Profeti, L.P.R., Ticianelli, E.A., Assaf, E.M., “Co/Al2O3catalysts promoted with noble metals for production of hydrogen by methane steam reforming”,, 87 (10/11), 2076-2081 (2008).
10 Ma, Y., Xu, Y., Demura, M., “Catalytic stability of Ni3Al powder for methane steam reforming”,.., 80 (1/2), 15-23 (2008).
11 Maluf, S.S., Assaf, E.M., “Ni catalysts with Mo promoter for methane steam reforming”,, 88 (9), 1547-1553 (2009).
12 Yoshida, K., Begum, N., Ito, S., Tomishige, K., “Oxidative steam reforming of methane over Ni/α-Al2O3modified with trace noble metals”,.., 358 (2), 186-192 (2009).
13 Nozaki, T., Hattori, A., Okazaki, K., “Partial oxidation of methane using a microscale non-equilibrium plasma reactor”,., 98 (4), 607-616 (2004).
14 He, J.X., Han, Y.Y., Gao, A.H., Zhou Y.S., Lu Z.G., “Investigation on methane decomposition and the formation of C2hydrocarbons in DC discharge plasma by emission spectroscopy”,...., 12 (1), 149-151 (2004).
15 Wang, Y., Liu, C.J., Zhang, Y.P., “Plasma methane conversion in the presence of dimethyl ether using dielectric-barrier discharge”,, 19 (3), 877-881 (2005).
16 Bo, Z., Yan, J.H., Li, X.D., Chi, Y., Cen, K.F., “Plasma assisted dry methane reforming using gliding arc gas discharge: Effect of feed gases proportion”,.., 33 (20), 5545-5553 (2008).
17 Li, X.S., Zhu, A.M., Wang, K.J., Xu, Y., Song, Z.M., “Methane conversion to C2hydrocarbons and hydrogen in atmospheric non-thermal plasma generated by different electric discharge techniques”,., 98 (4), 617-624 (2004).
18 Wang, B.W., Yang, E.C., Xu, G.H., Hao, J.K., “Theoretical study of reaction paths and transition states on conversion methane into C2hydrocarbons through plasma”,...., 15 (1), 44-50 (2007).
19 Wang, B.W., Xu, G.H., Sun, H.W., “Distribution of electrical field energy for conversion of methane to C2hydrocarbonsdissymmetrical electric field enhanced plasma”,, 15 (2), 115-121 (2006).
20 Wang, B.W., Yang, K.H., Xu, G.H., “Effect of cooling methods on methane conversiondielectric-barrier discharges”,, 10 (5), 575-580 (2008).
21 Wang, B.W., Zhang, X., Liu, Y.W., Xu, G.H., “Conversion of CH4, steam and O2to syngas and hydrocarbonsdielectric barrier discharge”,, 18 (1), 94-97 (2009).
2009-01-12,
2009-06-26.
the National Natural Science Foundation of China (20606023, 20490203).
** To whom correspondence should be addressed. E-mail: wangbw@tju.edu.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Removal of Uranium (VI) by Fixed Bed Ion-exchange Column Using Natural Zeolite Coated with Manganese Oxide*
- Phase Equilibrium of Isobutanol in Supercritical CO2
- Permeability and Selectivity of Sulfur Dioxide and Carbon Dioxide in Supported Ionic Liquid Membranes*
- Hydroxyapatite Coatings on Titanium Prepared by Electrodeposition in a Modified Simulated Body Fluid*
- Model Study on a Submerged Catalysis/Membrane Filtration System for Phenol Hydroxylation Catalyzed by TS-1*
- Molecular Dynamics Simulation of Effect of Salt on the Compromise of Hydrophilic and Hydrophobic Interactions in Sodium Dodecyl Sulfate Micelle Solutions*
