Permeability and Selectivity of Sulfur Dioxide and Carbon Dioxide in Supported Ionic Liquid Membranes*
2009-05-15JIANGYingying江滢滢WUYouting吴有庭WANGWenting王文婷LILei李磊ZHOUZheng周政andZHANGZhibing张志炳
JIANG Yingying (江滢滢), WU Youting (吴有庭), WANG Wenting (王文婷), LI Lei (李磊), ZHOU Zheng (周政) and ZHANG Zhibing (张志炳)
Permeability and Selectivity of Sulfur Dioxide and Carbon Dioxide in Supported Ionic Liquid Membranes*
JIANG Yingying (江滢滢), WU Youting (吴有庭)**, WANG Wenting (王文婷), LI Lei (李磊), ZHOU Zheng (周政) and ZHANG Zhibing (张志炳)
Key Laboratory of Mesoscopic Chemistry of MOE, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, China
Permeabilities and selectivities of gases such as carbon dioxide (CO2), sulfur dioxide (SO2), nitrogen (N2) and methane (CH4) in six imidazolium-based ionic liquids ([emim][BF4], [bmim][BF4], [bmim][PF6], [hmim][BF4], [bmim][Tf2N] and [emim][CF3SO3]) supported on polyethersulfone microfiltration membranes are investigated in a single gas feed system using nitrogen as the environment and reference component at temperature from 25 to 45ºC and pressure of N2from 100 to 400 kPa. It is found that SO2has the highest permeability in the tested supported ionic liquid membranes, being an order of magnitude higher than that of CO2, and about 2 to 3 orders of magnitude larger than those of N2and CH4. The observed selectivity of SO2over the two ordinary gas components is also striking. It is shown experimentally that the dissolution and transport of gas components in the supported ionic liquid membranes, as well as the nature of ionic liquids play important roles in the gas permeation. A nonlinear increase of permeation rate with temperature and operation pressure is also observed for all sample gases. By considering the factors that influence the permeabilities and selectivities of CO2and SO2, it is expected to develop an optimal supported ionic liquid membrane technology for the isolation of acidic gases in the near future.
permeation, gas separation, ionic liquid, supported ionic liquid membrane, acidic gas
1 INTRODUCTION
The selective separation of gases using supported liquid membranes (SLMs) is recognized as one of the most promising membrane-based processes [1-3]. In an SLM system, a defined solvent or solvent/carrier solution is immobilized inside the porous structure of a polymeric or ceramic membrane, which separates the feed phase, in which the gases of interest are loaded, from the receiving phase, in which the gas components will be transferred. This configuration has attracted a great deal of interest because it combines the processes of extraction and stripping and the amount of solvent required is much less than that in the conventional solvent extraction process. In addition, higher gas selectivity in SLMs is also obtained compared with conventional polymeric membranes [4, 5]. However, industrial applications of SLMs are still scarce, mainly due to the concern with SLM stability and long-term performance, leading to a reduction of gas permeance and membrane selectivity. These effects are attributed to the loss of solvent from the supporting membrane, either by evaporation or dissolution/dispersion into the adjacent phases [6].
Ionic liquids (ILs) are thermally stable liquid salts at room temperature, constituted by an organic cation and either an organic or an inorganic anion. Unlike traditional organic solvents, chemical modification of alkyl groups in the cation or the anion can produce task-specific solvents,.., ILs with an amine cation for acidic gas separations [7, 8]. The use of ILs as immobilized phases in porous supports produces supported ionic liquid membranes (SILMs), which is particularly interesting owing to the nonvolatile under most conditions [9] and insoluble nature of ILs, which leads to stable SLMs without any observable loss of the ILs to the atmosphere or the contacting gas phases. Higher stability of SILMs over conventional SLMs is also attained because of the greater capillary force associated with the high viscosity of ionic liquids, which could reduce the displacement of the liquids from the micron pores under pressure. In addition, as the supported materials in the membrane, the ionic liquid with different chemical and physical properties may make the permeance of gas components into the receiving phase substantially different.
The research has started only in recent several years to explore SILMs for gas isolation and separation. Scovazzo. [10] reported CO2permeability of (263-750)×10-10cm2·s-1·kPa-1(350-1000 barrers) in ionic liquids composed of common cation [emim]+and different water stable anions [Tf2N]-, [CF3SO3]-, and [dca]-. They obtained CO2/N2selectivity ranging from 15 to as high as 61, over the upper bound of a CO2/N2Roberson plot of representative polymers. Quinn. [11] worked with hydrated ammonium molten salts and found that the properties of molten salt membrane depended on the feed gas humidity. For the gas pair CO2/CH4, they reported a selectivity of 120, with the CO2permeability versus pressure also exhibiting a facilitated transport relationship. Gan. [12] investigated a new SILM supported on nanofiltration membrane and reported its good stability at operating pressures relevant to industrial application. Morgan. [13] studied the gas diffusivity and solubility using a lag-time technique that involved analysis of both the transient and steady-state permeation regimes through a supported liquid film. We examined the separation of SO2using the SILMs [14] and reported that the SO2permeability was even more promising, being an order of magnitude larger than that of CO2. However, despite the fact that the several pioneering papers of using SILMs mentioned above are significant for acidic gas separation, it is still necessary to explore the factors that influence the permeability and selectivity of acidic gases in the SILM processes.
Continuing to our previous work [14], this investigation seeks to develop a comprehensive understanding of the factors that affect the acidic gas separation and transport in the SILMs. Experimentally, a single gas feed system operating under constant pressure of gases is used to avoid the displacement of ionic liquids from the porous structure. Six ILs are selected from the combination of three cations [emim]+, [bmim]+and [hmim]+with four anions [BF4]-, [PF6]-, [CF3SO3]-and [Tf2N]-and used as membrane materials for acidic gas separations, especially for carbon dioxide and sulfur dioxide from nitrogen and methane. The fluxes and ideal selectivities for the gas pairs CO2/N2, CO2/CH4, SO2/N2, SO2/CH4and SO2/CO2are investigated, with emphasis on the exploration of influencing factors in the permeation of acidic gases.
2 EXPERIMENTAL
2.1 Materials
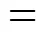
The support for the ILs is hydrophilic polyethersulfone (PES). The PES in contact with the ILs did not show physical change with time, such as swelling or warping. The PES membrane was purchased from Beijing Membrane Corporation with the following specifications: 0.22 μm pore size, 80% porosity, 150 μm thickness and 64 cm2cross-sectional area. SILMs were formed by soaking the PES-membrane into a selected ionic liquid overnight in a vaccum oven at 40°C to ensure that the membrane was saturated with the ionic liquid. The excessive ionic liquid on the membrane surface was carefully removed with soft tissue prior to the membrane installation into the stainless steel membrane filtration cell. The SILMs as formed above were exposed to cross-membrane pressures of 10 to 50 kPa, justifying the stability of the membrane. It is also experimentally examined that the SILMs can withstand a cross-membrane pressure up to 250 kPa, being close to the theoretical calculation based on a membrane pore size of 0.22 μm and the [bmim][Tf2N] surface tension of 37.5 mN·m-1. In addition, before the permeation experiments of gases, the SILMs were tested for leakage using nitrogen.
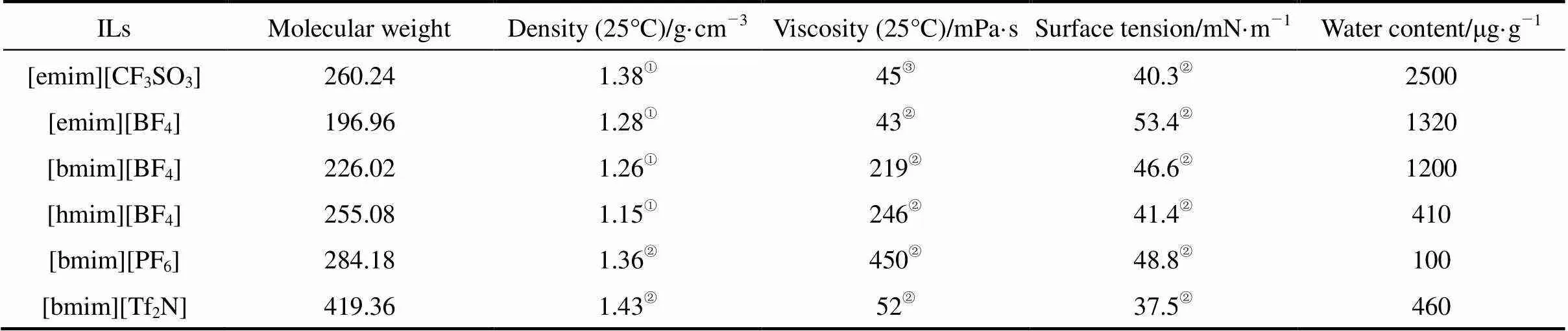
Table 1 Characteristic properties of ionic liquids used in this study
① -③from Refs. [19], [20], and [10], respectively.
2.2 Apparatus and procedure
The permeability experiments were performed using a stainless steel dual-chamber cell. The apparatus in this study has the same structure as that in Ref. [21]. The entire unit of the cell was thermostated in water bath to ±0.1°C. In the test cell, the membrane was a barrier between two sealed chambers containing thermodynamically equilibrated ammount of nitrogen (.. at the same pressure and temperature). The upper (feed) and lower (permeate) chambers had volumes of 245 and 215 cm3, respectively, as determined from the pressure of a known amount of gas in the container at a fixed temperature. The solute gas, CO2or SO2, was charged from a gas cylinder to the cell upstream side to a pre-set pressure. This loading produced a partial pressure difference in the solute gas across the membrane since the partial pressures (usually 100 to 400 kPa) of N2on both sides of the membrane were equal. The measurement of the solute gas flux across the membrane came indirectly from the measured total pressure that increased with time in the downstream side. The pressure downstream as well as the pressure change upstream was monitored respectively by two absolute pressure transducers (Omega PX811005AV) with an accuracy of ±0.1 kPa and a scale of 600 kPa. A computer data acquisition system was used to record the pressure from the sensors at an elapsed time interval (usually 1 min). The pressure curves exhibited a trend of linear increase with time when the ammount of permeate was less than 10% of the feed. Additionally, the initial slope of the measured total pressure over 20-30 min for SO2, after a lag time of about 3 min, was used to calculate the flux rate of SO2under the test conditions. In the case of CO2, the pressure over 30 min after a lag time of about 5 min was used to calculate the slope. To measure the flux rate of N2, the procedure was the same except that a known ammount of N2was loaded in the feed chamber as the solute gas. Due to the lower N2flux, the initial slope was taken over approximately 720 min. The same procedure as that for N2was followed to measure the CH4flux, except that the initial slope was taken over approximately 300 min. All the permeability data were calculated on the basis of the cross-sectional area and the thickness of the primitive PES membrane.
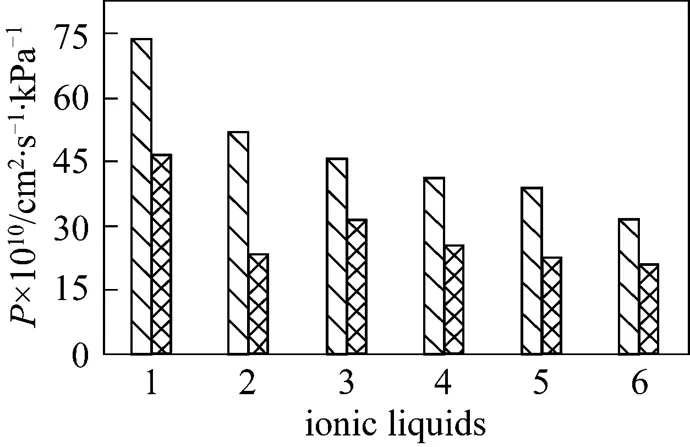

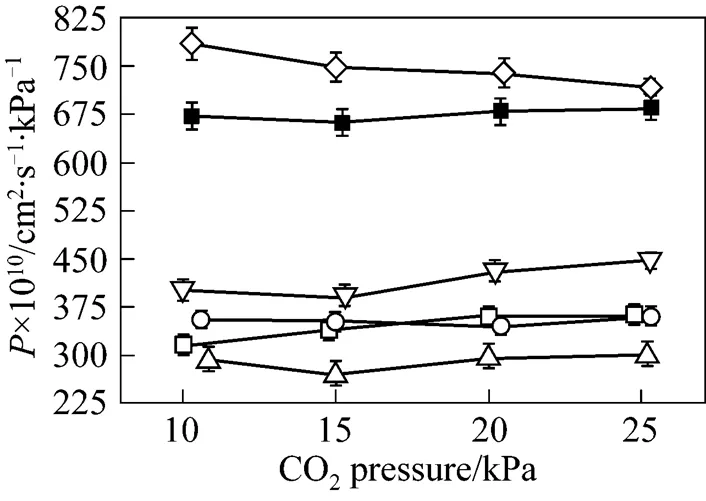
Figure 2 Permeability of CO2in the SILMs at 25°C as a function of transmembrane pressure difference■ [emim][CF3SO3]; □ [emim][BF4];◇ [bmim][Tf2N];○ [bmim][BF4]; ▽ [hmim][BF4];△ [bmim][PF6]
Figure 3 Permeability of SO2in the SILMs at 25°C as a function of transmembrane pressure difference ◄ [emim][CF3SO3];■ [emim][BF4];◆ [bmim][Tf2N];● [bmim][BF4];▼ [hmim][BF4];▲ [bmim][PF6]
3 RESULTS AND DISCUSSION
3.1 Effects of IL species on the permeability and selectivity
The permeabilities of gases at 25°C for each of the six SILMs are shown in Figs. 1-3, respectively. At 25°C, long time are required to obtain the permeabilities of N2and CH4. Since the permeation fluxes of N2and CH4are small, relative large experimental uncertainties (8%) are found, compared with the data (less than 3%) of SO2and CO2. The permeation experiments of N2and CH4are performed under several transmembrane pressure differences,.., 10, 20, and 40 kPa. No apparent change in the permeability is found at these transmembrane pressure differences, and only the data under 20 kPa transmembrane pressure are shown in Fig. 1. It is also illustrated in Fig. 2 that the CO2permeability of the six SILMs changes little with the transmembrane pressure difference. However, exception occurs in the SO2permeation, in which the permeability increases with the transmembrane pressure difference within the experimental range. This may be primarily due to the sensitive and non-linear increase of SO2transport in the ILs at elevated pressure. The fact that the permeability of SO2is over an order of magnitude larger than that of CO2as from the comparison between Fig. 2 and Fig. 3 shows a potential of SO2separation using SILMs. Similarly, the permeations of ordinary gases (N2and CH4) are over one to two orders of magnitude less than those of acidic gases (see Figs. 1-3), which means the easily realizable isolation of acidic gases.
The permeability (P) of a single gas on these dense films can be explained on the basis of the solution-diffusion mechanism, which is expressed as the product of solubility and diffusivity [15],

wherePis in cm2·s-1·kPa-1,Sis the solubility coefficient of gas in ionic liquid in cm3·cm-3·kPa-1that can be calculated from the Henry’s constant (see Table 2), andDis the diffusivity in cm2·s-1of gas moleculein the ionic liquid. The ideal diffusion coefficients can be determined for [Cmim][X] ionic liquids using a “semi-infinite volume” approach [13],

where2is the viscosity of ionic liquid in mPa·s and1is the solute molar volume in cm3·mol-1. Eqs. (1) and (2) can be applied to evaluate permeabilities of different gases in different ionic liquids from the data of solubility and viscosity. As shown in Table 3, the calculated permeabilities of N2and CH4in [emim][CF3SO3] and [bmim][BF4] are smaller than the experimental values, which may be due to the relative large experimental uncertainties both in the permeabilities of this study and in the Herny’s constants of N2and CH4reported [23]. However, the calculated SO2permeabilities are found to be 2 to 6 times larger than the experimental data (see Table 3), which can not be explained from the experimental uncertainty and the tortuosity (usually around 1.1-1.5) of the membrane support. It may be due to the difference between the saturated solubility, measured under equilibrium conditions or static conditions, and the dynamic solubility of the solute gas across the SILM. Since SO2is more soluble in the ILs than CO2, as known from the Henry’s constants in Table 2, the slow dynamic permeation of the solute gases causes a smaller actual solubility coefficient than the value under equilibium condition. The use of saturated solubility for the prediction is therefore questionable, especially for SO2, which has extremely large solubilities in the ILs. The higher the saturated solubility, the larger the difference between the actual and predicted permeabilities of gases in the SILMs. The accurately predicted permeability of CO2and the greatly overestimated permeability of SO2are in good agreement with such a conclusion.

Table 2 Henry’s constants (100 kPa) of gases in the six ionic liquids at 25°C
①With the same water contents as in Table 1.
②Calculated from the experimental solubility data measured in this study using the standard procedure [14].
③ —⑦ from Refs. [14], [10], [22], [15], and [23], respectively.
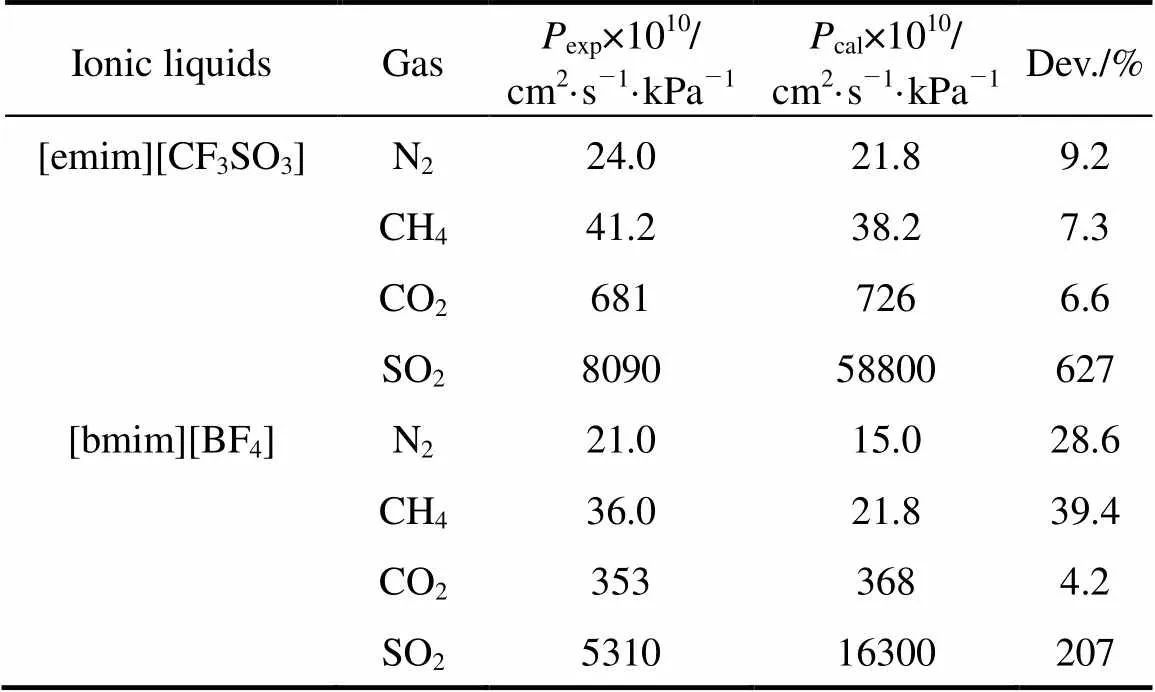
Table 3 Comparison of calculated gas permeabilities (corrected with porosity of support) with experimental data for the SILMs under 10 kPa transmembranepressure difference at 25°C
The comparison of calculations with experiments also reveals that there are different limiting steps for the permeation of different gases. Due to the easy dissolution of SO2in ILs, SO2permeation appears to be merely dependent on the diffusion in ILs. This is why the relative SO2permeabilities between the SILMs tested appear to be merely related to the viscosities of the ILs, with the tendency from the highest to the lowest SO2permeability, [emim][CF3SO3]> [emim][BF4]>[bmim][Tf2N]>[bmim][BF4]>[hmim][BF4]> [bmim][PF6], which is similar to the trend of the lowest to the highest viscosity, [emim][CF3SO3]≈ [emim][BF4]<[bmim][Tf2N]<[bmim][BF4]<[hmim][BF4]< [bmim][PF6]. As for the permeation of CO2, both dissolution and diffusion have an impact on the CO2separation and the dissolution is the dominant step. For example, the three SILMs based on [BF4]-anion exhibit similar CO2permeabilities (see Fig. 2), though the viscosities of three ILs are much different from each other (see Table 1), whereas an apparent difference in CO2permeability between the [bmim][BF4] and [bmim][PF6] membranes is found in spite of the similarity of CO2solubility in these two ILs. This is primarily due to the limitation of CO2dissolution in these [BF4]--based ILs (see the Henry’s constants in Table 2) for the former case, and due to the dominant influence of diffusion on the permeation for the latter case. Bearing in mind the limiting steps of gas transport as analyzed above, it is easy to understand how and to what extent the different permeations of different gases are in the SILMs.
In fact, the permeation of gases in ILs has to be ascribed to the nature of ILs, which is typically composed of cations and anions. Keeping the anion the same ([BF4]-), it can be seen from Figs. 1-3 that the change in the permeabilities of ordinary gases and acidic gases by increasing the alkyl chain length of cation is minuscule, which may be attributed to the little different solubilities of gases in ILs by changing the cation from [emim]+to [hmim]+. The effect of anion with different character on the permeability can be also seen from Figs. 1-3, by paying attention to the ILs of the same cation [bmim]+. It is found that [Tf2N]-improves dramatically the permeations of two ordinary gases and CO2. In addition, [CF3SO3]-shows a positive effect on the permeability of SO2, whereas [PF6]-apparently reduces the gas permeabilities and [BF4]-has a neutral effect on the permeation. Similar trend of gas permeability in SILMs was also observed by Scovazzo. [10] and Morgan. [13]. They found that the SILMs with [Tf2N]-anion had higher CO2permeability compared to those with other anions and the permeabilities decreased, in the orders of [Tf2N]->[CF3SO3]->[dca]->[Cl]-[10] and [Tf2N]-> [PF6]-[13]. Particularly, it is impressive to note the permeation difference of acidic gases between [emim][CF3SO3] and [emim][BF4] supported membranes. Both ILs have the same cation, similar viscosities (see Table 1) and CO2or SO2solubility (see the Henry’s constant in Table 2). Both the permeabilities of CO2and SO2in [emim][CF3SO3] are found to be much greater than those in [emim][BF4] (see Figs. 2 and 3), which is in contradiction to the analysis based on the solution-diffusion theory and the limiting steps. As a possible explanation, the preferred permeation of acidic gases in [emim][CF3SO3] may be attributed to the nature of [CF3SO3]-anion, which enables the strong absorption of water vapor. In fact, the water content in [emim][CF3SO3] are the highest among the six ILs investigated (see Table 1), not to mention the fact that further water absorption occurs during the membrane installation. The presence of water in the ILs very probably leads to the facilitated transport of acidic gases in the SILMs, as evidence from the work by Scovazzo. [10]. Therefore, as shown in the paper, the regulation of acidic gas separation and transport can be easily realized by selecting the IL with different character for the SILMs and modifying the IL with groups of facilitated transport such as water and amine.
To effectively separate CO2from the ordinary gases, the first choice of ILs should be [emim][CF3SO3], which has the highest ideal selectivities for CO2/N2and CO2/CH4pairs among six SILMs, as evidenced from Table 4. The CO2/N2ideal selectivity in [emim][CF3SO3] membrane is up to 28, which is close to 35 obtained by Scovazzo. [10]. However, there is no apparent difference for the other five SILMs to separate CO2from the ordinary gases, which can be manifested from their similarity in CO2/N2or CO2/CH4ideal selectivities. For instance, from [emim][BF4] to [bmim][Tf2N], the CO2/N2ideal selectivity ranges from 13 ([hmim][BF4]) to 17 ([bmim][Tf2N]), with an increase of about 31%. In the same way, there exists only a 57% difference in CO2/CH4ideal selectivity among [emim][BF4], [bmim][BF4], [bmim][PF6], [hmim][BF4] and [bmim][Tf2N], with [emim][BF4] having the lowest value (down to 7). The investigations above shows that the SILMs with facilitated transport can apparently improve the CO2separation from ordinary gases. It also implies that the ideal selectivities of CO2/N2and CO2/CH4can hardly be enhanced greatly by only changing cations or anions in the ILs.

Table 4 also shows the ideal gas selectivities for SO2/N2, SO2/CH4 and SO2/CO2. As the cation changing from [emim]+ to [bmim]+ and [hmim]+, for the ILs with the same anion [BF4]-, the ideal selectivities of SO2/N2 and SO2/CO2 decrease from approximately 246 and 19 for [emim][BF4] to 159 and 13 for [hmim][BF4]. The relative change in SO2/CH4 ideal selectivities by increasing the alkyl chain length of the cation is also found to be miniscule and irregular, for example, the SO2/CH4 ideal selectivity in [bmim][BF4] is 136, only a little higher than those in [emim][BF4] and [hmim][BF4]. However, the change in the anion is significant in separating SO2 from N2, CH4 or CO2. As the anion switched from [BF4]- to [TF2N]- and [PF6]- for the ILs with a common cation [bmim]+, the ideal selectivities of SO2/N2, SO2/CH4 and SO2/CO2 gradually decrease in the order of [BF4]->[PF6]->[Tf2N]-. It is clearly illustrated that the anion [BF4]-is highly effective for the selective separation of SO2 from ordinary gases and CO2.
In addition, for the same reason as presented in the discussion of influencing factors of anion on the permeability, [CF3SO3]-is also shown to enable good ideal selectivity for the SO2separation from the ordinary gases. In fact, the [emim][CF3SO3] supported membrane shows both excellent permeability and ideal selectivities of SO2over ordinary gases, which makes itself a potential application for the SO2separation in the near future. Thus, it is a meaningful and challenging study to understand how the characteristics of IL affect acidic gas separation and transport in SILMs, which is helpful for the design of “perfect” SILM,.., the SILM that possesses both high gas permeability and gas selectivity.
3.2 Effects of operation temperature and pressure on the gas permeability and selectivity
As the operation temperature and pressure are important parameters in the gas separation using ILs [13, 16], it is of great interest to further investigate experimentally the gas permeation at different operation pressure and temperature. The influences of temperature on the gas transport across [emim][CF3SO3] membrane are shown in Figs. 4 and 5. The temperature shows a postive effect on all gases permeabilities. The permeation rates of gases increase more rapidly when the temperature changes from 35 to 45°C than from 25 to 35°C. The permeability curves of N2and CH4in Fig. 5 exhibit a nonlinear relation with temperature, indicating that the gas permeability is a power function of the temperature (the power is approximately 2.5 for N2and 2.0 for CH4). Since only three temperatures are examined, the experimental uncertainty in the power is relatively large. Despite this, it does appear that the gas permeability is essentially sensitive to temperature. However, the selectivity of gas pairs undergoes on a contrary trend. Almost all the ideal selectivities of gas pairs, as shown in Fig. 6, decrease with increasing temperature, a reverse performance over the permeability. For example, the ideal selectivity of CO2/N2in [emim][CF3SO3] membrane decreases from 27 to 16, and that of SO2/CH4changes from 208 to 151, whereas the SO2/CO2selectivity keeps unchanged. It was found that the trend of the ideal selectivity. temperature was similar to that of the gas solubility selectivity in ILs. temperature [16]. In fact, since both the solubility and diffusivity of gases in the ILs are a function of temperature, the selectivity of gas pairs, being a product of the solubility selectivity and diffusivity selectivity according to the solution-diffusion theory, depends strongly and complicatedly on the temperature. Therefore, both the experimental investigation and theoretical development for the influence of temperature on the ideal selectivity are significant to the membrane-based gas separations.
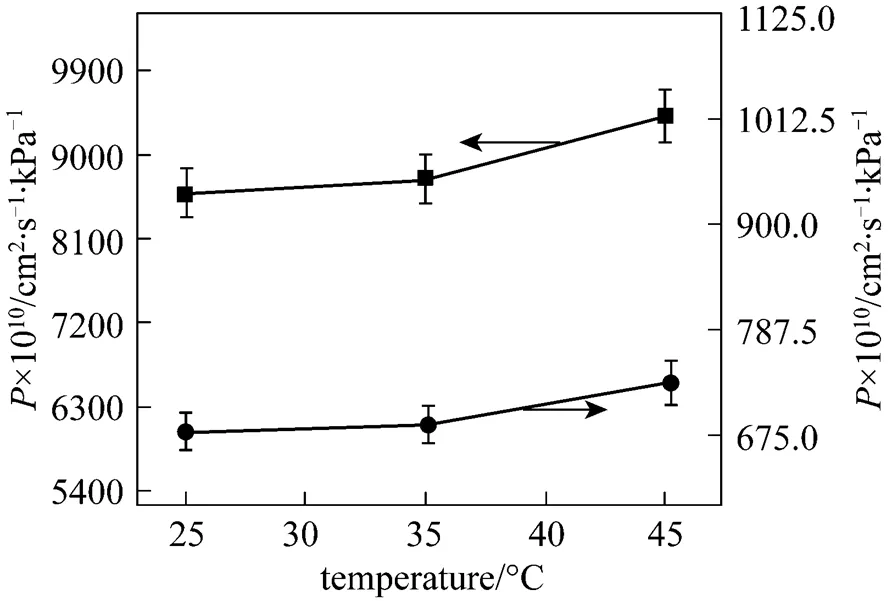
Figure 4 Permeabilities of SO2and CO2in [emim][CF3SO3] membrane under 20 kPa transmembrane pressure difference as a function of temperature■ SO2;● CO2
Figure 5 Permeabilities of N2and CH4in [emim][CF3SO3] membrane under 20 kPa transmembrane pressure difference as a function of temperature■ N2;● CH4

Figure 6 Ideal selectivities of gas pairs in [emim][CF3SO3] membrane under 20 kPa transmembrane pressure difference as a function of temperature left axis:▼ SO2/N2; ◄ SO2/CH4right axis: ◆ SO2/CO2;● CO2/N2;▲ CO2/CH4
As pointed out in the previous section, the transmembrane pressure difference has a positive effect on the SO2permeation and no effect on the permeability of other three gases. However, the driving force across the membrane is the transmembrane pressure difference, rather than the operation pressure. The operation pressure is referred to the thermodynamically equilibrated pressure of N2resident both in the upstream and downstream during the experiments. As shown in Figs. 7 and 8, when the pressure of thermodynamically equilibrated nitrogen is increased from 100 to 400 kPa in [bmim][Tf2N] membrane at ambient temperature (25°C), the permeability of CO2is enhanced by 273.75×10-10cm2·s-1·kPa-1, whereas the SO2permeation rate increases by 482.25×10-10cm2·s-1·kPa-1, measured at 10 kPa of gases driving force. The same trend as that of CO2and SO2is observed for the case of N2and CH4(see Fig. 8). It is evident that the operation pressure does have an impact on the gas permeation. However, as shown in Fig. 9, the gas ideal selectivities of gas pairs only have a weak dependence on the equilibrate gas pressure, which implies the feasibility of realizing high permeability and keeping the selectivitythe regulation of the operation pressure. Because of the limitation of SO2saturation pressure (344 kPa at 25°C), it is difficult to further explore the effect of higher N2pressure on the SO2permeability and selectivity. However, the data above provide the insight of the influence of operation pressure on the gas separation. Therefore, it may be expected that a careful selection for the combination of operation temperature and pressure can enhance the isolation and recovery of CO2and SO2from the industrial gas stream in the SILM processes.
It is shown in this paper that the SILM separation of SO2and CO2from two ordinary gases is influenced by many factors, such as the transmembrane pressure difference, nature of IL species, operation temperature and pressure. The exploration of these influencing factors is helpful to optimize the membrane separation processes. For example, to separate 0.2% (by volume) SO2from a flue gas stream at a pressure of 100 kPa, [emim][CF3SO3] can be selected as the ionic liquid for the SILM process. Before the flue gas stream entering into the SILM, it is better to pressurize the stream to 300 kPa to provide a high transmembrane pressure difference and operation pressure for high SO2permeation, as well as to humidify and cool the stream using water to 25°C to provide a low operation temperature for high SO2/N2selectivity. Since the parameter range explored in this paper is limited, the example above represents only a preliminary optimization. However, it does illustrate the significance of this work. As for the optimization for CO2separation, since all the six ILs in this study show a CO2selectivity not high enough, more efforts are required to find a suitable ionic liquid species that can realize the purpose.
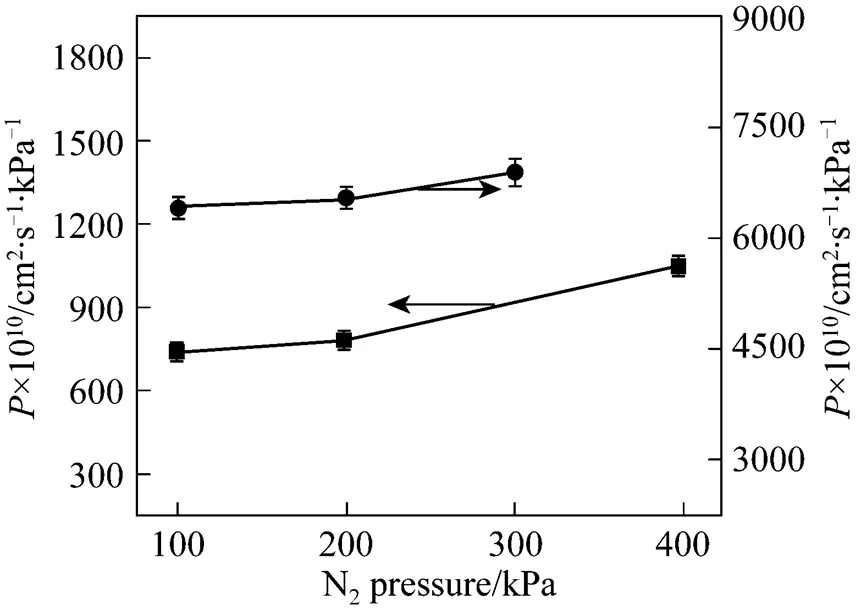
Figure 7 Permeabilities of CO2and SO2in [bmim][Tf2N] membrane under 10 kPa transmembrane pressure difference as a function of equilibrated gas pressure at 25°C■ CO2;● SO2
Figure 8 Permeabilities of CH4and N2in [bmim][Tf2N] membrane under 10 kPa transmembrane pressure difference as a function of equilibrated gas pressure at 25°C ● CH4;▲ N2
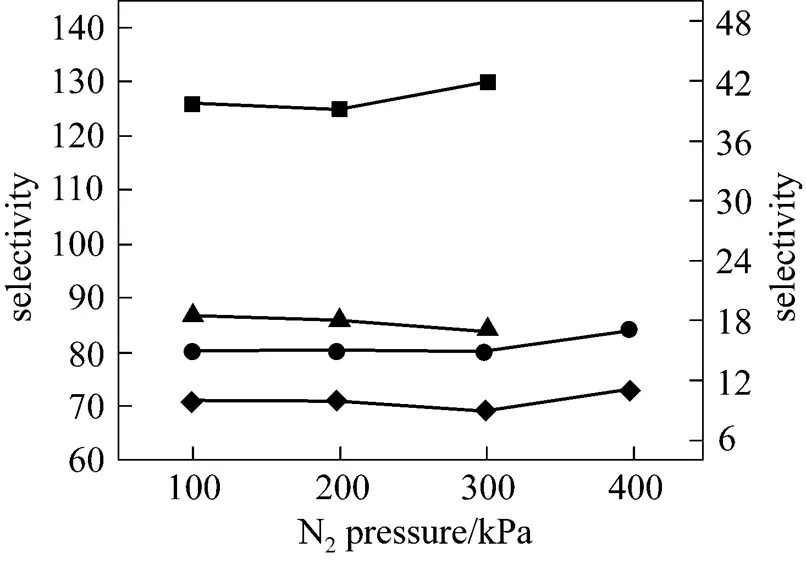
Figure 9 Ideal selectivities of gas pairs in [bmim][Tf2N] membrane under 10 kPa transmembrane pressure difference as a function of operation pressure at 25°Cleft axis:■ SO2/N2;▲ SO2/CH4right axis:● CO2/N2;◆ CO2/CH4
4 CONCLUSIONS
This work provides a systematical insight into the permeabilities and selectivities of CO2and SO2in supported ionic liquid membranes. The solution- diffusion transport mechanism can reasonably predict the permeabilities of two ordinary gases (N2and CH4) and CO2and overestimate SO2permeabilities, probably due to the use of saturated solubility in the calculation and the governing steps from either dissolution or diffusion. It is also found that the SILMs not only offer very good permeabilities of CO2and SO2, but also provide high ideal selectivities of gas pairs. The different performance of SILMs in gas transport and separation should be referred to the nature of ILs, such as cations and anions, as well as the operation temperature and pressure. All these illustrate that the tested SILMs is competitive or superior to existing membrane materials with their outstanding performance in the selectivity and permeability. Therefore, owing to the negligible vapor pressures of SILMs, together with the possibility of producing task-specific SILMschemical modification of the ions, it is expected that SILMs possess the potential for producing highly selective membranes with high permeabilities and are more hopeful for industrial applications in the near future in comparison to the conventional SLMs.
1 Gorri, D., Ibanez, R., Ortiz, I., “Comparative study of the separation of methanol-methyl acetate mixtures by pervaporation and vapor permeation using a commercial membrane”,..., 280, 582-593 (2006).
2 Teramoto, M., Takeuchi, N., Maki, T., Matsuyama, H., “Gas separation by liquid membrane accompanied by permeation of membrane liquid through membrane physical transport”,..., 24, 101-112 (2001).
3 Ito, A., Duan, S., Ikenori, Y., Ohkawa, A., “Permeation of wet CO2/CH4, mixed gas through a liquid membrane supported on surface of a hydrophobic microporous membrane”,..., 24, 235-242 (2001).
4 Noble, R.D., Way, J.D., “Description of facilitated transport and environmental applications”, Membrane Processes in Separation and Purification, Kluwer Academic Publishers, Dordrecht, 316-342 (1994).
5 Bartsch, R.A., Way, J.D., Chemical Separations with Liqiud Membranes, ACS Symposium Series 642, American Chemical Society, Washington, DC (1996).
6 Takeuchi, H., Takahashi, K., Goto, W., “Some observations on the stability of supported liquid membranes”,..., 34, 19 (1987).
7 Bates, E.D., Mayton, R.D., Ntai, I., Davis, J.H., “CO2capture by a task-specific ionic liquid”,...., 124, 926-927 (2002).
8 Wu, W., Han, B., Gao, H., Liu, Z., Jiang, T., Huang, J., “Sulfurization of flue gas: SO2absorption by an ionic liquid”,...., 43, 2415-2417 (2004).
9 Earle, M.J., Esperanca, J.M.S.S., Gilea, M.A., Lopes, J.N.C., Rebelo, L.P.N., Magee, W.J., Seddon, K.R., Widegren, J.A., “The distillation and volatility of ionic liquids”,, 439, 831-834 (2006).
10 Scovazzo, P., Kieft, J., Finan, D.A., Koval, C., DuBois, D., Noble, R., “Gas separations using non-hexafluorophosphate [PF6]-anion supported ionic liquid membranes”,..., 238, 57-63 (2004).
11 Quinn, R., Appleby, J.B., Pez, G.P., “Hydrogen sulfide separation from gas streams using salt hydrate chemical absorbents and immobilized liquid membranes”,..., 37, 627-638 (2002).
12 Gan, Q., Rooney, D., Xue, M., Thompson, G., Zou, Y., “An experimental study of gas transport and separation properties of ionic liquids supported on nanofiltration membranes”,..., 280, 948-956 (2006).
13 Morgan, D., Ferguson, L., Scovazzo, P., “Diffusivities of gas in room-temperature ionic liquids: data and correlations obtained using a lag-time technique”,...., 44, 4815-4823 (2005).
14 Jiang, Y.Y., Zhou, Z., Jiao, Z., Li, L., Wu, Y.T., Zhang, Z.B., “SO2gas separation using supported ionic liquid membranes”,..., 111 (19), 5058-5061(2007).
15 Camper, D., Bara, J., Koval, C., Noble, R., “Bulk-fluid solubility and membrane feasibility of Rmim-based room-temperature ionic liquids”,...., 45, 6279-6283 (2006).
16 Finotello, A., Bara, J.E., Camper, D., Noble, R.D., “Room-temperature ionic liquids: temperature dependence of gas solubility selectivity”,...., 47, 3453-3459 (2007).
17 Hagiwara, R., Ito, Y., “Room temperature ionic liquids of alkylimidazolium cations and fluoroanions”,..., 105, 221-227 (2000).
18 Bonhote, P., Dias, A., Papageorgiou, N., Kalyanasundaram, K., Gratzel, M., “Hydrophobic, highly conductive ambient-temperature molten salts”,.., 35, 1168-1178 (1996).
19 Branco, L.C., Rosa, J.N., Moura, J.J., Ramos, C.A., Asonso, M., “Preparation and characterization of new room temperature ionic liquids”,..., 8, 3671-3677 (2002).
20 Huddleston, J.G., Visser, A.E., Reichert, W.M., Willauer, H.D., Broker, G.A., Rogers, R.D., “Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation”,., 3, 156-164 (2001).
21 Hu, X., Tang, J., Blasig, A., Shen, Y., Radosz, M., “CO2permeability, diffusivity and solubility in polyethylene glycol-grafted polyionic membranes and their CO2selectivity relative to methane and nitrogen”,..., 281, 130-138 (2006).
22 Anthony, J.L., Anderson, J.L., Maginn, E.J., Brennecke, J.F., “Anion effects on gas solubility in ionic liquids”,..., 109, 6366-6374 (2005).
23 Jacquemin, J., Gomes, C., Husson, M.F., Majer, P.V., “Solubility of carbon dioxide, ethane, methane, oxygen, nitrogen, hydrogen, argon, and carbon monoxide in 1-butyl-3-methylimidazolium tetrafluoroborate between temperatures 283 K to 343 K and at pressures close to atmospheric”,..., 38, 490-502 (2006).
2008-11-10,
2009-03-02.
the National Natural Science Foundation of China (20776065), the Natural Science Foundation of Jiangsu Prov ince (BK2008023), and the National Found for Fostering Talents of Basic Science (J0630425).
** To whom correspondence should be addressed. E-mail: ytwu@nju.edu.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Removal of Uranium (VI) by Fixed Bed Ion-exchange Column Using Natural Zeolite Coated with Manganese Oxide*
- Phase Equilibrium of Isobutanol in Supercritical CO2
- Conversion of Methane by Steam Reforming Using Dielectric-barrier Discharge*
- Hydroxyapatite Coatings on Titanium Prepared by Electrodeposition in a Modified Simulated Body Fluid*
- Model Study on a Submerged Catalysis/Membrane Filtration System for Phenol Hydroxylation Catalyzed by TS-1*
- Molecular Dynamics Simulation of Effect of Salt on the Compromise of Hydrophilic and Hydrophobic Interactions in Sodium Dodecyl Sulfate Micelle Solutions*
