Kinetic and Microstructure of SiC Deposited from SiCl4-CH4-H2*
2009-05-14YANGYan杨艳andZHANGWeigang张伟刚
YANG Yan (杨艳) and ZHANG Weigang (张伟刚)
Kinetic and Microstructure of SiC Deposited from SiCl4-CH4-H2*
YANG Yan (杨艳)1,2and ZHANG Weigang (张伟刚)1,**
1State Key Lab of Multi-phase Complex Systems, Institute of Process Engineering, CAS, Beijing 100190, China2Graduate University of Chinese Academy of Sciences, Beijing 100049, China
Silicon carbide was prepared from SiCl4-CH4-H2gaseous precursors by isothermal, isobaric chemical vapor deposition (CVD) at atmospheric pressure and temperatures ranging from 900°C to 1100°C. Kinetic studies showed that carbosilane of SiH2Cl2, SiHCl3and SiCl2formed from decomposition of SiCl4and CH4contributed to the deposition of hexangular facet and granular pebble structured SiC. An average apparent activation energy of 152 kJ·mol-1was determined. The overall CVD process was controlled not only by the surface reactions but also by complex gas phase reactions. The as-deposited thin film was characterized using scanning electron microscopy, X-ray diffraction and transmission electron microscopy, these analysis showed that the deposited thin film consisted of pure phase of the β-SiC, the growth morphology of β-SiC differs from hexangular facet to granular pebble structures, which varied with substrate length and CVD temperature.
chemical vapor deposition, SiC, kinetics, microstructure
1 INTRODUCTION
Chemical vapor deposition (CVD) of silicon carbide is one of the most frequently investigated deposition processes not only because of the outstanding mechanical and physical properties of the materials prepared by this technique, but also because of this versatile process control on the kinetics and microstructure of the deposits with many variables such as temperature, pressure and dilution ratios [1-5]. Numerous studies differ with one another in either precursor, objective, or experimental procedure, but most of them were performed using methyltrichlorosilane (SiCH3Cl3, MTS) [6-10]. Deposition of SiC was studied in different aspects. Some authors were interested in the deposition rate [11]. In some papers the composition of the deposit was determined, additionally [1, 8, 12, 13]. Other papers are concerned with the gas phase chemistry [14]. Deposition rate and composition of the gas phase were simultaneously determined only in two studies [2]. However, no detailed study related to the deposition rate, gas phase composition as well as composition and structure of the deposit was available.
CVD of SiC from the H2/MTS system has been studied previously in our lab, with a new chemical reaction model being proposed [15]. According to this model, SiC was not deposited from MTS or its primary decomposed radicals directly but from chlorocarbosilanes with higher molecular size formed in the gas phase, and this process was mainly controlled by surface area/volume ratio () of the reactor. Co-deposition of free Si with SiC occurred at temperature lower than 1050°C from the surface reactions of chlorosilanes and chlorocarbosilanes, depending also on substrate length and. Pure SiC could only be deposited from chlorocarbosilanes and polychlorocarbosilanes at temperatures above 1050°C, which were formed from the gas phase reactions between H2, SiCl4and CH4. In other words, deposition of pure SiC at high temperature above 1050°C depended strongly on the concentration of SiCl4and CH4, which were formed from those species of SiCl3, CH3, SiHCl3,. Therefore, SiCl4-CH4-H2systems should be an alternative and even better system compared to the SiCH3Cl3-H2system for the CVD of SiC without co-deposition of free silicon, which deteriorate the high temperature properties of SiC served as semi-conductor or hard coatings. However, CVD of SiC from the SiCl4-CH4-H2gaseous precursors was not studied systematically before. On the other hand, SiCl4is much more cheaper and easier for handling due to its lower volatility compared to mono-silanes or MTS.
This study aimed to deposit pure SiC using SiCl4-CH4-H2system to confirm the previous conclusions concluded from the chemical reaction models [16, 17]. However, the more important purposes of this study are as follows: (1) to develop a CVD process using SiCl4-CH4-H2gaseous precursors, which are much more stable at high temperature compared to MTS and monosilanes and promote suppression of silicon co-deposition; (2) to investigate the correlations of the kinetics and microstructure of deposits with the process variables such as temperature and substrate length, aimed to an improvement of material quality of SiC.
2 EXPERIMENTAL
2.1 CVD apparatus

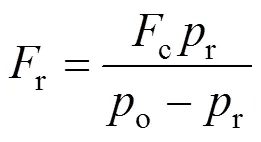
whereo(Pa): the output pressure;r(g·min-1): mass flow rate of the reactant;c(g·min-1): mass flow rate of the carrier;r(Pa): vapor pressure of SiCl4.
Figure 1 Scheme of the chemical vapor deposition reactor
1—mass flow meters; 2—saturator of SiCl4; 3—three-way valve; 4—hot wall furnace; 5—vacuum pump; 6—thermocouple; 7—temperature controller
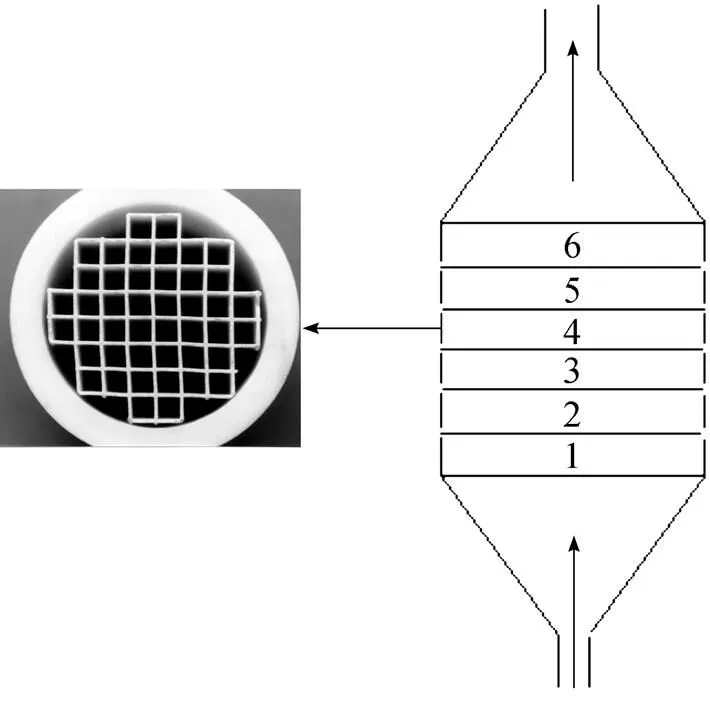
Figure 2 The channel structured substrates and deposition reactor (Left, cross-section of a cordierite substrate; right, arrangement of 6 substrates in the graphite deposition space)
2.2 Preparation of samples

Eight substrates (each 5 mm in height) are stacked into the deposition space in all experiments. This procedure makes it possible to determine the deposition rate as a function of the length of the reactor or substrate. Two or three subsequent deposition experiments of about 2 h are performed under a given deposition condition. Steady-state deposition rates are determined from the linear mass increase as a function of deposition time. Before the SiC deposition, pores of the channel structures are filled or sealed by deposition of pyrolytic carbon using CH4as carbon source at 1100°C, a deposition time of about 24 h is sufficient for sealing the pores with a thickness of coating about 50 μm (Fig. 3). Accomplishment of pre-coating should be judged from a steady-state deposition rate of carbon. The pre-coated carbon exhibited similar thermal expansion coefficient (4.5×10-6K-1, 293-673 K) to SiC, which benefited a thick SiC coating formation without any cracks.
2.3 Deposition procedure
SiCl4-CH4was used as the source gas, hydrogen was not only the carrying gas to transport the reactants to the reactor, but also essential for chemical reactions to form SiC. Hydrogen was also necessary for removing organic contamination existed on the substrate surface immediately before the film deposition [18].
Experiments were performed under the following conditions: the input gas mole ratio of SiCl4︰CH4︰H2was 1︰1︰8, with a total pressure of 101.3 kPa, temperature ranged from 900°C to 1100°C, the residence time of gas was kept constant of 1 s.

Table 1 Some geometric properties of the channel structure of cordierite substrate (Mg2Al4Si5O18)
① Channels per square centimeter.
② Based on a stack of 5 substrates.
According to the simulated results [16] of temperature distribution in the reactor using commercial Fluent 6.0 (Fluent Inc.), the substrates with a length of 4 cm for deposition were placed on the isothermal zone of the deposition space. The reactor was purged with pure argon during heating up to avoid oxidation of substrate. When the desired temperature was reached, the carrier gas was supplied to the saturator and the pressure was controlled by an automatically controlled pump. Constant deposition rates (only the deposition on the outer cylindrical surface of substrate being measured) were obtained after several runs of CVD process which was determined by the mass gain per hour of each slice of substrates. Experimentally, the steady-state deposition rates were determined from the linear mass increase as a function of deposition time.

Figure 3 Cross-section micrograph of the porous substrate and the pre-coated carbon layer
3 RESULTS AND DISCUSSION
3.1 Thermodynamic calculations
Calculation of chemical equilibrium composition is always necessary for the selection of specific CVD process. Thermodynamic analysis of the equilibrium gas composition can not only suggest the degree of variation of the chemical deposition conditions but also the thermodynamic yields and the formation of intermediate species [19].



Figure 4 Equilibrium compositions of the gas phase from the reactions of SiCl4-CH4-H2as a function of temperature
Figure 5 Equilibrium compositions of the solid phase as a function of temperature□ SiC; △ Si;○ C

The equilibrium compositions of the solids corresponding to the gas phase composition of Fig. 4 are presented in Fig. 5, from which it can be concluded that the pure SiC can be deposited between 682°C and 1100°C if co-deposition of SiC and Si exists; SiC and carbon can be co-deposited if there is co-deposition of SiC, Si and C. The mole of SiC increases rapidly with the temperature while carbon is the opposite, which shows that more pure SiC can be obtained with the increase of temperature.
3.2 Kinetics of deposition
Deposition rate of SiC films strongly depends on chemical reaction kinetics, which additionally influences the formation of micro-structures. Among the parameters dominating the CVD process, temperature, substrate length and concentration of reactants (or supersaturation) play the most important roles.
3.2.1
Deposition rates as a function of substrate length are shown in Fig. 6. A substrate length of 40 mm corresponds to a residence time of 1.0 s for all the experiments. Deposition rates at 900 and 950°C are negligible, which are not included in the figures.

Figure 6 Surface-related deposition rates as a function of substrate length at various temperatures□ 1000°C;○ 1050°C;△ 1100°C
Deposition rates at 1000 and 1050°C increase with substrate length, which means deposition of SiC is not only from the reactions between feeding gases of methane and silicon tetrachloride but also from their derived reactive species formed in the gas phase [17]. This conclusion is more prominent if the decreasing of the SiCl4/CH4concentrations with prolongation of substrate length is considered. Considering the very low deposition rate when the substrate length is extrapolated to zero even at a temperature as high as 1100°C, contribution of more reactive species progressively formed in the gas phase to the deposition rate is dominated. As the temperature is low (1000°C), deposition rate increases slowly with increasing of substrate length, but a dramatic increase of deposition rate is achieved with the substrate length above 3.5 cm. This phenomenon exists also in the cases of higher temperatures of 1050 and 1100°C, but the substrate lengths shift to lower values,.. about 1.5 cm at 1050°C, and 1 cm at 1100°C, respectively. This shift should be caused by an acceleration of reactive precursors formation in the gas phase. A maximum deposition rate is obtained at a substrate length of 3 cm with the temperature of 1100°C. Decreasing of the deposition rate afterward therefore is caused by a depletion of reactive species in the gas phase. Deposition rates obtained at 1050°C and 1100°C increase when the substrate length is above 1 cm or 1.5 cm, which clearly shows an overcoming of induction period for the formation of reactive precursors [5], such as SiH2Cl2, SiHCl3, or SiCl2,.
3.2.2
The total deposition rate as a function of temperature is shown in Fig. 7, which indicates a two-stage deposition process, low temperature (below 1000°C) and high temperature regimes (above 1000°C). By analyzing the results it can be concluded that decomposition of SiCl4mainly leads to the formation of small molecular or radical species, such as SiCl3, HSiCl3, SiCl2,(see Fig. 3).
Decomposition of methane below 1000°C mainly forms CH3and C2species when the residence time is shorter than 1s, which results in the small deposition rate of SiC from these small species because of limitation of surface active sites [20]. However, decomposition of SiCl4and CH4at the high temperature regime leads to the formation of carbosilanes or high molecule of carbosilanes. Major reactions are as follows according to the model suggested by Zhang and Hüttinger [17]:
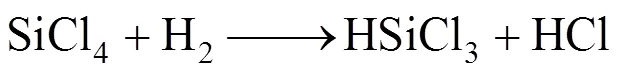




Influence of temperature on the deposition rate are additionally analysed by plotting of a logarithmic deposition rate as a function of reciprocal temperature. Fig. 7 (b) shows the calculated results based on Fig. 7 (a) according to the well-known Arrhenius equation, which gives the dependence of the rate constantof chemical reactions on the temperature(in Kelvin) and activation energy, as shown below:

whereis the pre-exponential factor or simply the prefactor andis the gas constant.
The calculated result shows an apparent activation energy of 152 kJ·mol-1while temperature above 1050°C where the rate increases with temperature, which is close with MTS as precursor in the literature [2].
According to the above kinetic results, chemical vapor deposition of SiC is therefore a result of strong interaction between homogeneous gas phase and heterogeneous surface reactions. Decomposition of precursors and their reactions in the gas phase are combined with surface nucleation or growth. The microstructure of such layer is therefore influenced strongly by the nucleation or growth of reactive species from the gas phase.

Figure 7 Total surface-related deposition rates as a function of temperature
3.3 Microstructure and morphologies of the deposits
If decomposition of SiCl4and CH4is followed by a gas phase nucleation, the resulting product is a powder. If the reaction steps or nucleation occur on the surface of a substrate, surface layers therefore are formed. The structure-property relationship of such layers is best approached by considering the mechanism of nucleation and growth in condensation from the vapor phase. For a given substrate-vapor combination, the nucleation and growth kinetics are decided by two major factors [21]: the supersaturation (related to the concentration of adsorbed species on the surface) and the temperature (related to their mobility). Therefore, the CVD process of layer growth or surface nucleation can be understood by analysis of the microstructures of the deposits.
3.3.1
Figure 8 shows X-ray diffraction (XRD) results of the coatings deposited at 1050°C (a) and 1100°C (b). For two cases, pure SiC is obtained with good crystal types at all substrate lengths. Diffraction angles on 35.73°, 60.13°, 71.95° and 75.70° are attributed to cubic silicon carbide and correspond to the crystal planes of (111), (220), (311) and (222), respectively [Figs. 8 (a) and 8 (b)]. Therefore, the preferred deposition orientation in the SiC layer is (111) planes (planes distance is 0.25193 nm and the crystal lattice constant is 0.452 nm), which is aligned parallel to the substrate, and the preferred orientation is enhanced additionally by increasing the temperature from 1050 to 1100°C.

Figure 8 XRD patterns of CVD SiC at 1050 and 1100°C
Figure 9 SEM micrographs of CVD SiC at different temperatures
3.3.2
According to the mechanism of chemical vapor deposition, the microstructure of deposit is controlled by two processes [22]: the formation and the growth of the crystalline. The temperature of the reaction system and the supersaturation of the reactants are the driving forces during the process.
3.3.2.1
The scanning electron microscopy (SEM) morphologies of the films at various temperatures are shown in Fig. 9, which indicates formation of hexangular facet structure of SiC (1100 and 1050°C). Average size of these facet structures is about 200 nm, which maybe or may be not correspond to a real crystal size of SiC. A granular pebble structure is obtained at low temperatures (1000 and 900°C), with an average size of pebble structure between 30 and 50 μm.
3.3.2.2
The concentration of reactants changes with the substrate position, which results in the different morphology and crystal size of the deposits.
Surface morphologies of SiC deposited at 1050°C with various substrate lengths are shown in Fig. 10. A ratio of carbon to silicon is 1.0034 detected with energy- dispersive X-ray (EDAX) elementary analysis indicates the pure silicon carbide (not shown). Surface morphology changes greatly with the substrate length. With increasing of substrate length more compact and smooth surface is obtained and formation of hexangular facet structure of SiC is promoted even with an increased deposition rate [see Fig. 7 (b)], which proves that the facet crystallinity can be improved with increasing of substrate length.
According to the deposition rate, the granular pebble structure is from small species leading to formation of very fine nucleus. However, the facet structure of SiC are from more reactive gas phase species with much higher deposition rate, and the facet structure with crystallite sizes above 200 nm normally can only be observed at very high temperature CVD process.
SiC crystal size is sensitive to concentration of SiCl4. The relation of supersaturation and the nucleus size by Gibbs-Thomson law [23] is

From Eq. (7) it follows that increasing the supersaturation of reactants, the nucleus sizeincreases exponentially. When the concentration is low, high temperature liquid drop containing elements of Si, C, H and Cl can not be formed on the boundary layer over the substrate materials because of lower supersaturation. The controlling step of chemical vapor deposition is changed from liquid nucleation to solid nucleation. The gas phase diffuses rapidly in the boundary layer and formed the tiny SiC grain by the inorganic nucleation. The deposition rate decreases rapidly with the decrease of concentration, which results in the thinner layers of coating at the same temperature, pressure and time.
Therefore, the induction of active species such as SiH2Cl2, SiHCl3and SiCl2formed from the decomposition of SiCl4influences not only the deposition rate but also the quality of formed film. Because the concentration of these reactive species increases as the gas flows down the reactor, an improved crystallization with larger size of SiC can be enhanced by a prolonged reactor length, or higher initial SiCl4partial pressure, which was not changed in the study.

Figure 10 SEM micrographs (with lower and high magnifications) of thin film formed at various substrate lengths
4 CONCLUSIONS
Summarize the above results and discussions, some conclusions can be drawn as follows:
(1) Deposition of SiC from the system of SiCl4-CH4-H2is a result of strong interaction between homogeneous gas phase and heterogeneous surface reactions, in which carbosilanes such as SiH2Cl2, SiHCl3, or SiCl2are formed from the decomposition of SiCl4and CH4. The apparent activation energy is about 152 kJ·mol-1.
(2) The deposit film consists of pure β-SiC with the (111) plane as the preferred deposition orientation, with a surface morphology of hexangular facet structure and granular pebble.
(3) Microstructures of the deposits are influenced strongly by the nucleation or growth of species from the gas phase, which is decided by the temperature and the supersaturation. Hexangular facet structure of SiC is the typical morphology of high temperature deposition, and granular pebble structure occurs at lower temperatures.
1 Kim, H.S., Choi, D.J., “Effect of diluent gases on growth behavior and characteristics of chemically vapor deposited silicon carbide films”,...., 82, 331-337 (1999).
2 Cagliostro, D.E., Riccitiello, S.R., “Model for the formation of silicon carbide from the pyrolysis of dichlorodimethylsilane in hydrogen (I) Silicon formation from chlorosilane”,...., 76 (1), 39-48 (1993).
3 Cagliostro, D.E., Riccitiello, S.R., “Model for the formation of silicon carbide from the pyrolysis of dichlorodimethylsilane in hydrogen (II) Silicon carbide formation from silicon and methane”,...., 76 (1), 49-53 (1993).
4 Cagliostro, D.E., Riccitiello, S.R., “Comparison of the pyrolysis products of dichlorodimethylsilane in the chemical vapor deposition of silicon carbide on silica in hydrogen or argon”,....,77 (10), 2721-2726 (1994).
5 Takeuchi, T., Egashira, Y., Osawa, T., Komiyama, H., “A kinetic study of the chemical vapor deposition of silicon carbide from dichlorodimethylsilane precursors”,..., 145 (4), 1277-1284 (1998).
6 Papasouliotis, G.D., Sotirchos, S.V., “On the homogeneous chemistry of the thermal decomposition of methyltrichlorosilane: Thermodynamic analysis and kinetic modeling”,..., 141 (6), 1599-1611 (1994).
7 Besmann, T.M., Sheldon, B.W., Lowden, R.A., Stinton, D.P., “Vapor-phase fabrication and properties of continuous-filament ceramic composites”,, 253, 1104-1109 (1991).
8 Sotirchos, S.V., Papasouliotis, G.D., “Experimental study of the atmospheric pressure chemical vapor deposition of silicon carbide from methyltrichlorosilane”,..., 14, 3397-3409 (1999).
9 Loumagne, F., Langlais, F., Naslain, R., “Reactional mechanisms of the chemical vapour deposition of SiC-based ceramics from CH3SiCl3/H2gas precursor”,.., 155, 205-213 (1995).
10 Loumagne, F., Langlais, F., Naslain, R., “Reactional mechanisms of the chemical vapour deposition of SiC-based ceramics from CH3SiCl3/H2gas precursor”,.., 155, 198-204 (1995).
11 Sone, H., Kaneko, T., Miyakawa, N., “measurements and growth kinetics of silicon carbide chemical vapor deposition from methyltrichlorosilane”,.., 219, 245-252 (2000).
12 Kaneko, T., Okuno, T., Yumoto, H., “Growth kinetics of silicon carbide CVD”,.., 91 (4), 599-604 (1988).
13 Lu, Y.M., Leu, I.C., “Microstructural study of residual stress in chemically vapor deposited B-SiC”,., 124, 262-265 (2000).
14 Vorobev, A.N., Karpov, S.Y., Zhmakin, A.I., Lovtsus, Y.N., “Effect of gas-phase nucleation on chemical vapor deposition of silicon carbide”,.., 211, 343-346 (2000).
15 Reznik, B., Gerthsen, D., Zhang, W.G., Hüttinger, K.J., “Microstructure of SiC deposited from methyltrichlorosilane”,...., 23, 1499-1508 (2003).
16 Zhang, W.G., Hüttinger, K.J., “CVD of SiC from methyltrichlorosilane. Deposition rates”,..., 7 (4), 167-172 (2001).
17 Zhang, W.G., Hüttinger, K.J., “CVD of SiC from methyltrichlorosilane. Composition of the gas phase and the deposit”,..., 7 (4), 173-181 (2001)
18 Habuka, H., Watanabe, M., Nishida, M., Sekiguchi, T., “Polycrystalline silicon carbide film deposition using monomethylsilane and hydrogen chloride gases”,., 201, 8961-8965 (2007).
19 Jung, Y.G., Park, S.W., Choi, S.C., “Effect of CH4and H2on CVD of SiC and TiC possible fabrication of SiC/TiC/C FGM”,.., 30, 339-345 (1997).
20 Hu, Z.J., Hüttinger, K.J., “Mechanisms of carbon deposition—A kinetic approach”,, 40 (4), 624-8 (2002).
21 Zheng, C.Q., Ran, J.G., New Inorganic Materials, Science Press, Beijing, 87-89 (2003). (in Chinese)
22 Zhang, W.G., “Chemical vapor deposition of carbon”, Chemical Vapor Deposition—From Hydrocarbon to Carbon, Science Press, Beijing, 44-51 (2007). (in Chinese)
23 Givargizou, E.J., Current Topic in Materials Science, North-Holland, New York, 56 (1978).
2008-07-05,
2009-04-18.
the One Hundred Talents Program of Chinese Academy of Sciences.
** To whom correspondence should be addressed. E-mail: wgzhang@home.ipe.ac.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Position Group Contribution Method for Estimation of Melting Point of Organic Compounds
- Process Intensification of VOC Removal from High Viscous Media by Rotating Packed Bed*
- Adsorption of Dye from Wastewater by Zeolites Synthesized from Fly Ash: Kinetic and Equilibrium Studies*
- Modeling of Isomerization of C8 Aromatics by Online Least Squares Support Vector Machine*
- Resolution of Ibuprofen Ester by Catalytic Antibodies in Water-miscible Organic-solvents*
- Reaction Characteristics of Asymmetric Synthesis of (2S,5S)-2,5-Hexanediol Catalyzed with Baker’s Yeast Number 6*
