Resolution of Ibuprofen Ester by Catalytic Antibodies in Water-miscible Organic-solvents*
2009-05-14YANGGensheng杨根生YINGLi应黎OUZhimin欧志敏andYAOShanjing姚善泾
YANG Gensheng (杨根生), YING Li (应黎), OU Zhimin (欧志敏) and YAO Shanjing (姚善泾)
Resolution of Ibuprofen Ester by Catalytic Antibodies in Water-miscible Organic-solvents*
YANG Gensheng (杨根生)1,2, YING Li (应黎)2, OU Zhimin (欧志敏)2and YAO Shanjing (姚善泾)1,**
1Department of Chemical and Biochemical Engineering, Zhejiang University, Hangzhou 310027, China2College of Pharmaceutical Sciences, Zhejiang University of Technology, Hangzhou 310032, China
The asymmetric hydrolysis of racemic ibuprofen ester is one of the most important methods for chiral separation of ibuprofen. In this work, a catalytic antibody that accelerates the rate of enantioselective hydrolysis of ibuprofen methyl ester was obtained against an immunogen consisting of tetrahedral phosphonate hapten attached to bovine serum albumin (BSA). The catalytic activity of the catalytic antibody in the water-miscible organic-solvent system composed of a buffer solution and,-dimethylformamide (DMF) was studied. With 6% DMF in the buffer solution (containing catalytic antibody 0.25 μmol, 0.2 mol·L-1phosphate buffer, pH 8) at 37°C for 10 h, a good conversion (48.7%) and high enantiomeric excess (>99%) could be reached. The kinetic analysis of the catalytic antibody-catalyzed reaction showed that the hydrolysis in the water-miscible organic-solvent system with DMF in buffer solution followed the Michaelis-Menten kinetics. The catalytic efficiency (cat/m) was enhanced to 151.91 L·mmol-1·min-1, twice as large as that for the buffer solution only.
catalytic antibody, ibuprofen, enantioselective hydrolysis, water-miscible organic-solvent, conversion
1 INTRODUCTION
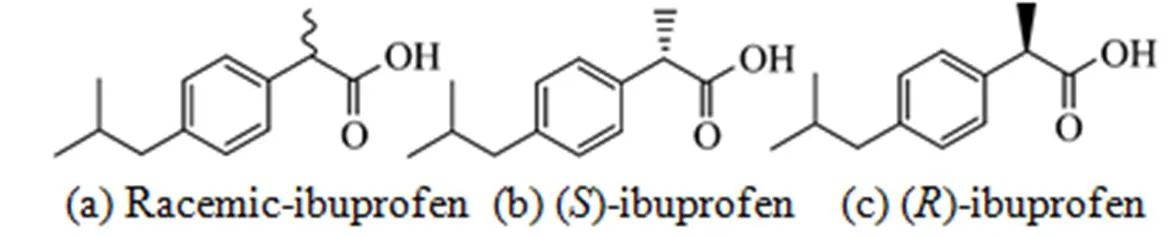
Figure 1 Structures of racemic-ibuprofen, ()-ibuprofen and ()-ibuprofen
Resolution of racemic ibuprofen has been achieved by the enzyme-catalyzed enantioselective hydrolysis of the corresponding racemic esters, amides, and nitriles [8, 9]. However, some disadvantages, such as toxicity of ibuprofen derivatives toward microorganisms and low catalytic activity of lipases, have been observed in previous investigations. Some new methods, such as ionic liquids [10] and biphasic enzymatic membrane reactors (EMRs) [11], have emerged in applications for enzyme-catalyzed kinetic resolution.
Catalytic antibodies, as a new class of man-made biocatalysts, have had very promising applications since they were first reported by two groups [12, 13]. To date, catalytic antibodies have shown the ability to catalyze a variety of chemical reactions, such as hydrolysis, the Diels-Alder reaction, and the retro-aldol process [14, 15]. When using catalytic antibodies for chemical synthesis, a dilemma often encountered is that many of the interesting and synthetically useful organic molecules have low solubility in water, while catalytic antibodies, like all proteins, function ideally in aqueous environments. The potential benefits of using catalytic antibodies for organic synthesis have motivated many researchers to develop optimized systems for circumventing this problem [16]. The proposed solutions include enclosing catalytic antibodies in reverse micelles [17, 18], or using a biphasic aqueous/ organic solvent system [19]. However, the significant features of catalytic antibodies in the organic mediaor in the immobilized systems have yet to be established.
A catalytic antibody as a new biocatalyst, generated from the transition-state phosphonate analog, will be used in this study to produce ibuprofen in an enantiomerically pure-configuration from its corresponding racemic esters in a water-miscible organic-solvent system. The objectives are to explore the enzymological characteristics of catalytic antibodies solubilized in an organic solvent and some aspects of the application of catalytic antibodies as catalysts in anhydrous media.
2 EXPERIMENTAL
2.1 Materials
Racemic ibuprofen and isobutylbenzene were supplied by Quhua Group Corporation and CNPC Chemical (China) respectively.- and-ibuprofen were obtained from Sigma. Ibuprofen methyl ester was synthesized by the following procedure. Thionyl chloride was dropped into a round-bottom flask containing racemic,-ibuprofen and the solution was refluxed for 10 h. After removal of unreacted thionyl chloride, the alcoholysis of acyl chlorides with methanol was carried out for 5 h. From the reaction mixture ibuprofen methyl ester was extracted and purified by repeated extraction with methylene chloride and 5% sodium bicarbonate solution. No remaining ibuprofen could be detected in the product as tested by thin-layer chromatography (TLC) and infrared spectroscopy (IR). l-ethyl-3-(3-dimethylaminopropyl) carbodiimide was purchased from Shanghai Medpep Co., Ltd. Bovine serum albumin (BSA), peroxidase labeled goat anti-rabbit IgG, Freund’s complete and incomplete adjuvants, and Sephadex G-25 were purchased from Sigma. Other chemicals were of G.R. grade and used without further purification.
2.2 Preparation and purification of the catalytic antibody
2.2.1
One phosphonate 2 was designed as a hapten on the concept of transition-state stabilization and conjugated with BSA for use as antigens for production of antibodies. The synthetic route for hapten 2 is illustrated in Fig. 2. The procedure for the synthesis of hapten is as follows:
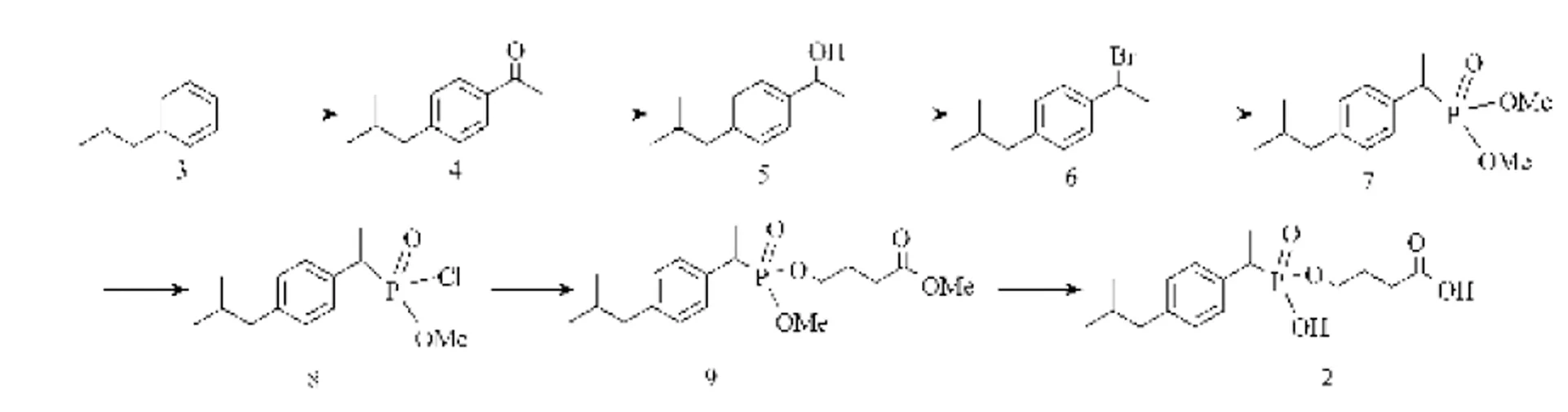
Figure 2 Synthesis route for hapten 2
Synthesis of 4-isobutylacetophenone 4
Acetyl chloride (22 g, 180 mmol) was added to a suspension of AlCl3(22 g, 165 mmol) in CH2Cl2(200 ml) at 0°C. After the mixture was stirred at 0°C for 10 min, isobutylbenzene (20 ml, 180 mmol) was added slowly and stirring was continued at the same temperature for 5 h. The reaction mixture was poured into a mixture of hydrochloric acid and ice water. The organic layer was separated, washed with water and brine, and dried with anhydrous Na2SO4. After evaporation of the solvent, the residue was distilled (170°C at 0.267 kPa) to give 4-isobutylacetophenone 4 (22.5 g, 85% yield) as a colorless oil.1H-NMR (400 MHz, CDCl3):0.91 (d, 6H, CH3), 1.90 (m, 1H, CH), 2.53 (d, 2H, CH2), 2.58 (s, 3H, COCH3), 7.23 (d, 2H, ArH), 7.88 (d, 2H, ArH).
Synthesis of 1-(4-isobutylphenyl)-1-ethanol 5
NaBH4(5.8 g, 0.16 mol) was added twice to a solution of 4-isobutylacetophenone 4 (14 g, 0.32 mol) in methanol (120 ml) at 0°C. The reaction was monitored by TLC. After addition of ice water (100 ml), the solution was acidified to pH 3 with 30% HCl. The mixture was then stirred at room temperature for 15 min and extracted with Et2O (3×100 ml). The combined ether layers were washed with brine, dried over Na2SO4, and concentrated to give a colorless liquid 5 (13.3 g, 95% yield).1H-NMR (400 MHz, CDCl3):0.97 (d, 6H, CH3), 1.30 (d, 3H, CH3), 1.80 (m, 1H, CH), 2.41 (d, 2H, CH2), 4.67 (m, 1H, CHOH), 5.13 (s, 1H, OH), 7.08 (d, 2H, ArH), 7.23 (d, 2H, ArH).
Synthesis of 1-Bromo-1-(4-isobutylphenyl) ethane 6
Synthesis of 1-(4-isobutylphenyl)-1-ethyl- dimethyl phosphonates 7
10 ml of triethyl phosphite was added dropwise to the above crude product 6 (12.27 g, without further purification) with stirring at 110-120°C, then refluxed for 4.5 h, and nitrogen was continuously bubbled through the mixture, using CaCl2as drier. The reaction mixture was cooled to room temperature, and then diluted by 150 ml acetic ester. The dilution was washed with water and brine, and then dried over anhydrous Na2SO4. Evaporation of the solvent gave 1-(4-isobutylphenyl)-1-ethyl-dimethyl phosphonates 7 (13.13 g, 88% yield of two reactions) as a wheat oil, which was subjected to chromatographic separation with silica gel column.1H-NMR (400 MHz, CDCl3):0.90 (d, 6H, CH3), 1.62 (d, 3H, ArCHCH3), 1.90 (m, 1H, CH), 2.51 (m, 2H, CH2), 3.27 (q, 1H, ArCHCH3), 3.84 (s, 6H, POCH3), 7.07(d, 2H, ArH), 7.17(d, 2H, ArH).
Synthesis of 1-(4-isobutylphenyl)-1-ethyl- chlormethylphosphonates 8
The mixture of 1-(4-isobutylphenyl)-1-ethyl- dimethyl phosphonates 7 (6 g, 22.3 mmol) and PCl5(6.63 g, 32 mmol) in CH3Cl3(50 ml) was stirred at 45°C for 3 h. Then SO2gas produced by heating Na2SO3was bubbled through the solution for 5 min. Evaporation of the solvent gave 1-(4-isobutylphenyl)- 1-ethyl-chlormethylphosphonates 8 (6.8 g, 110% yield) without being purified to the next reaction.1H-NMR (400 MHz, CDCl3):0.91 (d, 6H, CH3), 1.65 (d, 3H, ArCHCH3), 1.92 (m, 1H, CH), 2.50 (m, 2H, CH2), 3.34 (q, 1H, ArCHCH3), 3.69 (s, 3H, POCH3), 7.14 (m, 2H, ArH), 7.23 (m, 2H, ArH).
Synthesis of ethyl 4-((1-(4-isobutylphenyl) ethyl) (methoxy) phosphoryloxy) butanoate 9
Synthesis of 4-(hydroxy(1-(4-isobutylphenyl) ethyl) phosphoryloxy) butanoic acid 2
The compound 9 (15 mg, 0.047 mmol), which dissolved in ethanol (2 ml), was added with the aqueous solution of NaOH (2 mol·L-1, 2 ml) dropwise with stirring. The reaction mixture was heated to reflux for 30 min. After evaporation of ethanol, the residual solution was stirred for 2 h, then acidified with HCl (1 mol·L-1, 4.3 ml) and evaporated under reduced pressure to give the crude product. Purification by HPLC gave pure 4-(hydroxy (1-(4-isobutylphenyl) ethyl) phosphoryloxy) butanoic acid 2 (6.7 mg, 45% yield).1H-NMR (400 MHz, CDCl3/D2O, 1/1)︰0.91 (d, 6H, CH3), 1.63 (d, 3H, ArCHCH3), 1.82 (m, 1H, CH), 2.02 (m, 2H, CH2), 2.25 (d, 2H, CH2), 2.50 (d, 2H, CH2), 3.23 (q, 1H, ArCHCH3), 4.03 (q, 2H, POCH2), 7.05 (d, 2H, ArH), 7.25 (d, 2H, ArH). MS (ESI)/328(M+).
2.2.2
Hapten 2 was covalently attached to BSA to be used as an immunogen. The method of conjugation used was the standard 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)-promoted amide formation reaction between the carboxylic acid of hapten 2 and the δ-amine of the surface lysine of BSA. Briefly, hapten 2 (20 mg, 0.06 mmol) in DMF (2.2 ml) was dissolved in 4 ml of normal saline (pH 5.2). BSA (10 mg, 6.67×10-5mmol) and l-ethyl-3-(3-dimethylaminopropyl) carbodiimide (30 mg) were added to the solution. The mixture was stirred at room temperature for 5 h. The mixture solution was then dialyzed against PBS buffer (pH 7.3) for six days. The formation of the BSA-hapten conjugate was confirmed by UV/VIS spectroscopy.
2.2.3
The antigen was used to immunize five BALB/c mice and to generate monoclonal antibodies, following the standard protocols [20]. Five BALB/c mice were immunized with the hapten-BSA conjugate (100 μg) emulsified in complete Freund’s adjuvant. After 3 weeks, the second immunization (100 μg in incomplete Freund’s adjuvant) was administered, and similarly, the third immunization. Three days later, the spleen cells from the immunized mice were fused with SP2/0 according to routine procedures. Hybridomas that secreted antibodies capable of binding hapten 2 were identified using enzyme linked immunosorbent assay (ELISA), and binding titers in the assay were used to select one clone for further investigation. This clone was cultured in a rolling culture bottle and the monoclonal antibody was isolated by salt fractionation. A large amount of the catalytic antibody was prepared from ascites in BALB/c mice. The antibodies were sequentially purified through ammonium sulfate precipitation at various levels, protein A affinity chromatography, DEAE-52 ion exchange chromatography, and dialysis against 30 mmol·L-1PBS (pH 8.0). The protein concentration was measured using the Bradford method [21].
2.3 Catalytic hydrolysis of the ibuprofen methyl ester in buffer solution
In aqueous media, the hydrolysis of the ibuprofen methyl ester was carried out in a mixture that had various substrate concentrations (,- and-, and-ibuprofen methyl esters) and purified monoclonal antibodies in 0.05 mol·L-1sodium phosphate buffer pH 8.0 at 37ºC. The mixture solution was kept at 37°C for the prescribed time interval, and the catalytic activity was destroyed by thermal denaturation of the catalytic antibodies at 95°C for 10 min following by rapid cooling to 0°C.
Kinetic parameters were determined using a Lineweaver-Burk plot of reaction for the antibody- catalyzed hydrolysis of substrate. The uncatalyzed rate (sp) was determined by measuring the substrate hydrolysis rates with normal IgG of mouse instead of catalytic antibody.
2.4 Catalytic hydrolysis of the ibuprofen methyl ester in water-miscible organic solvents
Ibuprofen methyl ester was solubilized in the mixture of water-miscible organic solvent (1%-10%, by volume) and phosphate buffer (0.2 mol·L-1, pH 8.0). The catalytic antibodies were added to the reaction medium, and then the reaction mixture was gently shaken in a shaking water bath at 250 r·min-1. The reaction temperature was 37°C unless otherwise specified. With the prescribed time interval, the production of ibuprofen and the disappearance of the ester were followed by HPLC.
To determine its precise optical purity, ibuprofen was separated from the reaction mixture by a two-step extraction procedure prior to analysis: methylene chloride was added to a solution prepared from the reaction mixture, and after the phase separation, 5% sodium bicarbonate solution was added to the organic phase (methylene chloride). Using this procedure, ibuprofen was successfully extracted to the aqueous phase (bicarbonate solution) without any detectable amount of ibuprofen methyl ester in the aqueous phase. The aqueous-phase solution was neutralized and used to determine the optical purity of ibuprofen. For determination of kinetic parameters, the similar procedure was performed as in buffer solution.
2.5 HPLC analysis
The analysis of both enantiomers of ibuprofen was conducted by HPLC [22] (instrument, Aligent 1100) with a Chiralcel OD-H (5 μm, 4.6 mm×250 mm),and a UV detector (254 nm). The mobile phase was-hexane and isopropanol (volume ratio 98︰2). The flow rate was set at 0.5 ml·min-1. Identification and quantification of the substrates and the products were performed by comparing the retention time and the peak area to those of the authentic samples, respectively.
The conversion (), enantiomeric ratio () and the enantiomeric excess of the product () were calculated by the following equations [23].
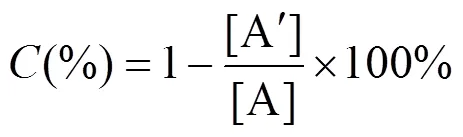
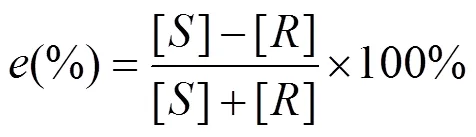
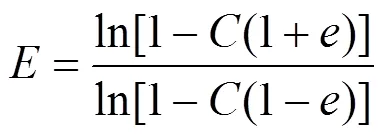
where [] and [] represent the concentration ofandisomer, respectively, and [A] and [A′] represent the initial concentration and residual concentration of ibuprofen methyl ester, respectively.
3 RESULTS AND DISCUSSION
3.1 Preparation of hapten and catalytic antibodies

A preliminary assay of the hydrolytic activity of the purified antibodies was accomplished by HPLC, and 3 of 7 antibodies were found to catalyze the hydrolysis at a rate significantly higher than the uncatalyzed background reaction. Of these, one antibody (A3) was ultimately chosen as the active catalytic antibody. As shown in Fig. 3, this catalytic antibody can accelerate catalysis of the-ibuprofen methyl ester but not the-ibuprofen methyl ester.
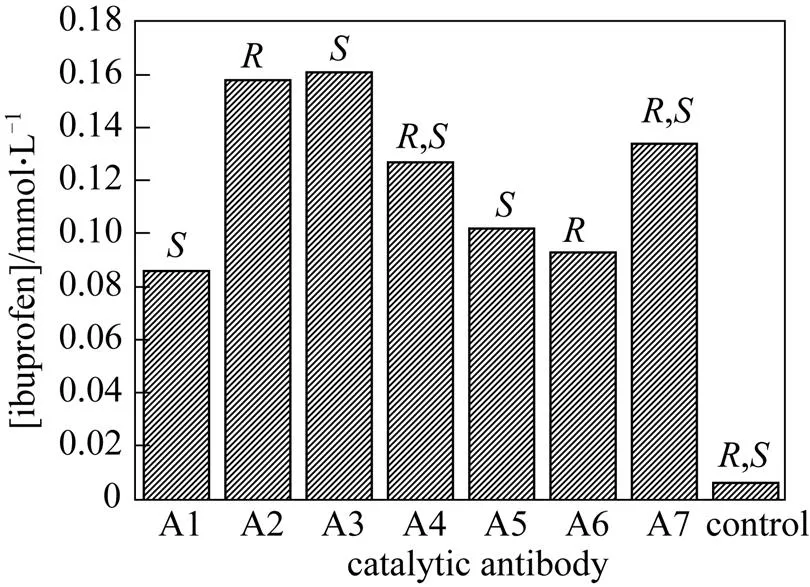
Figure 3 Data for the initial screen of antibody-catalyzed hydrolyses of ibuprofen methyl ester
[The column represents the amount of ibuprofen methyl ester in the background reaction (control) and in the monoclonal antibody-catalyzed hydrolyses of ibuprofen methyl ester (columns A1-A7).,indicates that the product is,-ibuprofen,indicates-ibuprofen, andindicates-ibuprofen. Reaction conditions: substrate,-ibuprofen methyl ester; ratio of molar concentrations of substrate to catalytic antibody 25︰1; 0.2 mol·L-1phosphate buffer (pH 8) at 37°C; reaction time 2 h]
The kinetic results revealed that the catalytic antibody catalyzed enantioselective hydrolysis in aqueous solution, with an initial rate consistent with Michaelis-Menten kinetics. The values ofcatandmobtained from the linear least-squares analysis were 1.47 min-1and 21.74 μmol·L-1, respectively, at 37ºC. The first order rate constant,sp, of spontaneous hydrolysis of ibuprofen methyl ester under the same conditions was 1.71×10-4min-1, so thatcatin this study corresponds to a rate constant enhancement factor of 8.6×103over the spontaneous reaction, indicating that the catalytic reaction rates mediated by catalytic antibodies are much higher. The ratio ofcat/spreflects the acceleration of the hydrolytic reaction.
3.2 Effects of organic solvents on the reactivity for kinetic resolution of ibuprofen methyl ester
In order to screen the water-miscible organic- solvent with regard to the reactivity and selectivity, 1% (by volume) solvent was added to the reaction medium, and the extent of hydrolysis and enantiomeric excess of the product were determined after 10 h reaction. Table 1 shows the experimental results obtained for the hydrolysis of ibuprofen methyl ester. Among the 9 organic solvents tested,,-dimethylformamide (DMF) shows enhanced conversion compared to the control experiment. It is also found that the conversion is not closely correlated to solvent parameters such as lg(solvent hydrophobicity), dielectric constant or dipole moment. On the other hand, it appears that catalytic activity is influenced by the hydrophobicity of the cosolvent. In the case of-alkanols (methanol, ethanol, and 1-propanol), for example, the relative activities decrease as the carbon number of solvent increases.
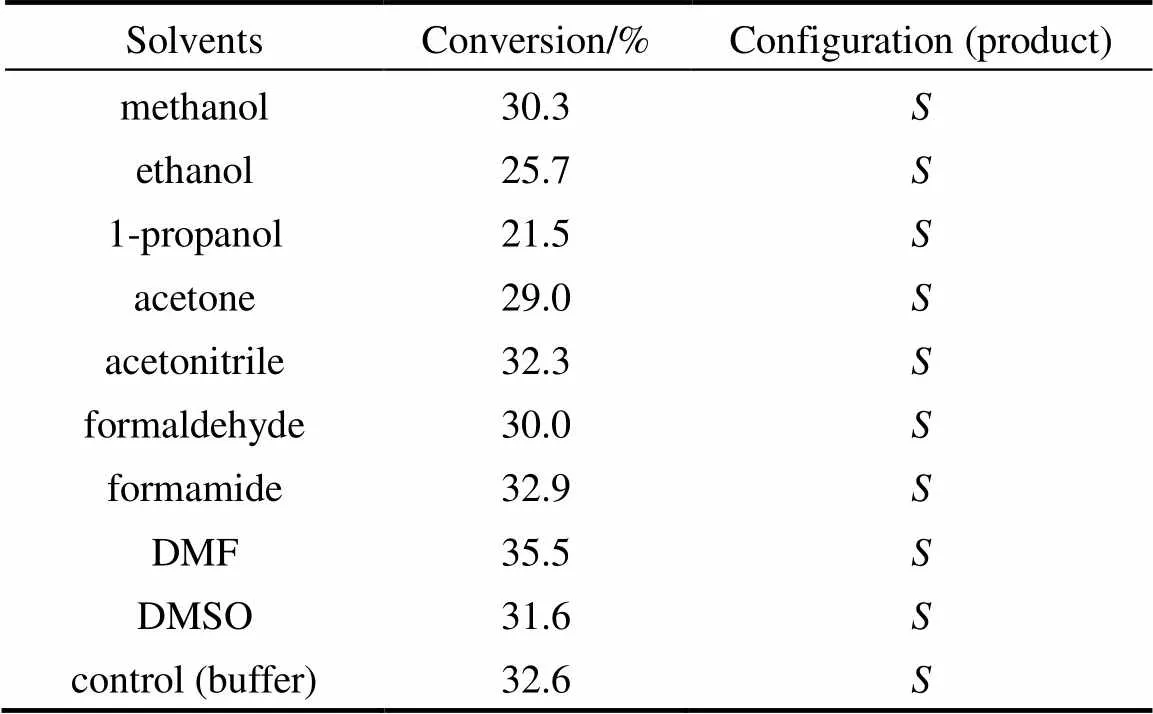
Table 1 Effects of water-miscible organic-solvents on the reactivity for kinetic resolution of ibuprofen methyl ester
Figure 4 shows the effect of DMF content in buffer solution on the conversion at 37ºC. In the range of 1%-12% (by volume) of DMF in buffer solution, the substrates were soluble completely. The catalytic activity of the catalytic antibody in the pure buffer solution was lower since the substrate was hardly soluble in it, so that the spontaneous and catalytic hydrolysis was hardly observed in pure buffer solution. By increasing the volume of DMF, the enantiomeric excess was maintained at high level (>99%). The conversion was enhanced by adding a small volume of DMF. The improvement in catalytic activity was observed at 4%-8% DMF. A further increase in the DMF volume resulted in decrease of conversion, because of the denaturation of the catalytic antibodies.
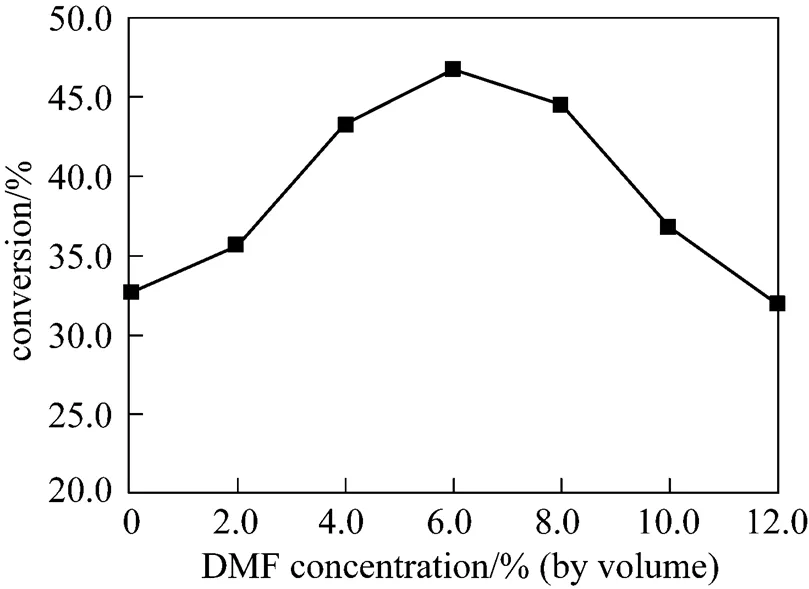
Figure 4 Effects of DMF content in buffer solution on kinetic resolution of ibuprofen methyl ester
3.3 Kinetic resolution of ibuprofen methyl ester bycatalytic antibody in water-miscible organic-solvent
Typical time courses of hydrolysis of ibuprofen methyl esters in buffer solution (pH 8.0) containing 6% (by volume) DMF at 37ºC are shown in Fig. 5. The substrate was hydrolyzed slowly and spontaneously at a very low rate, the hydrolysis rate was largely increased by the addition of a catalytic antibody, and further increased by the addition of DMF. Thus, the catalytic antibody in water-miscible organic-solvent showed higher catalytic activity than in the buffer system, and the reaction was completed after 10 h under this condition. The catalytic hydrolysis by a catalytic antibody both in the buffer solution and in the DMF buffer solution were for-ibuprofen methyl ester. These results indicate that the catalytic antibodies have enantioselectivity and substrate specificity.
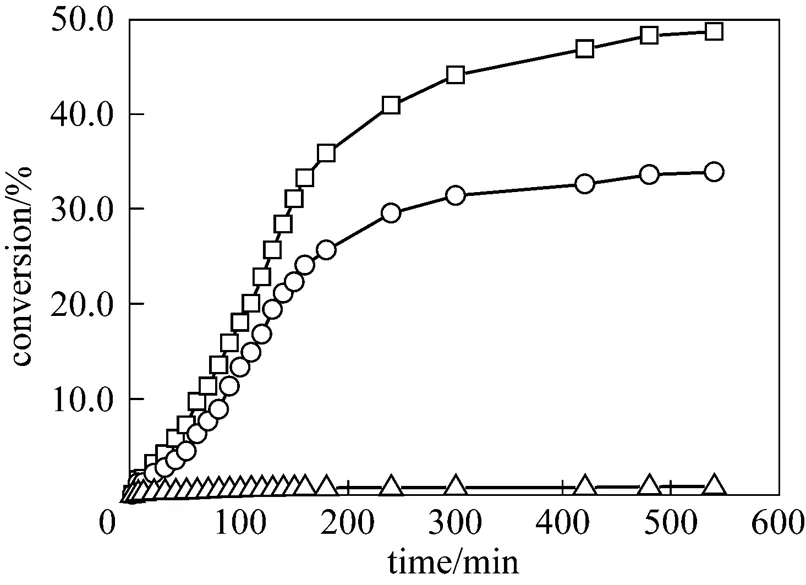
Figure 5 Typical time courses of hydrolysis of ibuprofen methyl ester at 37ºC□ hydrolysis in buffer solution with 6% DMF;○ hydrolysis in buffer solution;△ spontaneous hydrolysis
3.4 Michaelis-Menten kinetic parameters in catalytic hydrolysis of ibuprofen esters in the buffer solution with 6% DMF
Michaelis-Menten kinetics was studied in the buffer solution with 6% (by volume) of DMF at 37ºC. Fig. 6 shows the typical behavior of initial rates. Linear Lineweaver-Burk plots of the steady data give kinetic parameters ofmandcat, and the results are summarized in Table 2. In 6% (by volume) of DMF content, the catalytic antibody showscatof 3.05 min-1andmof 23.42 μmol·L-1. Compared with the rate constant of uncatalyzed reaction, thiscatvalue gives a 1.8×104fold increase. For the substrate,mvalues in the DMF buffer solution and in the aqueous medium are similar, but thecatvalue in the DMF buffer solution is slightly higher. The increase inmindicates a decrease in the affinity between the substrate and the catalytic antibodies. The increase in catalytic efficiency (cat/m) and inmcould be explained by dissolubility of the substrates, and the products in the buffer solution with polar solvent (DMF) are beneficial to the reaction. This result is similar to the resolution of ibuprofen ester bylipase [24]. Thus, the catalytic antibody can maintain the catalytic activity even in water-miscible organic-solvents, although the catalytic antibody is partly denatured in these solvents.
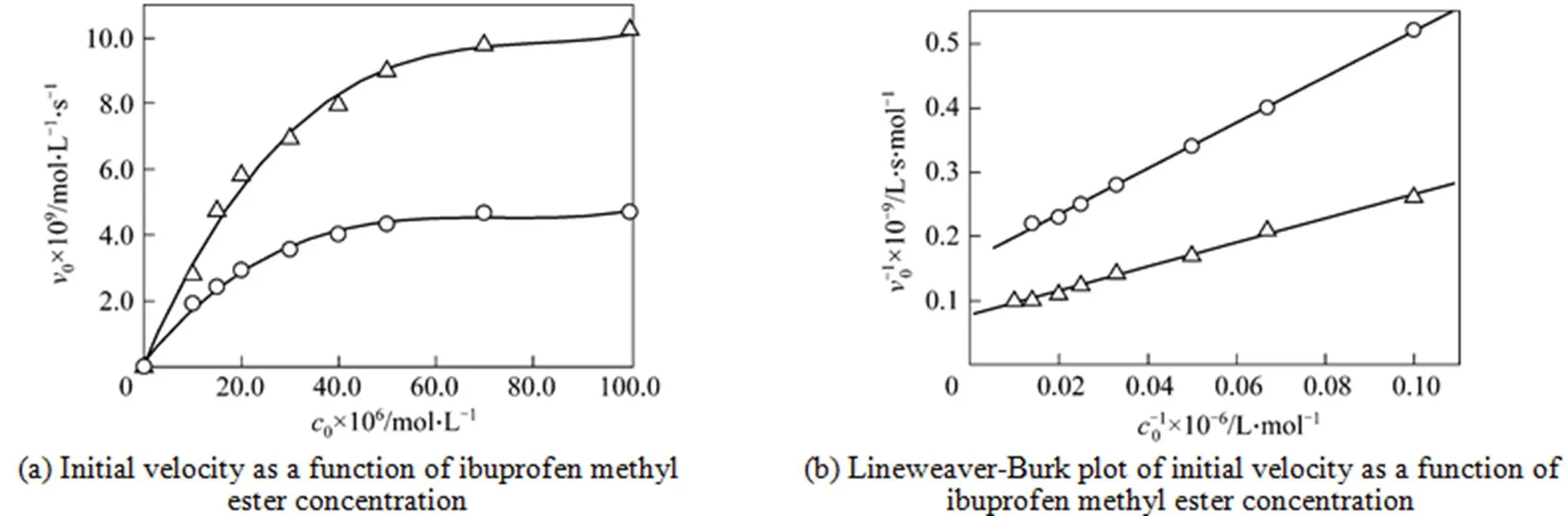
Figure 6 Michaelis-Menten kinetics of hydrolysis of ibuprofen methyl ester catalyzed by a catalytic antibody at 37°C△ hydrolysis in buffer solution with 6% DMF (with 0.25 μmol catalytic antibody, 0.2 mol·L-1phosphate buffer, pH 8);○ hydrolysis in buffer solution (with 0.25 μmol catalytic antibody, 0.2 mol·L-1phosphate buffer, pH 8)
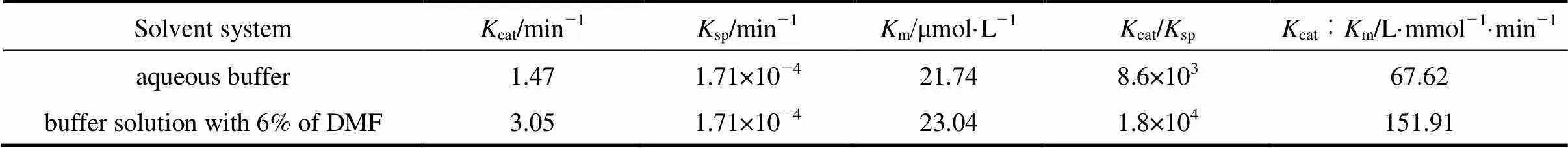
Table 2 Comparison of kinetic results in the buffer solution and in buffer solution with 6% (by volume) DMF at 37ºC
3.5 Effect of temperature on catalytic antibody activity
The influence of temperature on the activity of the catalytic antibody was investigated between 20 and 50ºC (Fig. 7). The DMF buffer solutions containing substrate were preincubated at each temperature before adding the catalytic antibody solution. The activity of the catalytic antibody reached a maximum between 30 and 40ºC but declined rapidly at higher temperatures. It is possibly because the antibody is of animal origin, higher temperature results in partial denaturation of the antibody protein.
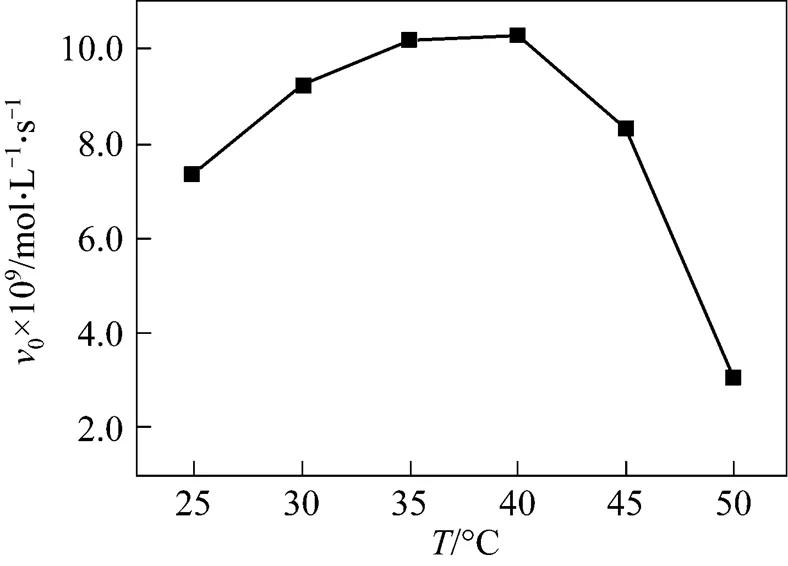
Figure 7 Effects of temperature on catalytic antibody catalytic activity (Reaction conditions: catalytic antibody concentration 0.25 μmol·L-1, ibuprofen methyl ester concentration 100 μmol·L-1, pH 8.0)
3.6 Comparison of resolution results of racemic ibuprofen by lipase and by catalytic antibody

Table 3 shows that the conversion and enantiomeric ratio are higher using catalytic antibody than using lipase, because the catalytic antibody is generated against special hapten. On the other hand, the resolution results in the hydrolysis of racemic ibuprofen esters demonstrate that the enantioselectivity of the catalytic antibody could be changed by altering the reaction medium. The selectivity enhancement achieved by the addition of polar organic cosolvents may be of practical importance for the catalytic antibody production of S-(+)-ibuprofen.
① The data from Ref. [24].
4 CONCLUSIONS
In this article, a catalytic antibody that accelerates the rate of enantioselective hydrolysis of ibuprofen methyl ester was successfully elicited against hapten 2. This study demonstrates that enantioselective hydrolysis can be catalyzed by antibodies generated against the carefully designed haptens. Since the catalytic antibodies are designed to catalyze the wide range organic reactions for lipophilic substrates, it is important to use antibodies in water-miscible or hydrophilic organic solvents. In this case, we have shown that water-miscible organic-solvent reaction conditions are compatible with preparative-(+)-ibuprofen with catalytic antibodies. A good conversion (48.7%) and high enantiomeric excess (>99%) have been obtained in the buffer solution with 6% DMF (containing catalytic antibody 0.25 μmol, 0.2 mol·L-1phosphate buffer, pH 8) at 37°C for 10 h. The kinetic analysis of the catalytic antibody-catalyzed reaction shows that the hydrolysis in the water-miscible organic-solvent system composed of DMF and buffer solutions follows the Michaelis- Menten kinetics. The catalytic efficiency (cat/m) is enhanced to 151.91 (L·mmol-1·min-1), which is two-fold increase compared with that for the buffer solution only. The conversion enhancement achieved by the addition of polar organic cosolvents may be of practical importance for the production of-(+)-ibuprofen catalyzed by catalytic antibodies. Although the catalytic activity of this antibody is not higher than natural enzymes, the ability of the antibodies to preserve their catalytic activity in nonaqueous media should significantly expand the versatility of antibodies as catalysts in biotechnological processes.
NOMENCLATURE
conversion rate, %
0substrate initial concentration, mol·L-1
enantiomeric ratio
enantiomeric excess of the product, %
catturnover number of catalytic antibody, min-1
cat/mcatalytic efficiency, L·mmol-1·min-1
mMichaelis-Menten constant, μmol·L-1
sprate constant for spontaneous reaction, min-1
0initial velocity, mol·L-1·s-1
1 Mayer, J.M., Testa, B., “Pharmacodynamics, pharmacokinetics and toxicity of ibuprofen enantiomers”,., 22, 1347-1366 (1997).
4 Mills, R.F., Adams, S.S., Cliffe, E.E., Dickinson, W., Nicholson, J.S., “The metabolism of ibuprofen”,, 3, 589-598 (1973).
5 Caldwell, J., Hutt, A.J., Gigleux-Fournel, S., “The metabolic chiral inversion and dispositional enantioselectivity of the 2-arylpropionic acids and their biological consequences”,.., 37, 105-114 (1988).
6 Williams, K., Day, R., Knihinicki, R., Duffield, A., “The stereoselective uptake of ibuprofen enantiomers into adipose tissue”,.., 35, 3403-3405 (1986).
7 Sheldon, R.A., Chirotechnology, Industrial Synthesis of Optically Active Compounds, Marcell Dekker, New York (1993).
8 Kumar, I., Manju, K., Jolly, R.S., “A new biocatalyst for the preparation of enantiomerically pure 2-arylpropanoic acids”,:, 12, 1431-1434 (2001).
9 Long, W.S., Kamaruddin, A.H., Bhatia, S., “Enzyme kinetics of kinetic resolution of racemic ibuprofen ester using enzymatic membrane reactor”,..., 60, 4957-4970 (2005).
10 Naik, P.U., Nara, S.J., Harjani, J.R., Salunkhe, M.M., “Ionic liquid anchored substrate for enzyme catalysed kinetic resolution”,...:., 44, 93-98 (2007).
11 Wang, Y.J., Hu, Y., Xu, J., Luo, G.S., Dai, Y.Y., “Immobilization of lipase with a special microstructure in composite hydrophilic CA/hydrophobic PTFE membrane for the chiral separation of racemic ibuprofen”,..., 293, 133-141 (2007).
12 Tramontano, A., Janda, K.D., Lerner, R.A., “Catalytic antibodies”,, 234, 1566-1570 (1986).
13 Pollack, S.J., Jacobs, J.W., Schultz, P.G., “Selective chemical catalysis by an antibody”,, 234, 1570-1573 (1986).
14 Tanaka, F., “Catalytic antibodies as designer proteases and esterases”,.., 102, 4885-4906 (2002).
15 Jovic, F., Louise, L., Mioskowski, C., Renard, P.Y., “Immunologically driven antibodies chemical engineering: Design and synthesis of a hapten aimed at nerve agent hydrolysis”,., 46, 6809-6814 (2005).
16 Reymond, J.L., Reber, J.L., Lerner, R.A., “Enantioselective, multigram-scale synthesis with a catalytic antibody”,....., 33, 475-477 (1994).
17 Durfor, C.N., Bolin, R.J., Sugasawara, R.J., Jacobs, J.W., Schultz, P.G., “Antibody catalysis in reverse micelles”,...., 110, 8713-8714 (1988).
18 Franqueville, E., Loutrari, H., Mellou, F., Stamatis, H., Friboulet, A., Kolisis, F.N., “Reverse micelles, a system for antibody-catalysed reactions”,...:., 21, 15-17 (2003).
19 Turner, J.M., Bui, T., Lerner, R.A., Barbas, C.F., List, B., “An efficient benchtop system for multigram-scale kinetic resolutions using aldolase antibodies”,..., 6, 2772-2774 (2000).
20 Harlow, E., Lane, D., Antibodies, A Laboratory Manual, Cold Spring Harbor Laboratory Press, New York (1988).
21 Bradford, M.M., “A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding”,.., 72, 248-254 (1976).
22 Guo, H.Y., Tang, L.H., Su, M., Xue, J.P., Xu, X.P., “Screening of a lipase with high enantioselectivity and its application in the resolution of racemic ibuprofen”,, 7, 1169-1174 (2007).
23 Chen, C.S., Fujimoto, Y., Girdaukas, G., Sih, C.J., “Quantitative analyses of biochemical kinetic resolutions of enantiomers”,...., 104, 7294-7299 (1982).
24 Lee, W.H., Kim, K.J., Kim, M.G., Lee, S.B., “Enzymatic resolution of racemic ibuprofen esters: Effects of organic cosolvents and temperature”,..., 80, 613-615 (1995).
2008-10-15,
2009-03-17.
the Natural Science Foundation of Zhejiang Province (Y404353).
** To whom correspondence should be addressed. E-mail: yaosj@che.zju.edu.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- On-line Monitoring for Phosphorus Removal Process and Bacterial Community in Sequencing Batch Reactor*
- Mechanism Study of Rice Straw Pyrolysis by Fourier Transform Infrared Technique*
- Simultaneously Designing and Targeting for Networks with Multiple Resources of Different Qualities*
- Modeling and Control of Nonlinear Discrete-time Systems Based on Compound Neural Networks*
- Effect of Doping Cerium in the Support of Catalyst Pd-Co/Cu-Co-Mn Mixed Oxides on the Oxidative Carbonylation of Phenol
- Biodegradation of Aniline by a Newly Isolated Delftia sp. XYJ6*
