Process Intensification of VOC Removal from High Viscous Media by Rotating Packed Bed*
2009-05-14LIWoyuan李沃源WUWei毋伟ZOUHaikui邹海魁CHUGuangwen初广文SHAOLei邵磊andCHENJianfeng陈建峰
LI Woyuan (李沃源), WU Wei (毋伟), ZOU Haikui (邹海魁), CHU Guangwen (初广文), SHAO Lei (邵磊) and CHEN Jianfeng (陈建峰)
Process Intensification of VOC Removal from High Viscous Media by Rotating Packed Bed*
LI Woyuan (李沃源), WU Wei (毋伟), ZOU Haikui (邹海魁), CHU Guangwen (初广文), SHAO Lei (邵磊) and CHEN Jianfeng (陈建峰)**
Research Center of the Ministry of Education for High Gravity Engineering and Technology, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029, China
The removal of a volatile organic compound (VOC) from high viscous liquid was carried out in a rotating packed bed (RPB) in this study. The mixed liquid of syrup and acetone was used as simulated high viscous polymer solution with acetone as the volatile compound. The influence of the rotating speed of RPB, liquid viscosity, liquid flow rate, vacuum degree, and initial acetone content in the liquid on acetone removal efficiency was investigated. The experimental results indicated that the removal efficiency increased with increasing rotating speed and initial acetone content in the viscous liquid and decreased with increasing liquid viscosity and flow rate. It was also observed that acetone removal efficiency increased with an increasing vacuum degree and reached 58% at a vacuum degree of 0.1 MPa. By the comparison with a flash tank devolatilizer, it was found that acetone removal efficiency in RPB increased by about 67%.
polymer devolatilization, rotating packed bed, high viscosity, mass transfer, volatile organic compound
1 INTRODUCTION
In the manufacture of a wide variety of polymers, the presence of impurities is very common, which are unwanted in final products. Such impurities typically include residual monomers, solvents that are used in the preparation of the polymers, and low molecular weight organic species such as dimers and trimers that may be formed during the polymerization process [1]. The removal of volatile compounds constitutes an important “unit operation” in the processing of polymeric materials. This operation is commonly referred to as “polymer devolatilization” since these compounds in most cases are volatile relative to their polymeric hosts and usually deleterious in polymer applications [2]. These volatiles will adversely affect the final use of polymeric products since they change the taste, color, toxicity, or thermophysical properties of polymers through plasticization or depolymerization [3]. For example, some polymers are used as food packaging where the migration of residual monomers from the polymer into the food will cause problems of undesirable odor, and/or flavor (taint). Thus, volatile impurity contents in polymers are strictly limited.
In many industrial processes, a great number of apparatuses and methods for the removal of volatile compounds from polymers are used. The volatile compounds are commonly removed from polymers in its molten state through a sudden pressure reduction (application of vacuum [4-6]), which causes the volatile compounds to be evaporated into a contiguous gas phase. Most equipment currently used to devolatilize polymer melts contains a rotating shaft for conveying and mixing. Examples are thin-film evaporators with pitched scraper blades [7-9], single-screw extruders, and twin-screw extruders [10-13], both corotating and counter-rotating types. The basic process elements, which comprise the active loci for devolatilization, are rotating melt pools and stationary melt layers or films in such equipment. Also, this kind of equipment is usually called “Surface Renewal Apparatuses”, which can increase mass transfer rate by enhancing the surface renewal rate and forming thin-film, namely, obtain better devolatilization efficiency in limited residence time.
The surface renewal rate and liquid film thickness in above equipment are affected by many factors, such as liquid viscosity and apparatus structure. The viscous liquid commonly includes low viscosity liquid (<5 Pa·s), medium viscosity liquid (5-50 Pa·s), high viscosity liquid (50-500 Pa·s), and superhigh viscosity liquid (>500 Pa·s) [14]. High and superhigh viscosity liquid usually has poor fluidity. Although increasing the liquid film thickness can enhance the fluidity of the liquid, it also increases the resistance of mass transfer, leading to a contradictory effect on devolatilization. Compared with above equipment, rotating packed bed (RPB) provides an ideal way to address this problem. Rotating packed bed is an apparatus consisting mainly of a packed rotator, which can enhance micromixing and mass transfer by the generation of a simulated high-gravity environmentthe action of centrifugal force. Rotating packed bed has found a variety of applications, such as nanomaterials preparation [15-18], polymer devolatilization [19, 20], absorption [21-24], distillation [25], and ozone oxidation [26, 27]. In RPB, high viscosity liquid flows rapidly in the forms of droplets, threads, and thin-films with fast surface renewal rate, which is favorable for the mass transfer and the removal of volatile compounds [19]. In addition, the physical size of RPB can be dramatically reduced, and as a result, the investment and operating costs can also be reduced.
Haw applied RPB to polymer devolatilization in the system of polystyrene/styrene/ethylbenzene with high viscosity (400 Pa·s) [19]. The devolatilization was performed at 260°C and at the pressure of 1333.22 Pa (10 mmHg), and the contents of styrene and ethylbenzene were reduced to less than 65×10-6(65 ppm) and 16×10-6(16 ppm), respectively. Yang. [20] used RPB for removing volatile compounds from a viscous liquid of less than 3 Pa·s, where a high pressure gas was introduced into the RPB peripherally and/or a suction force source was connected to a position near the rotation axis, so that a volatile component contained in the viscous liquid was entrained in the gas counter currently flowing through the RPB, and thus the volatile component was removed from the viscous liquid.
In this article, RPB was used for the removal of a volatile organic compound (VOC) from high viscous liquid with acetone as the VOC and syrup as the viscous liquid. The effects of operating parameters on acetone removal efficiency were investigated. In addition, a comparison of devolatilization effects between RPB and a laboratory-scale flash tank was carried out.
2 EXPERIMENTAL
2.1 Materials
Syrup containing 78% (by mass) of caramel and 22% (by mass) of water (8-100 Pa·s at 12°C) was purchased from Beijing Caramel Factory. Acetone (A.R.) was obtained from Beijing Chemical Corporation and used as received. Acetone was selected as the volatile component for avoiding the azeotropy, as well as the good dissolvability of acetone in syrup. The viscosity of syrup was measured using a HAAKE RS150L Rheometer (Germany).
2.2 Devolatilization
The packed rotator in the RPB has an inner radius of 4.6 cm, an outer radius of 11 cm, and an axial length of 3 cm. As a result, the radial thickness and the volume of the rotator are 6.4 cm and 947 cm3, respectively. Stainless wire mesh was used for the packing, which is composed of interconnected wires with a mean diameter of 0.22 mm and an average mesh diameter of 4 mm.
Figure 1 schematically depicts the experimental setup in this work. Rotating packed bed was connected to a vacuum system and the pressure, in the range of 1325.22 Pa (9.94 mmHg) to atmospheric pressure, was measured using a set of manometers. The rotating speed was controlled by a frequency modulation device. The acetone and syrup solution was stored in a tank and driven by nitrogen gas to flow into the inner side of the rotatora liquid distributor. Liquid stream moved outward through the packing under the action of the centrifugal force and was split into very small droplets, threads, and thin films, whereas acetone evaporated into the gas phase rapidly in this process. Finally, the liquid was collected at the liquid outlet of the RPB under atmospheric pressure.
The devolatilization was performed at 12°C. The rotating speed varied from 556 to 1390 r·min-1and the liquid flow rate from 44 to 164 ml·min-1, whereas the vacuum degree varied from 0.084 to 0.1 MPa. The initial content of acetone in syrup was maintained in the range of 0.33% to 0.81% (by mass) throughout all the experiments.
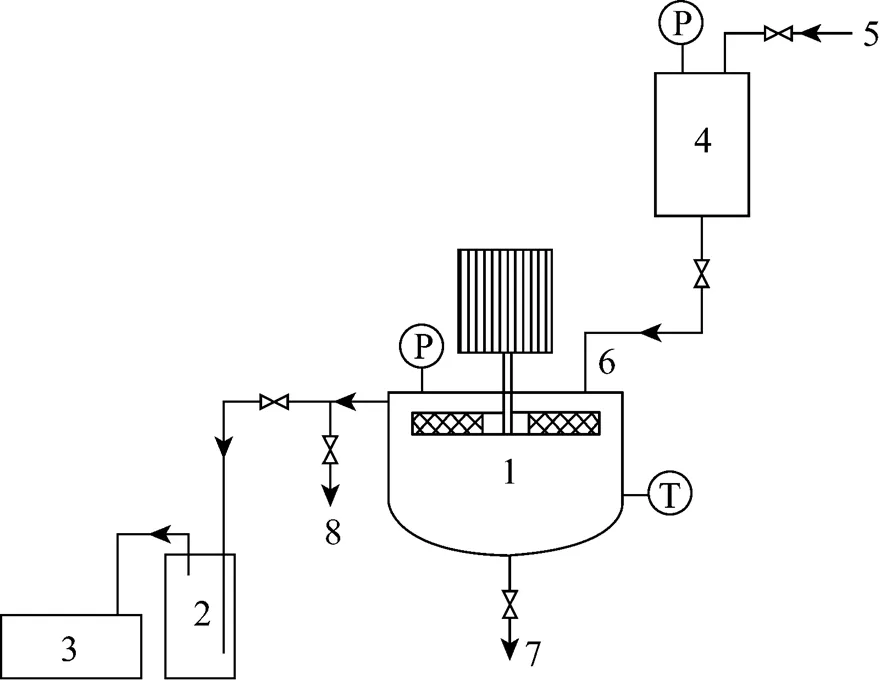
Figure 1 Experimental set-up for devolatilization 1—RPB; 2—surge flask; 3—vacuum pump; 4—tank; 5—nitrogen inlet; 6—liquid inlet; 7—liquid outlet; 8—vacuum degree control valve
2.3 Calculation of the volatile content and volatile removal efficiency
The mass percent of acetone before and after devolatilization was measured using a spectrophotometer (UV-2501PC, Shimadzu Corporation, Japan). The syrup and acetone solution was diluted by deionized water with the mass ratio of the solution to deionized water as 6 to 4. Acetone content was determined by UV-Vis absorption spectra of acetone at 265 nm and calculated by the following equation:
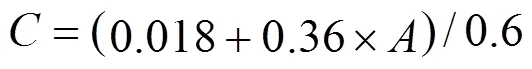
Acetone removal efficiency is defined as follows:
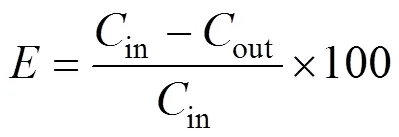
3 RESULTS AND DISCUSSION
3.1 Effect of rotating speed
Figure 2 illustrates the effects of the rotating speed on acetone removal efficiency. The vacuum degree, initial acetone content in syrup, liquid flow rate, and viscosity were 0.0987 MPa, 0.66% (by mass), 100 ml·min-1, and 70 Pa·s, respectively. It can be observed from Fig. 2 that acetone removal efficiency initially increased and then decreased with the increase of the rotating speed.
The high viscous media were thrown outwards by the centrifugal force in the high-gravity environment of RPB in the form of microsized or nanosized films, threads, and droplets, leading to a significant increase of the gas-liquid contact area. The high-gravity environment also enhances the turbulent motion and the surface renewal rate of the fluid. The high-gravity level can be calculated by the following equation:
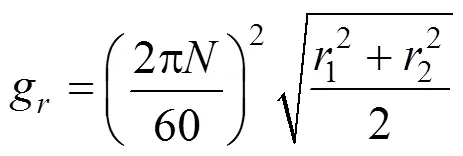
Equation 3 illustrates that the high-gravity level depends strongly on the rotating speed of RPB and varies with the second power of the rotating speed.
The residence time of liquid in the packing and the liquid film thickness decreased with the increase of the rotating speed, whereas acetone removal efficiency increased with an increasing residence time or decreasing liquid film thickness due to the decrease of the diffusion distance. The liquid film thickness decreased quickly as the rotating speed increased at low rotating speed, overwhelming the unfavorable effects of the decrease of the residence time and leading to the increase of acetone removal efficiency. However, according to the result of Munjal [28], as the rotating speed increased at high rotating speed, the liquid film thickness remained almost unchanged due to the high viscosity and shearing strength inside the liquid film, whereas the residence time of liquid decreased significantly, resulting in the decrease of acetone removal efficiency.
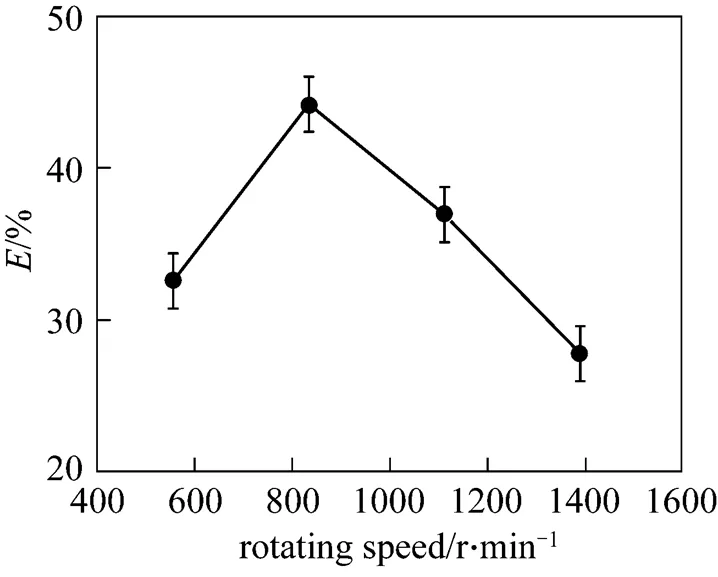
Figure 2 Effect of rotating speed on acetone removal efficiency
Furthermore, the surface renewal of high viscous liquid is required for promoting mass transfer of the volatile compounds with a concurrent displacement of the equilibrium composition. The removal of dissolved gases from polymer melts is also commonly carried out as a physical separation process in which surface renewal rate has an important effect [29]. Since the surface renewal rate of the liquid was enhanced by increasing the rotating speed of RPB, better acetone removal efficiency resulted.
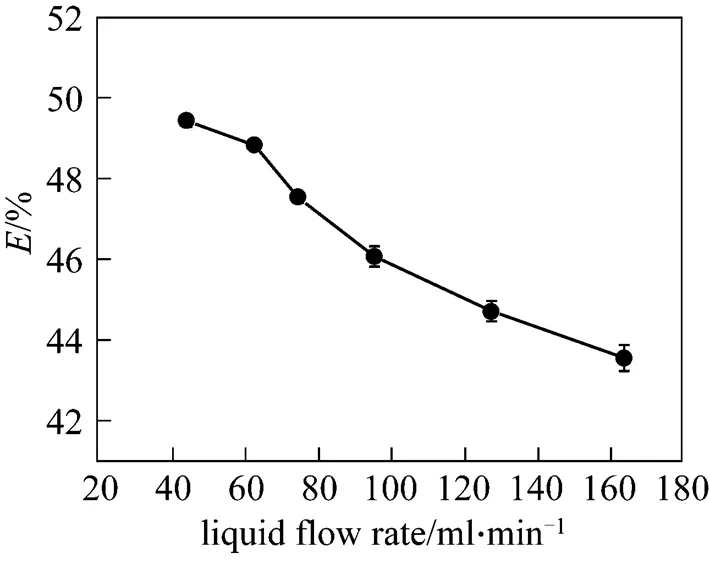
Figure 3 Effect of liquid flow rate on acetone removal efficiency
3.2 Effect of viscosity
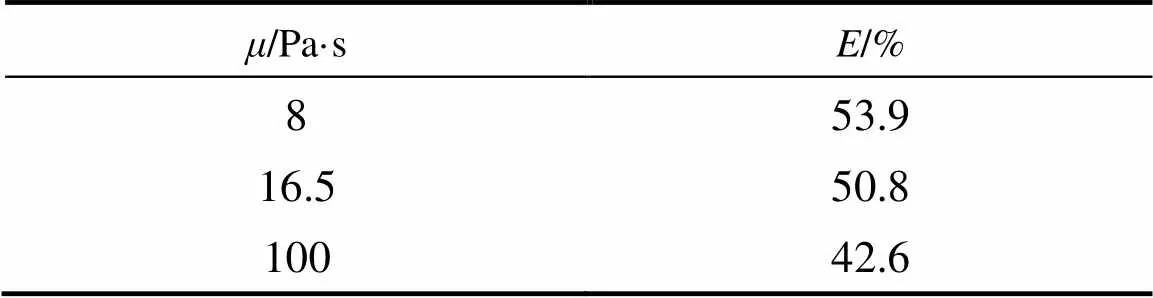
Table 1 lists the effect of viscosity on acetone removal efficiency. The vacuum degree, initial acetone content in syrup, rotating speed, and liquid flow rate were 0.0983 MPa, 0.79% (by mass), 1112 r·min-1, and 130 ml·min-1, respectively.
As shown in Table 1, acetone removal efficiency decreased with the increase of viscosity. The liquid film thickness in packing was calculated as follows [28]:
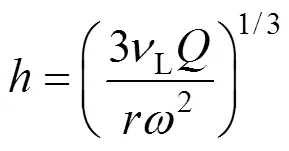
The liquid film thickness increased with the increase of viscosity, which is unfavorable for the devolatilization. Furthermore, the diffusion resistance also increased with the increase of viscosity, resulting in the deterioration of acetone removal efficiency.
3.3 Effect of liquid flow rate
Figure 3 presents the effect of liquid flow rate on acetone removal efficiency. The vacuum degree, initial acetone content in syrup, rotating speed, and viscosity were 0.0986 MPa, 0.81% (by mass), 1112 r·min-1, and 100 Pa·s, respectively. It can be seen from Fig. 3 that acetone removal efficiency decreased with an increasing liquid flow rate. According to Fick’s first law of diffusion, the molecular diffusion rate of acetone is a constant at certain temperature, vacuum degree, liquid viscosity, and initial acetone content. The liquid film thickness increased as the liquid flow rate increased, leading to the increase of the mass transfer resistance of acetone and the decrease of acetone removal efficiency.
3.4 Effect of vacuum degree
Figure 4 indicates the effect of vacuum on acetone removal efficiency. The initial acetone content in syrup, liquid flow rate, rotating speed, and liquid viscosity were 0.71% (by mass), 59 ml·min-1, 834 r·min-1, and 70 Pa·s, respectively. It was observed from Fig. 4 that the removal efficiency increased with the increase of the vacuum degree from 0.084 MPa to 0.1 MPa, where the saturated vapor pressure of acetone was higher than the pressure in RPB. It was observed that acetone removal efficiency increased with an increasing vacuum degree and reached 58% at a vacuum degree of 0.1 MPa. An increase in vacuum degree reduced the partial pressure of acetone in the gas phase, enhancing the driving force for acetone mass transfer from the liquid to the gas phase.
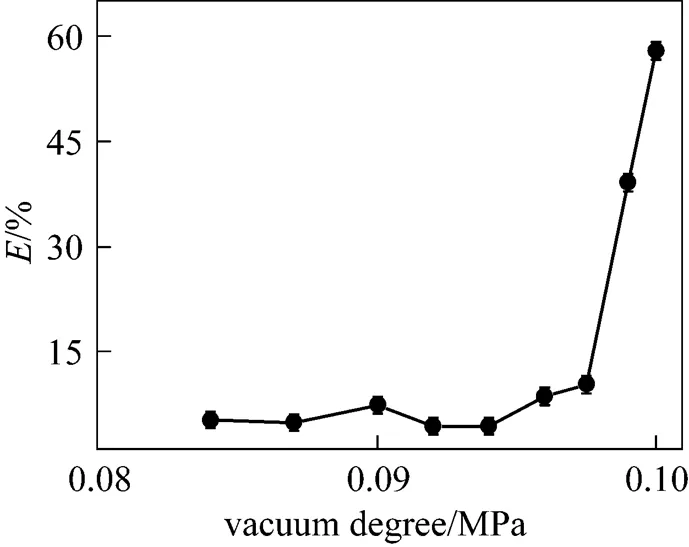
Figure 4 Effect of vacuum degree on acetone removal efficiency
As shown in Fig. 4, the sensitivity of acetone removal efficiency to the variations of vacuum degree at low vacuum degree was much lower than that at high vacuum degree. This may be attributed to the fact that the diffusion rate of acetone is slow and the diffusion flux is low at low vacuum degree. As the vacuum degree increased, the diffusion rate of acetone increased accordingly, leading to an increase in acetone removal efficiency.
3.5 Effect of initial acetone content in syrup
Figure 5 illustrates the effect of initial acetone content on acetone removal efficiency. The vacuum degree, liquid flow rate, rotating speed, and liquid viscosity were 0.098 MPa, 62.5 ml·min-1, 1112 r·min-1, and 100 Pa·s, respectively. It was found from Fig. 5 that acetone removal efficiency increased with the increase of the initial acetone content in syrup. Since the viscous liquid was split into very small droplets, threads, and films when flowing through the packing, the mass transfer of acetone inside the liquid can be neglected and acetone diffusion process can be assumed to carry out on the surface of liquid film, and acetone is removed immediately by the vacuum when entering the gas phase. The increase of acetone content enhanced the driving force for mass transfer on liquid film surface and boosted acetone removal efficiency.
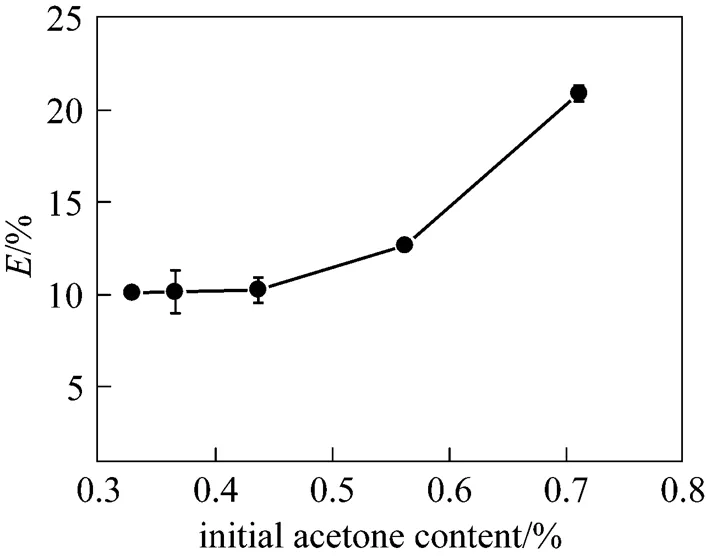
Figure 5 Effect of initial acetone content in syrup on acetone removal efficiency
3.6 Comparison of devolatilization effects of RPB and flash tank
Flash tank devolatilizer, devolatilizing extruder, and heavy-duty, wiped-film evaporator are commonly used to perform devolatilization. Among these three apparatuses, the flash tank devolatilizer has the simplest structure and been widely used commercially [4]. A flask equipped with a vacuum pump, agitating device, and temperature controller was used as a flash tank devolatilizer to compare with RPB for devolatilization effects. As shown in Table 2, acetone removal efficiency enhanced significantly from 6.1% (by mass) in the flash tank to 10.2% (by mass) in RPB, an increase by about 67%, indicating that RPB can boost the removal efficiency of volatile compounds from polymers and is an excellent apparatus for polymer devolatilization.
4 CONCLUSIONS
This work has investigated the devolatilization of high viscous liquid in RPB using acetone as the volatile compound and syrup as the viscous liquid. Experimental results indicated that RPB boosted the removal efficiency of volatile compound because the desorption of the volatile compound from the viscous liquid was enhanced due to the increase of the surface renewal rate of the liquid and the decrease of the mass transfer resistance of the volatile compound, which resulted from the very small liquid droplets, threads, and films generated in the packed rotator. By the comparison with the laboratory-scale flash tank devolatilizer, it was found that the removal efficiency of acetone in RPB increased by about 67%. These results demonstrated that RPB could be used as an ideal devolatilizer for volatile compound removal from high viscous liquid.

Table 2 Comparison of devolatilization effects of RPB and flash tank
NOMENCLATURE
height of the absorption peak of acetone at 265 nm in UV-Vis spectra
mass percent of acetone in the sample, %
inmass percent of acetone in the inlet liquid stream, %
outmass percent of acetone in the outlet liquid stream, %
acetone removal efficiency, %
ghigh-gravity degree, m·s-2
liquid film thickness, cm
rotating speed, r·min-1
vacuum degree, MPa
liquid flow rate per unit width on a flat surface, cm2·s-1
ddevolatilization rate, ml·min-1
radial distance, cm
1inner radius of packing, m
2outer radius of packing, m
temperature, °C
liquid viscosity, Pa·s
Lkinematic viscosity, cm2·s-1
rotational speed, rad·s-1
1 Craig, T.O., “Polymer devolatilization process”, U.S. Pat., 6410683 (2002).
2 Biesenberger, J.A., Lee, S.T., “A fundamental study of polymer melt devolatilization (I) Some experiments on foam-enhanced devolatilization”,..., 26 (14), 982-988 (1986).
3 Ye, S.M., Jiang, J.K., Jiang, C.Y., Pan, Q.M., “Dynamic supercritical fluid devolatilization of polymers”,...., 13 (6), 732-735 (2005).
4 Meister, B.J., Platt, A.E., “Evaluation of the performance of a commercial polystyrene devolatilizer”,...., 28, 1659-1664 (1989).
5 Yang, C.T., Smith, T.G., Bigio, D.I., Anolick, C., “A model for foam devolatilization in an extruder”,...., 37, 1464-1472 (1998).
6 Wang, H., Hossan, R.J., Gohr, E.T., “Method for removing volatile components from solid polymeric materials”, U.S. Pat., 6365710 (2002).
7 Matarrese, S., Rossi, G.A., Consolo, M.L., Cigna, G., Borghi, I., “Process for the continuous production in solution of styrene thermoplastic resins”, Eur. Pat., 0286071 (1988).
8 Feres, V., “Thin-film evaporators”, U.S. Pat., 4707220 (1986).
9 Parrillo, D., Singh, P., “Method for isolating polyphenylene ether polymer resins from solution”, U.S. Pat., 6860966 (2001).
10 Hasson, A., Gerhart, R.J., “Method for removing volatile substances from polyphenylene ether resin blends”, U.S. Pat., 5102591 (1989).
11 Khanin, D., Scarola, L.S., “A process for devolatilization”, Eur. Pat., 0920966 (1998).
12 Schmidt, L.R., Caraher, J.M., Maxam, J.L., “Method for extruder devolatilization of spiro(bis)indane polycarbonates”, U.S. Pat., 4980105 (1989).
13 Tukachinsky, A., Talmon, Y., Tadmor, Z., “Foam-enhanced devolatilization of polystyrene melt in a vented extruder”,., 40, 670-675 (1994).
14 Feng, L.F., Cao, S.F., Gu, X.P., Wang, K., “Highly viscous stirred polymerization reactor”,..., 24, 257-261 (2001). (in Chinese)
15 Wang, M., Zou, H.K., Shao, L., Chen, J.F., “Controlling factors and mechanism of preparing needle like CaCO3under high-gravity environment”,., 142, 166-174 (2004).
16 Chen, J.F., Shao, L., Zhang, C.G., Chen, J.M., Chu, G.W., “Preparation of TiO2nanoparticles by a rotating packed bed reactor”,...., 22, 437-439 (2003).
17 Chen, J.F., Shao, L., Guo, F., Wang, X.M., “Synthesis of nano-fibers of aluminum hydroxide in novel rotating packed bed reactor”,..., 58, 569-575 (2003).
18 Wang, D.G., Guo, F., Chen, J.F., Liu, H., Zhang, Z.T., “Preparation of nano aluminium trihydroxide by high gravity reactive precipitation”,..., 121, 109-114 (2006).
19 Chen, J.F., High Gravity Technology and Application—Novel Reactive and Separation Technology, Chemical Industry Press, Beijing (2002).
20 Yang, S., Lin, C.C., Tseng, I.M., Liu, W.T., Yu, H.T., “Method for removing volatile components from a high viscosity liquid by using rotation pack bed”, U.S. Pat., 6884401 (2005).
21 Lin, C.C., Su, Y.R., “Performance of rotating packed beds in removing ozone from gaseous streams”,..., 61, 311-316 (2008).
22 Lin, C.C., Jian, G.S., “Characteristics of a rotating packed bed equipped with blade packings”,..., 54, 51-60 (2007).
23 Lin, C.C., Wei, T.Y., Hsu, S.K., Liu, W.T., “Performance of a pilot-scale cross-flow rotating packed bed in removing VOCs from waste gas streams”,..., 52, 274-279 (2006).
24 Cheng, H.H., Tan, C.S., “Reduction of CO2concentration in a zinc/air battery by absorption in a rotating packed bed”,., 162, 1431-1436 (2006).
25 Li, X.P., Liu, Y.Z., Li, Z.Q., Wang, X.L., “Continuous distillation experiment with rotating packed bed”,...., 16 (4), 656-662 (2008).
26 Chen, Y.H., Chang, C.Y., Su, W.L., Chen, C.C., Chiu, C.Y., Yu, Y.H., Chiang, P.C., Chiang, S.I.M., “Modeling ozone contacting process in a rotating packed bed”,...., 43, 228-236 (2004).
27 Chen, Y.H., Chiu, C.Y., Chang, C.Y., Huang, Y.H., Yu, Y.H., Chiang, P.C., Shie, J.L., Chiou, C.S., “Modeling ozonation process with pollutant in a rotating packed bed”,...., 44 (1), 21-29 (2005).
28 Munjal, S., Dudukovic, M.P., Ramachandran P., “Mass-transfer in rotating packed beds (I) Development of gas-liquid and liquid-solid mass-transfer correlations”,..., 44 (10), 2245-2256 (1989).
29 Secor, R.M., “A mass transfer model for a twin-screw extruder”,..., 26, 647-652 (1986).
2008-11-07,
2009-02-17.
the National Natural Science Foundation of China (20821004), the National High Technology Research and Development Program of China (2006AA030202), and the Program for New Century Excellent Talents in University of China (NCET-07-0053).
** To whom correspondence should be addressed. E-mail: chenjf@mail.buct.edu.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- On-line Monitoring for Phosphorus Removal Process and Bacterial Community in Sequencing Batch Reactor*
- Mechanism Study of Rice Straw Pyrolysis by Fourier Transform Infrared Technique*
- Simultaneously Designing and Targeting for Networks with Multiple Resources of Different Qualities*
- Modeling and Control of Nonlinear Discrete-time Systems Based on Compound Neural Networks*
- Effect of Doping Cerium in the Support of Catalyst Pd-Co/Cu-Co-Mn Mixed Oxides on the Oxidative Carbonylation of Phenol
- Biodegradation of Aniline by a Newly Isolated Delftia sp. XYJ6*
