Effect of Doping Cerium in the Support of Catalyst Pd-Co/Cu-Co-Mn Mixed Oxides on the Oxidative Carbonylation of Phenol
2009-05-14LIANGYinghua梁英华GUOHongxia郭红霞CHENHongping陈红萍Jingde吕敬德andZHANGBobo张波波
LIANG Yinghua (梁英华), GUO Hongxia (郭红霞), CHEN Hongping (陈红萍), LÜ Jingde(吕敬德) and ZHANG Bobo (张波波)
Effect of Doping Cerium in the Support of Catalyst Pd-Co/Cu-Co-Mn Mixed Oxides on the Oxidative Carbonylation of Phenol
LIANG Yinghua (梁英华)1,*, GUO Hongxia (郭红霞)1,2, CHEN Hongping (陈红萍)1, LÜ Jingde(吕敬德)3and ZHANG Bobo (张波波)1
1School of Chemical Engineering and Biological Technology, Hebei Polytechnic University, Tangshan 063009, China2College of Light Industry, Hebei Polytechnic University, Tangshan 063000, China3Tangshan Zhongrun Coal Chemical Co., Ltd., Tangshan 063611, China
Effect of doping cerium in the support on the catalytic activity and side product of the reaction in the oxidative carbonylation of phenol to diphenyl carbonate (DPC) over the catalyst Pd-Co/Cu-Co-Mn mixed oxides was studied. The specific surface areas, crystal phase, valency, and content of the element on the surface of the catalysts were determined, and the products were detected by gas chromatograph/mass spectrometry (GC-MS). It is found that the catalyst without Ce shows higher activity than that with Ce, and the yields of DPC for the two catalysts can reach 30% and 23%, respectively. However, doping cerium can prevent the formation of 2-hydroxyphenyl benzoate and-bromophenyl phenyl carbonate.
diphenyl carbonate, oxidative carbonylation, doping cerium, sol-gel method
1 INTRODUCTION
Polycarbonates (PCs) are the important engineering thermoplastics and substitutes for metal and glass, which have excellent mechanical, electrical and optical properties [1]. In recent years, there has been an increasing demand for safer and environmentally favorable processes for PC synthesis. The transesterification process using 2,2-bis(4-hydroxyphenyl)-propane (bisphenol A) and diphenyl carbonate(DPC) instead of the interfacial polycondensation of diphenols with phosgene has been expected because of the following advantages: no toxic phosgene, no solvent, and no salt formation. Therefore, the synthesis of DPC has received an increasing interest in recent years [2-4].
Several methods have been developed for manufacturing DPC [2-4]. Among them, oxidative carbonylation of phenol with CO and O2has attracted keen attention in recent years, since it is a one-step process with H2O being the sole by-product in theory, and the use of highly toxic phosgene can be avoided.
Homogeneous palladium compounds with cocatalystshave been reported to be the highly efficient catalytic system for this process [5, 6]. To facilitate the separation of catalysts from products and increase the thermal stability and amenability of the catalyst in the continuous processing, heterogenization of homogeneous catalysts was also explored [7-16]. Iwane. [17] used palladium catalysts supported by porous carriers, such as carbon, alumina, silica and the like, to promote the synthesis of DPC and the yield of DPC could reach 12.6%. Heterogeneous Pd supported on activated carbon (Pd/C) has been studied for the oxidative carbonylation of phenol to DPC widely. Takagi. [18] reported that 9.55% DPC yield was obtained when using a Pd/C-Pb-NMe4Br catalytic system. Song. [19] studied the effects of the amount of various inorganic cocatalysts and bases coupled with Pd/C catalyst, and investigated an optimized catalytic system for the reaction. To find out the optimized catalyst compositions with the heterogeneous Pd/C catalyst system, DPC yields were compared with different ratios of Ce(OAc)3/Pd and Bu4NBr/Pd. The highest DPC yield of 26.8% could be obtained using the catalyst with optimum ratios of the catalytic components. Although there had been some achievement already, the yield of DPC was still relatively low.
It was reported that Ce played a vital role on the selectivity and life of the catalyst, which was used for inorganic redox cocatalyst, but the selectivity of the catalyst required to be improved further [20, 21]. Goyal. [21] investigated the effect of inorganic redox cocatalyst in detail. Among the various metal complexes studied, Cu(OAc)2and Ce(OAc)3×H2O were found to be the most efficient in terms of DPC yield. However, Cu(OAc)2led to formation of-phenylene carbonate as a byproduct, and Ce(OAc)3×H2O gave DPC in a yield comparable to that obtained with Cu(OAc)2without any-phenylene carbonate formation.
In order to overcome the drawbacks listed above, the Cu-Co-Mn mixed oxides were prepared by sol-gel method [22], which were used as the support of catalyst for direct synthesis of DPC by heterogeneous catalytic reaction. In this paper, the effect of doping cerium into the support on the catalytic activity and byproduct of the reaction in the oxidative carbonylation of phenol was studied.
2 EXPERIMENTAL
2.1 Support preparation
The Cu-Co-Mn mixed oxide supports were prepared by sol-gel method. Cu(OAc)2·H2O, Co(OAc)2·4H2O, Mn(OAc)2·4H2O, Ce(OAc)3·3H2O, citric acid, and ethanediol were used as the raw materials. Cu(OAc)2·H2O, Co(OAc)2·4H2O, Mn(OAc)2·4H2O, citric acid were dissolved with distilled water, and a series of concentrations of them were prepared as 1.5, 1.5, 1.5, 3.0 mol·L-1, respectively. When the cerium- doped support was prepared, Ce(OAc)3×3H2O was also dissolved with concentration of 0.75 mol·L-1. The above solutions were mixed together, and about 2 ml ethanediol was added. The mixtures were stirred for 12 h in the water bath at 85°C, and the pH value was adjusted to ~10 by dropwise addition of aqua ammonia. Then they were dried at 85°C in vacuum overnight and the precursor was obtained. Finally the precursor was calcined at 600°C for 6 h to prepare black nanometer powder support. The cerium-undoped and cerium-doped supports were named as a and b for short, respectively.
2.2 Catalyst preparation
The catalysts were prepared by impregnation method, which used the supports prepared above. PdCl2was the key catalyst and Co(OAc)2·4H2O was the cocatalyst. The catalysts prepared by supports a and b were named as A and B for short, respectively.
Catalyst A was prepared by impregnating 1 g support a in 5 ml aqua ammonia solution of PdCl2and Co(OAc)2×4H2O with concentration of 0.034 and 0.24 mol·L-1, respectively. Then the mixture was aged in air at the room temperature for 13 h. The powder was recovered by vaporizing the water in vacuum at 55°C for 2 h. Finally the catalyst was calcined at 600°C for 6 h.
Catalyst B was prepared by impregnating support b and the procedure was similar to the preparation of catalyst A.
2.3 Catalyst characterization
The specific surface areas (BET, m2·g-1) of all samples were determined on a constant volume adsorption apparatus (Gemini V 3365/2380) by the N2BET method at liquid nitrogen temperature.
The size of the particles and dispersibility of catalyst were measured by the transmission electron microscopy (TEM, JEM-100CX II).
The X-ray power diffraction (XRD) patterns were obtained at the room temperature using BDX-3200 with Ni-filtered Cu Kαradiation. The X-ray tube was operated at 40 kV and 20 mA. 2angle was scanned from 10° to 90°.

The element content of the catalysts was determined by energy dispersive X-ray spectroscopy (EDS) performed in conjunction with a scanning electron microscope (SEM, KYKY-2800).
2.4 Catalyst evaluation

The reaction products were quantified by a capillary gas chromatography (GC7900) with a FID detectorand identified by gas chromatography/mass spectrometry (Hewlett Packard 5971, GC-MS), respectively.
3 RESULTS AND DISCUSSION
3.1 Effect of cerium doping on the catalytic activity
3.1.1
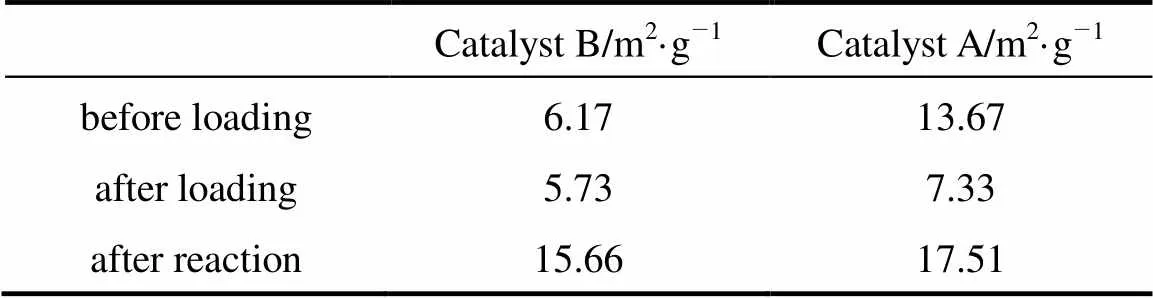
Table 1 shows the specific surface areas of the catalysts. The results indicate that the specific areas of catalyst A are bigger than those of catalyst B in any case, which includes before loading, after loading and after reaction. The reason may be that doping cerium in the support of the catalyst makes the surface of the catalyst get together, so the specific surface areas decrease. In addition, for any one of the two catalysts, the specific surface areas get smaller after loading compared with before loading. The result implies that some Pd species may enter into the channels. However, the specific surface areas after reaction is higher than those after loading, even higher than those before loading due to the addition of 4A molecular sieve (used as desiccant) with very high surface areas.
In order to investigate the size and dispersibility of particles in the catalyst after loading, the TEM images are shown in Fig. 1. The results show that the size of the particles in both cases is in the range of 40-50 nm. However, it can be seen clearly that catalyst A has more dispersive particles than catalyst B, which consists with the results of specific surface areas mentioned above. Doping cerium makes the particles of the catalyst aggregate to larger ones with ununiformity.

Figure 1 TEM images of the catalysts after loading
3.1.2

Regarding catalyst B, the patterns show the peaks corresponding not only to complex oxides Co2MnO4, Pd0.5Pd3O4, and SiO2, but also to CeO2. It can be seen that Ce is not fused with other crystal phases and exists as CeO2all the time.

Figure 2 XRD patterns of catalysts △ CeO2; # SiO2;○ Co2MnO4; * Pd0.5Pd3O4
3.1.3
The valency of catalysts was analyzed with XPS. The peak deconvolution fits were used to study the oxygen content in the crystal lattice and surface. The results are shown in Figs. 3 to 6. The XPS peaks (Fig. 3) illustrate that the catalyst A contains Pd, Cu, Co, Mn, O, and C, and the catalyst B contains Ce besides the elements included in the catalyst A. Ce3dof catalyst B corresponds to Ce (IV), which exhibits a peak near 881.9eV.
As for Cu(Fig. 4), it exists as Cu (I) and Cu (II) mostly in catalyst A, because the binding energies are 930.9eV and 934.5eV, respectively. However, it is mainly Cu (I) in catalyst B for the binding energy of 931.5eV.
Mn is mostly existed as Mn(III) in catalysts A and B, in which the peak location are 641.9eV and 641.5eV, respectively (Fig. 4).
Co is mainly existed as Co (II) in catalysts A and B due to the binding energies of 780.3eV and 780.0eV, separately (Fig. 4).
As for Pd (Fig. 5), it mainly exists as Pd (II). However, the binding energy of Pd3din catalyst A(337.5eV) is higher than that in catalyst B (336.0eV). The reason might be that part of Ce interacts with Pd. According to the electronegativity value scaled by Pauling, Cu (1.9), Co (1.9), and Mn (1.5) have stronger electronegativity than Ce(1.0). When they react with Pd (2.2), the density of electrons around the Pd atomic nucleus provided by Ce is higher than that supplied by Cu, Co, and Mn. Thus, the electrostatic attraction between Pd nucleus and electrons in the 3d orbit caused by Ce is lower than that caused by Cu, Co, and Mn. In conclusion, cerium doping makes the binding energy of Pd3ddecrease.

The change of active element contents in catalysts before and after reaction was determined by EDS. The results are summarized in Table 2. It can be seen that the dissolution of Co and Mn is not severe as a whole, which may be attributed to the exist of stable crystal phase Co2MnO4, but the leaching of Pd and Cu is still serious. However, the active constituent in catalyst B is easier to leach to the solvent than that in catalyst A during the reaction. The reason lies in that the surface areas of catalyst B is smaller than catalyst A, and the dispersivity of particles in catalyst B is not better than that in catalyst A as shown in Section 3.1.1.

Figure 3 Comparison of catalyst A and catalyst B’s XPS spectra
Figure 4 Comparison of catalyst A and catalyst B’s Co2p, Mn2p, and Cu2pXPS spectra

Figure 5 Comparison of catalyst A and catalyst B’s Pd3dXPS spectra
Figure 6 Comparison of O1sXPS spectra of catalyst A and catalyst B
3.1.4
The catalytic activity of the two catalysts is investigated at the same conditions. The activity of catalyst A is higher than that of catalyst B, and the yields of DPC can reach about 30% and 23%, respectively. The reasons may be the following.
First, the surface area of catalyst A is higher than that of catalyst B and the dispersivity of particles in catalyst A is also better than that in catalyst B, leading to more active centers of the former than those of the latter. Cerium doping makes the particles of the catalyst aggregate to larger ones with ununiformity, so it shows negative effect on the catalytic activity.
Second, the crystal phase of catalyst A is mainly Co2MnO4, and the negative electron center formed in the crystal is caused by the low valence Mn (III). In order to maintain charge equilibrium, oxygen vacancies compensate the charge difference. The increase of oxygen vacancies destroys the crystal integrity and prompts the oxygen adsorption on the crystal surface. This accelerates the oxygen gas transition to crystal oxygen, and the regeneration of catalyst is also improved. However, the crystal phases in catalyst B are mainly Co2MnO4and CeO2, and Ce (IV) makes crystal phase CeO2maintain charge equilibrium. As a result, the content of Co2MnO4in catalyst B is lower than that in catalyst A, so oxygen vacancies in catalyst B are lower than those in catalyst A.
Third, the binding energy of Pd3din catalyst A is higher than that in catalyst B. The high oxidation state of the Pd species is responsible for the enhancement of the catalytic activity [24].

Table 2 Contents of active element in catalysts
3.2 Effect of cerium doping on the side product of oxidative carbonylation of phenol
Under the present conditions, there were some byproducts formed along with DPC. Unlike the results reported by Goyal. [21], the major byproducts were 2-hydroxyphenyl benzoate (molecular formula, C13H10O3) and-bromophenyl phenyl carbonate (molecular formula, C13H9BrO3) for catalyst A. In addition, phenyl acetate and tributylamine were also detected by GC-MS. However, phenyl salicylate, which was mentioned as one of the main byproducts in many literatures, was not found. For catalyst B, in addition to phenyl acetate and tributylamine, there was no other byproduct detected. It can be seen that cerium doping is not able to improve the catalytic activity, while it can prevent the formation of 2-hydroxyphenyl benzoate and-bromophenyl phenyl carbonate, which is important to the separation of the products.
It should be pointed out that there were still some ‘unknown’ compounds identified by GC-MS in the reaction solution. They were called as ‘unknown’ because their formation mechanisms were not known yet and GC-MS could not confirm them for there were multiprobabilities for the results of GC-MS. It might be the oxidation product of phenol.
4 CONCLUSIONS
In summary, cerium doping has an important effect on the catalytic activity and byproduct of the reaction in the oxidative carbonylation of phenol to diphenyl carbonate over the catalyst Pd-Co/Cu-Co-Mn mixed oxides.

(2) For byproduct of the reaction, cerium doping can prevent the formation of 2-hydroxyphenyl benzoate and-bromophenyl phenyl carbonate.
1 Meenakshi, G., Ritsuko, N., Mitsuru, U., “Direct synthesis of diphenyl carbonate by oxidative carbonylation of phenol using Pd-Cu based redox catalyst”,...:., 137, 147-154 (1999).
2 Meenakshi, G., Ritsuko, N., Sugiyama, J., “Effect of inorganic redox cocatalyst on Pd-catalyzed for direct synthesis of diphenyl carbonate”,.., 54, 29-31 (1998).
3 Hirotoshi, I., Mistsuru, U., Kazuhiko, T., “Oxidative carbonylation of phenol carbonate catalyzed by Pd-Sn complexes with redox catalyst”,...:., 138, 311-313 (1999).
4 Hirotoshi, I., Mistsuru, U., Kazuhiko, T., “Oxidative carbonylation of phenol carbonate catalyzed by Pd-Sn heterotrinuclear complexes along with Mn redox catalyst without any addition of ammonium halide”,...:., 144, 369-372 (1999).
5 Liu, H.W., Zhao, Q., Zhao, X.Q., “Oxidative carbonylation of phenol to diphenyl carbonate over palladium complexes catalysts”,.. 34 (1), 47-49 (2002). (in Chinese)
6 Okuyama, K., Sugiyama, J., Nagahata, R., “Oxidative carbonylation of phenol to diphenyl carbonate catalyzed by palladium-carbene complexes”,...., 203, 21-27 (2003).
7 Buysch, H.J., Hess, C., Jentsch, J.D., “Supported platinum-group metal preparation of diaryl carbonates”, EP 736325 (1996).
8 Buysch, H.J., Jentsch, J.D., Rechner. J., “Supported platinum catalysts and process for preparation of diary carbonates”, EP 736324 (1996).
9 Hesse, C., Nothesis, U., Rechner. J., “Supported platinum-group metal for producing diaryl carbonates form carbon monoxide”, WO 9908786 (1999).
10 Takagi, W., “Preparation of aromatic carbonates as materials for polycarbonates”, JP 09278716 (1997).
11 Xue, W., Wang, Y.J., Zhao, X.Q., “Oxidative carbonylation of phenol to diphenyl carbonate over supported embedded catalyst Pd-Cu-O/SiO2prepared through W/O microemulsion method”, In: The proceedings of the 13th International Conference on Catalysis, Paris, 7, 1-321 (2004).
12 Xue, W., Zhao,X.Q., Wang,Y.J., “Effect of promoter copper on the oxidative carbonylation of phenol over the ultrafine embedded catalyst Pd-Cu-O/SiO2”,...:., 232, 77-81 (2005).
13 Zhang, G.X., Wu, Y.X., Ma, P.S., Wu, G.W., Li, D.H., “Study on direct synthesis of diphenyl carbonate with heterogeneous catalyzing reaction (I) Effect of method of preparing Pd catalyst carriers on catalyst activity”,..., 23 (2), 130-132 (2002). (in Chinese)
14 Zhang, G.X., Ma, P.S., Wu, Y.X.,Wu, G.W., Li, D.H., “Study on direct synthesis of diphenyl carbonate with heterogeneous catalyzing reaction (II) Effect of calcination temperature on catalytic activity of catalyst”,...., 30 (4), 362-367 (2002). (in Chinese)
15 Zhang, G.X., Wu, Y.X., Ma, P.S., Wu, G.W., Li, D.H., “Study on direct synthesis of diphenyl carbonate with heterogeneous catalyzing reaction (III) Effect of lanthanum content on catalytic activity of catalyst”,....,16 (4), 292-297 (2002). (in Chinese)
16 Zhang, G.X., Wu, Y.X., Ma, P.S., Wu, G.W., Li, D.H., “Study on direct synthesis of diphenyl carbonate with heterogeneous catalyzing reaction (IV) Effect of active components and the supporting methods on catalytic activity of catalyst”,...., 23 (5), 410-416 (2002). (in Chinese)
17 Iwane, H., Miyagi, H., Imada, S., “Method of producing aromatic carbonate”, EP 0614876 (1993).
18 Takagi, M., Miyagi, H., Yoneyama, T., “Palladium-lead catalyzed oxidative of phenol”,...:., 129, 1-3 (1998).
19 Song, H.Y., Yark, D., Lee, J.S., “Oxidative carbonylation of phenol to diphenyl carbonate over supported palladium catalysts”,...:., 154, 243-250 (2000).
20 Zhang, G.X., Wu, Y.X., Ma, P.S., Tian, Q.F., Wu, G.W., Li, D.H., “Study on direct synthesis of diphenyl carbonate with heterogeneous catalytic reaction (VI) Effect of Sn loading method and content on activity of Sn-Pd supported catalyst”,...., 12 (2), 191-195 (2004).
21 Goyal, M., Nagahata, R., Sugiyama, J., “Effect of inorganic redox cocatalysts on Pd catalyzed oxidative carbonylation of phenol for direct synthesis of diphenyl carbonate”,.., 54 (3), 29-36 (1998 ).
22 Guo, H.X., Chen, H.P., Liang, Y.H., Rui, Y.L., Lü, J.D., Fu, Z.D., “Direct synthesis of diphenyl carbonate with heterogeneous catalyst and optimal synthesis conditions of the support prepared by sol-gel method”,...., 16 (2), 223-227 (2008).
23 Wu, G. W., Wu, Y.X., Ma, P.S., Jin, F., Zhang, G.X., Li, D.H., Wang, C.W., “Preparation of substitution-structure perovskite carriers and activity evaluation for synthesis of diphenyl carbonate”,.., 39 (4), 385-400 (2006).
24 Bi, Y.S., Lü, G.X., “Influence of transition metal additives on CO oxidation over NaZSM-5 supported Pd”,...., 62 (20), 1981-1987 (2004).
2008-10-17,
2008-12-05.
* To whom correspondence should be addressed. E-mail: Liangyh64@yahoo.com.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Position Group Contribution Method for Estimation of Melting Point of Organic Compounds
- Process Intensification of VOC Removal from High Viscous Media by Rotating Packed Bed*
- Adsorption of Dye from Wastewater by Zeolites Synthesized from Fly Ash: Kinetic and Equilibrium Studies*
- Modeling of Isomerization of C8 Aromatics by Online Least Squares Support Vector Machine*
- Resolution of Ibuprofen Ester by Catalytic Antibodies in Water-miscible Organic-solvents*
- Reaction Characteristics of Asymmetric Synthesis of (2S,5S)-2,5-Hexanediol Catalyzed with Baker’s Yeast Number 6*
