The Effect of Hydrophobic Modification of Zeolites on CO2 Absorption Enhancement*
2009-05-14LUSumin卢素敏MAYouguang马友光ZHUChunying朱春英SHENShuhua沈树华andHEQing何清
LU Sumin (卢素敏), MA Youguang (马友光)**, ZHU Chunying (朱春英) SHEN Shuhua (沈树华) and HE Qing (何清)
The Effect of Hydrophobic Modification of Zeolites on CO2Absorption Enhancement*
LU Sumin (卢素敏)1, MA Youguang (马友光)2,**, ZHU Chunying (朱春英)2, SHEN Shuhua (沈树华)2and HE Qing (何清)1
1Department of Material and Chemical, Tianjin Polytechnic University, Tianjin 300160, China2School of Chemical Engineering and Technology, State Key Laboratory of Chemical Engineering, Tianjin University, Tianjin 300072, China
Two methods of the modification of zeolite were employed: framework element modification and surface coating, and the influence of the zeolites before and after modification on the CO2absorption was investigated. It was found that although hydrophobicity of zeolite could be obtained by means of the surficial organic coating in the method of surface coating modification, partial channel of zeolite would be plugged, as a result, leading to the surface area reducing greatly. Distinctively, the framework element modification method could maintain not only complete lattice structure and adsorption capability of zeolite, but would also obtain a good hydrophobic property. Consequently, significant enhancement on gas absorption by this modified zeolite was achieved and up to a maximum enhancement factor of 2.62. This shows that the solid particles with good enhancement role to gas absorption need not only good adsorptive capability but also certain hydrophobicity. An unsteady heterogeneous model was employed to predict enhancement factor and the calculated results agree well with the experimental data.
zeolite, modification, enhancement of gas absorption, hydrophobicity
1 INTRODUCTION
Many researches [1-3] have reported that mass transfer rate may be enhanced significantly by the presence of certain fine solid particles. Various mechanisms and models were developed to explain the effect. According to Demmink. [4] and Wimmer. [5, 6], the solid particles with enhancement effect to gas absorption should be hydrophobic and tend to adhere themselves to the gas-liquid interface. The hydrophobic solid particle commonly used is activated carbon [7, 8], which requires to be pretreated before usage due to the strong adsorption to organic molecules, and accordingly its application is limited. Zeolite is also an adsorbent available commercially and easy to get with a high adsorption affinity to many gases. Undesirably, little enhancement of gas absorption was observed in aqueous solutions due to its strong hydrophilicy. Recently, hydrophobic zeolites have gained much attention due to their ability to selectively remove organic pollutants from the air stream [9]. However, study on their absorption enhancement effect has not been reported. In this study, hydrophobic modification of zeolite was carried out by different methods, and the influence of zeolite before and after modification on gas absorption was investigated experimentally. An unsteady heterogeneous model was employed to predict the enhancement factors.
2 ExperimentAL
2.1 Modification of zeolite
Two modification methods were employed. (1) Framework element modification by which the hydrophobicity was improved through changing the silica- alumina ratio in the skeleton in two ways: (a) acid treatment, the slurry of zeolite with suitable water added was boiled, then fitting HCl (concentration varied from 1mol·L-1to 8 mol·L-1) was dripped gradually and refluxed for 3 h at 100°C, followed by filtrating, washing, drying, and finally calcining for 5 h at 550°C; (b) hydrothermal synthesis combined with acid treatment, zeolite was treated with steam at 550°C in a fixed-bed reactor to dealuminate from the structural skeleton and then dissolving the aluminum residues in the micropores with HCl. The product obtained was then filtrated, dried, and calcinated for 5 h at 500°C to give modified zeolites. (2) Surface coating method, the surface of zeolite was coated by organic molecules without changing silica-alumina ratio. A certain amount of raw zeolite was put into cyclohexane solution with ethyl orthosilicate (PDMS) of a certain concentration, and then impregnated for 18 h at room temperature under electromagnetic stirring. The resulting sample was filtrated and dried at 110°C for 3 h to finally obtain the modified zeolite.
The silica-alumina ratios were measured by GENESIS EDS from EDAX Corp. of US. The specific surface area was determined using a Gemini V 2380 instrument from Micrometitics Instrument Corp. X-ray diffraction patterns(XRD) were recorded in DISCOVER diffractometer with Cu Karadiation.
2.2 Gas absorption experiment
CO2/water(distilled water) was selected as the experimental material and the zeolite particles (particle size: 1-10mm) were introduced to enhance CO2(>99.5% mass fraction) absorption. The absorption experiments were carried out in a thermostatic vessel (Fig. 1). Four symmetrical baffles were mounted to prevent the formation of a horizontal vortex. Two stirrers were employed to mix the gas phase and liquid phase respectively. A cooling coil in the vessel was connected to the thermostatic bath to maintain a constant reaction temperature (298 K±0.1 K).

Figure 1 Experimental set-up for gas absorption
1—air inlet valve; 2—junction valve; 3—balance tank; 4—pressure transmitter; 5—pressure difference transmitter (connected with the computer); 6—temperature sensor; 7—magnetic stirrer; 8—cooling coil; 9—gas outlet valve; 10—vacuum value; 11—gas stirrer; 12—liquid stirrer; 13—baffles; 14—stainless steel top; 15—stainless steel vessel; 16—thermostatic bath
Before each experiment, the vessel was filled with the slurry. Then the liquid was degassed by opening valve 10 until the slurry was equilibrated under the vapor pressure of water, then valve 10 was closed. Open valves 1 and 2 and CO2was fed into cell 3 and vessel 15 up to a fixed pressure. Valves 1 and 2 were then closed rapidly. Turning on the motor of the magnetic stirrer 7, the absorption was started. The absorption processes began with an initial pressure of 0.1MPa. Vessel 3 was a reference cell, a pressure difference transducer was connected between cell 3 and vessel 15. The transducer signal was transmitted to the computer and recorded online. With the value of the recorded pressure difference, the absorption rate could be calculated. Determining the absorption rate of CO2in slurry and pure water respectively, the enhancement factor was obtained.
3 Theoretical Model
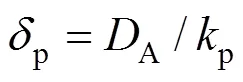
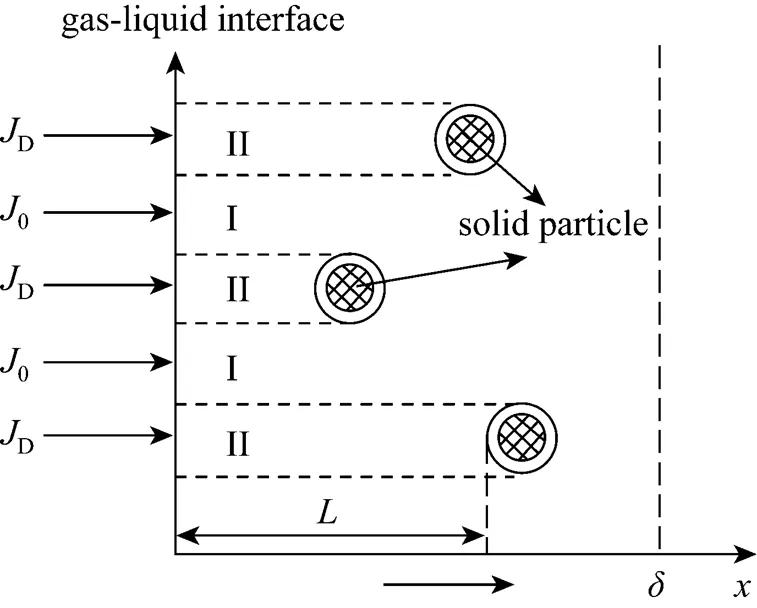
Figure 2 Sketch map of mass transfer in the slurry
According to the assumptions, the unsteady-state species balance for the solute can be derived respectively in the two zones.
3.1 Zone I
A species balance for the solute in the liquid phase is given by:
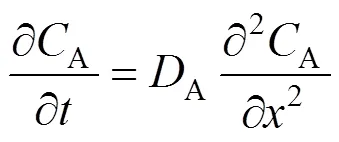
with the following conditions:
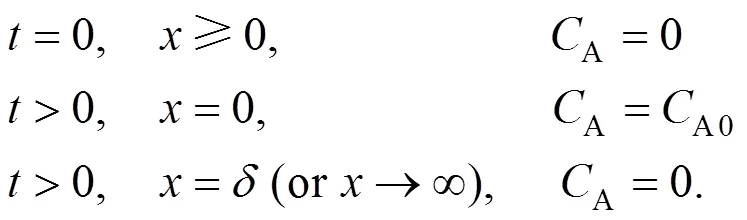
3.2 Zone II
In the continuous phase of this zone, the balance of the solute can be written as:
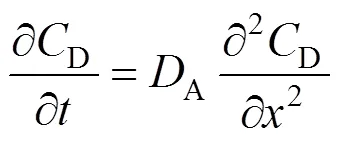
The relevant conditions of Eq. (3) are given by

In the liquid film wrapping the particles,
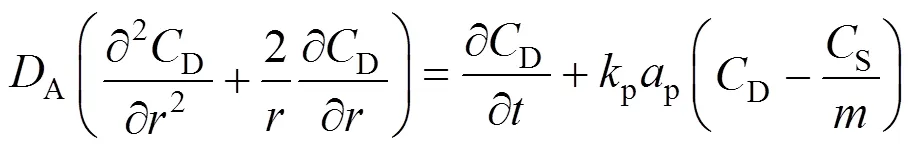
The necessary initial and boundary conditions are:
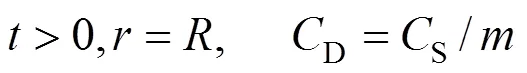
wherepis the surface area of the particles, m-1.

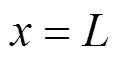
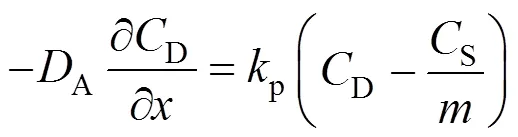
And the boundary condition can be given by
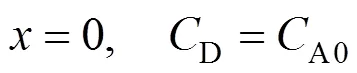
Sin Eq. (8) is the adsorbed amount of A on solid per unit volume of particle, mol·m-3, andis the average particle-to-interface distance related to the solute concentration. Assuming a uniform distribution of the particle at the gas-liquid interface, thencan be calculated as follows [12]


The balance for the accumulation of the solute within or on a single particle can be written as:
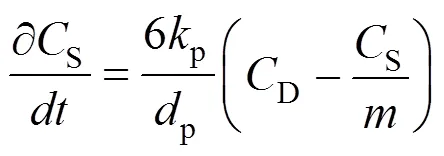
with the following initial condition:
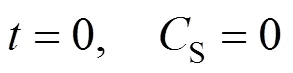
3.3 Absorption rate
Based on the Higbie penetration model, the absorption rate can be written:

Ifis the residence time of liquid element, the time-averaged flux follows from:
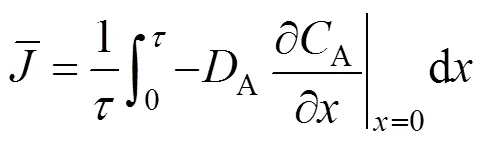
3.4 Enhancement factor
The enhancement factor is defined as
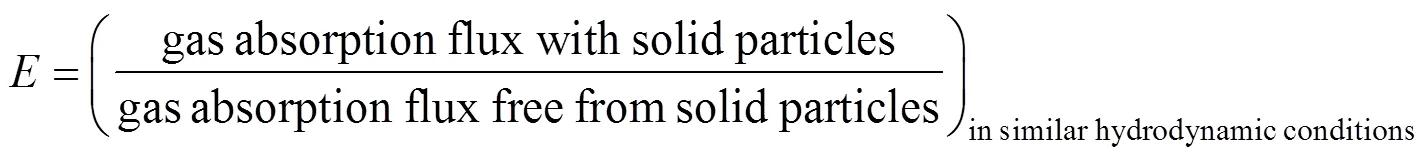
Assuming that the coverage of the gas-liquid interface can be described by Langmuir-type adhesion isotherm [6, 10]:

Then the enhancement factors can be written as:
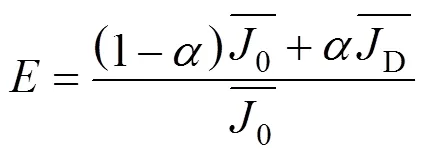
where the subscript 0 and D are average gas absorption rate in uncovered and covered zone respectively.
The above equations are solved numerically by gPROMs modeling software (Process System Enterprise Ltd.).
4 Results and Discussion
4.1 A comparison of particle properties obtained by different modification methods
After pressing modified and unmodified zeolite powder into disks, the liquid-solid contact angle was determined with JY-82 angle measuring instrument, and the results are shown in Table 1. From the results, good hydrophobicity can be obtained by both of the two modification methods. Contrarily, when putting a drop of water on the surface of unmodified zeolite, water can spread completely and permeate into the particle quickly, indicating the strong hydrophilicity of unmodified zeolite.

Table 1 Liquid-solid contact angle by different treatments
The surface modification method did not change the silica alumina ratio of zeolite and the hydrophobicity of zeolite obtained was mainly dependent on the property of the organic coating. However, the hydrophobicity of zeolite modified by skeleton modification was obtained by changing the silica alumina ratio of the zeolite skeleton.
The zeolite crystalline consists of structural units of silicon-oxygen tetrahedrons and alumina-oxygen tetrahedrons. The silicon-oxygen tetrahedrons in which one silica atom bonds to four oxygen atoms meet the valence desire of silica (+4), whereas, an electronegativity is present in Al-O tetrahedrons due to the structure of one trivalent aluminum atom bonding to four oxygen molecules. This imbalance of electrical charges needs to be compensated by some cations which result in the hydrophilicity of zeolites [13]. Thus in order to improve the hydrophobicity of zeolite, the content of aluminum in the crystalline should be decreased [9, 14, 15]. The higher the ratio of silica to aluminum, the stronger the hydrophobicity. The experimental Si/Al ratios are shown in Table 2.

Table 2 Silica-alumina ratios by different treatments
X-ray analyses were made for zeolite modified by framework element modification and the XRD patterns show that perfect framework structure and lattice structure of zeolites were maintained and no skeleton collapse was observed in the range of acid concentration discussed (Fig. 3). Furthermore, the experimental results of surface area also indicated that compared with unmodified zeolite, the specific surface area was somewhat increased because some impurities in the channel of zeolite were removed by acid used. However, surface area of zeolite by surface coating modification was reduced greatly because partial channel was covered with organic molecules.

Figure 3 XRD patterns of modified and unmodified zeolites
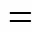
4.2 Influence of modified zeolite on gas absorption
4.2.1
Introducing modified zeolite particles to CO2gas absorption system, the enhancement factors of gas absorption as a function of solid concentration (s) are shown in Fig. 4. From the results, significant enhancement of gas absorption rate was obtained by modified zeolites, and the higher the silicon to aluminum ratio, the greater the enhancement factors. The highest enhancement factor was found to be 2.62 at a silicon-aluminum ratio of 123.

Figure 4 The influence of modified zeolitesby framework element modification method on gas absorption
SiO2/Al2O3(exp.):■ 63;● 86;▲ 128
Due to good hydrophobicity obtained, modified zeolite particles by framework element modification are preferably situated at the gas-liquid interface, resulting in higher concentration of particles near gas-liquid interface than in the bulk [16]. Near the interface, the adsorptive particles are loaded with solute and the concentration gradient of the solute in the mass transfer layer will be increased. After spending a certain time in the interfacial layer, the particles return to the bulk of the liquid where the solute is desorbed and the particles regenerated. With this so-called “shuttle” [16, 17] between the interface and the bulk, gas absorption rate can be enhanced.
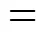
4.2.2
The results of CO2absorption experiments in suspensions of unmodified and modified zeolite by surface modification are given in Fig. 5. No enhancement of gas absorption rate was found for both the zeolite particles.
Figure 5 The influence of unmodified and modified zeolites by surface coating on gas absorption
■ unmodified zeolite;● modified zeolite
Unmodified zeolites with low Si/Al ratio show high affinity to water molecules. When in aqueous solution, most of the surface active sites of zeolites will be covered by water molecules, resulting in the loss of adsorption capability to the solute. On the other hand, although a good hydrophobicity of the modified zeolite by surface method was observed (Table 1), no beneficial effects on gas absorption were obtained. On the contrary, the CO2absorption rate decreased with the solid concentrations(Fig. 5). This phenomenon is due to the fact that the coating layer of ethyl orthosilicate formed on the surface of zeolite disabled its adsorption capability of CO2. When in aqueous solution, a layer of inertial attaching particles will cover part of the gas-liquid interface, leading to the drop of gas absorption rate.
5 Conclusions
Two methods of zeolite modification were employed: framework element modification and surface coating, and the influence of the zeolites before and after modification on the CO2absorption was studied. According to the results from both experiment and prediction by present model, the authors could draw out conclusions as follows:
(1) By means of the surficial organic coating, hydrophobicity was obtained for modified zeolite by surface modification, but meanwhile, the partial particle channel was plugged. As a result, specific surface area of zeolite was decreased greatly.
(2) The framework element modification method could maintain not only complete lattice structure and adsorption capability of zeolite, a good hydrophobic property could also be obtained. Consequently, significant enhancement on gas absorption by this modified zeolite was achieved and up to a maximum enhancement factor of 2.62, which shows that zeolite particles by framework element modification would be a promising material for the future application.
(3) Solid particles with good enhancement role to gas absorption need not only adsorptive capability but also certain hydrophobicity.
(4) An unsteady heterogeneous model was employed to predict enhancement factor and the calculated results agree well with the experimental data.
NOMENCLATURE
psolid-liquid interfacial area, m2×m-3
Asolute concentration, mol×m-3
A0solute concentration at the interface, mol×m-3
Dsolute concentration in particle covered zone, mol×m-3
Sadsorbed amount of A on solid per unit volume of particle, mol×m-3
Adiffusion coefficient, m2×s-1
pparticle diameter, m
enhancement factor
mass transfer rate, mol×m-2×s-1
pliquid-side mass transfer coefficient, m×s-1
sparticle adhesion coefficient, m3×kg-1
particle-to-interface distance, m
partition coefficient of the solute between the solid and the liquid
sparticle concentration, kg×m-3
diameter of particle, m
distance to the center of the particle, m
time, s
distance to the interface, m
fraction of the interface covered by particles
maxmaximum coverage
penetration depth, m
pliquid film thickness around the particle, m
pparticle density, kg×m-3
residence time, s
particle volume concentration
1 Kaya, A., Schumpe, A., “Surfactant adsorption rather than ‘shuttle effect’?”,, 60 (22), 6504-6510 (2005).
2 Ruthiya, K.C., Kuster, B.F.M., Schouten, J.C., “Gas-liquid mass transfer enhancement in a surface aeration stirred slurry reactors”,., 81 (5), 632-639 (2003).
3 Ruthiya, K.C., van der Schaaf, J., Kuster, B.F.M., Schouten, J.C., “Mechanisms of physical and reaction enhancement of mass transfer in a gas inducing stirred slurry reactor”,..., 96 (1-3), 55-69 (2003).
4 Demmink, J.F., Mehra, A., Beenackers, A.A.C.M., “Gas absorption in the presence of particles showing interfacial affinity: case of fine sulfur precipitates”,.., 53 (16), 2885-2902 (1998).
5 Wimmers, O.J., Fortuin, J.M.H., “The use of adhesion of catalyst particles to gas bubbles to achieve enhancement of gas absorption in slurry reactors (II) Determination of the enhancement in a bubbles containing slurry reactor”,., 43 (2), 313-319 (1988).
6 Wimmers, O.J., de Sauvage Nolting, H.J.J., Fortuin, J.M.H., “The effect of the size of catalyst particles adhering to bubbles on the enhancement of gas absorption in slurry reactors”,..., 43 (8), 2155-2159 (1988).
7 Kluytmans, J.H.J., van Wachem, B.G.M., Kuster, B.F.M., Schouten, J.C., “Mass transfer in sparged and stirred reactors: Influence of carbon particles and electrolyte”,., 58 (21), 4719-4728 (2003).
8 Tinge, J.T., Drinkenburg, A.A.H., “The enhancement of the physical absorption of gases in aqueous activated carbon slurries”,, 50 (6), 937-942 (1995).
9 Takeuchi, M., Kimura, T., Hidaka, M., Rakhmawaty, D., Anpo, M., “Photocatalytic oxidation of acetaldehyde with oxygen on TiO2/ZSM-5 photocatalysts: Effect of hydrophobicity of zeolites”,, 246 (2), 235-240 (2007).
10 Vinke, H., Hamersma, P.J., Fortuin, J.M.H., “Enhancement of the gas-absorption rate in agitated slurry reactors by gas-adsorbing particles adhering to gas bubbles”,..., 48 (12), 2197-2210 (1993).
11 Zhang, G.D., Cai, W.F., Xu, C.J., Zhou, M., “A general enhancement factor model of the physical absorption of gases in multiphase systems”,..., 61 (5), 558-568 (2006).
12 Junker, B.H., Wang, D.I.C., Hatton, T.A., “Oxygen transfer enhancement in aqueous/perfluorocarbon fermentation systems (II) Theoretical analysis”,.., 35 (2), 586-597 (1990).
13 Chen, N.Y., “Hydrophobic properties of zeolites”,..., 80 (1), 60-64 (1976).
14 Camblor, M.A., Corma, A., Iborra, S., Miquel, S., Primo, J., Valencia, S., “Beta zeolite as a catalyst for the preparation of alkyl glucoside surfactants: the role of crystal size and hydorphobicity”,, 172 (1), 76-84 (1997).
15 Cheng, H., Reinhard, M., “Sorption of trichloroethylene in hydrophobic micro pores of dealuminated Y zeolites and natural minerals”,..., 40 (24), 7694-7701(2006).
16 Holstvoogd, R.D., van Swaaij, W.P.M., van Dierendonck, L.L., “The absorption of gases in aqueous activated carbon slurries enhanced by adsorbing or catalytic particles”,..., 43 (8), 2181-2187 (1988).
17 Alper, E., Ozturk, S., “Effect of fine solid particles on gas-liquid mass transfer rate in a slurry reactor”,..., 46 (1), 147-158 (1986).
18 Holstvoogd, R.D., van der Swaaii, W.P.M., “The influence of adsorption capacity on enhanced gas absorption in activated carbon slurries”,..., 45 (1), 151-162 (1990).
19 Shen, S.H., Ma, Y.G., Zhu, C.Y., Lu, S.M., “Absorption enhancement of carbon dioxide in aqueous activated carbon slurries”,....(), 58 (4), 835-841 (2007).
20 Dagaonkar, M.V., Heeres, H.J., Beenackers, A.A.C.M., Pangarkar, V.G., “The application of fine TiO2particles for enhanced gas absorption”,.., 92 (1), 151-159 (2003).
2008-06-05,
2008-10-07.
the National Natural Science Foundation of China (20176036).
** To whom correspondence should be addressed. E-mail: ygma@tju.edu.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Modeling and Optimization for Scheduling of Chemical Batch Processes*
- Simulation of Droplet-gas Flow in the Effervescent Atomization Spray with an Impinging Plate*
- Numerical Investigation of Constructal Distributors with Different Configurations*
- The Kinetics of the Esterification of Free Fatty Acids in Waste Cooking Oil Using Fe2(SO4)3/C Catalyst
- Multiple Model Soft Sensor Based on Affinity Propagation, Gaussian Process and Bayesian Committee Machine*
- Measurement and Correlation of Solid-Liquid Equilibria of Phenyl Salicylate with C4 Alcohols
