慢性乙型肝炎功能性治愈不是梦
2025-02-27庄辉


摘要: 慢性乙型肝炎功能性治愈的定义是在停止抗病毒治疗后至少24周,HBsAglt;0. 05 IU/mL,血清HBV DNAlt;10 IU/mL。这需要抑制HBV复制、降低病毒抗原产生,同时恢复对HBV感染的免疫应答。约30%~50%接受核苷(酸)类似物治疗并经严格选择的慢性乙型肝炎患者,加用或单用聚乙二醇干扰素α治疗,或经核苷(酸)类似物有限疗程治疗后HBsAglt;100 IU/mL者,可获得功能性治愈。目前有40余种新的抗HBV药物和免疫调节剂正在进行临床试验。抑制HBV复制、降低病毒抗原,以及提高HBV感染免疫应答药物的联合应用,可能是慢性乙型肝炎功能性治愈的理想治疗策略。但确定最佳的联合、用药时间、用药顺序和治疗期限等尚需进一步研究。
关键词: 乙型肝炎, 慢性; 乙型肝炎病毒; 功能性治愈; 完全治愈; 抗病毒药
Functional cure of chronic hepatitis B is not a dream
ZHUANG Hui
Department of Microbiology and Center of Infectious Diseases, Peking University Health Science Center, Beijing 100191, ChinaCorresponding author: ZHUANG Hui, zhuangbmu@126.com (ORCID: 0000-0001-9119-6325)Abstract: Functional cure of chronic hepatitis B (CHB) is defined as HBsAglt;0.05 IU/mL and serum HBV DNAlt;10 IU/mL for at least24 weeks after discontinuation of antiviral therapy. This requires suppression of HBV replication and reduction of viral antigenproduction, as well as restoration of immune response to HBV infection. About 30% — 50% of highly selected CHB patients treated withnucleos(t)ide analogues can achieve functional cure after add-on therapy or monotherapy with pegylated interferon-α or a finitecourse of treatment with nucleos(t)ide analogues among patients with HBsAglt;100 IU/mL. At present, clinical trials are beingconducted for more than 40 types of novel anti-HBV drugs and immunomodulators. The combination of drugs that inhibit viralreplication, reduce antigen burden, and restore immune response to HBV infection may be an ideal strategy to achieve thefunctional cure of CHB. However, further studies are needed to determine the optimal drug combination, the timing and sequenceof medication, and the duration of treatment.
Key words: Hepatitis B, Chronic; Hepatitis B Virus; Functional Cure; Complete Cure; Antiviral Agents
HBV复制率高,每天约产生1万亿个完整的病毒颗粒,以及只含HBsAg的亚病毒颗粒,后者不能复制,也不能感染,但其含量较完整的HBV高1 000~100 000倍[1-2]。血液循环中的大量HBsAg可导致慢性HBV感染者免疫耗竭,而自发、或聚乙二醇干扰素α(PEG-IFN-α)或核苷(酸)类似物[nucleos(t)ide analogues, NAs]治疗后,发生HBeAg 或 HBsAg 消失者可恢复 HBV 特异性 T 淋巴细胞免疫应答[3-7] 。最近研究显示,小干扰 RNA (smallinterfering RNA, siRNA)治疗后,HBsAg显著下降的患者可恢复HBV T淋巴细胞特异性免疫应答[8],结果提示,至少一部分慢性乙型肝炎(CHB)患者在HBV复制和HBsAg产生被抑制后,HBV特异性T淋巴细胞免疫应答可以恢复。因此,HBsAg消失对HBV特异性T淋巴细胞免疫应答恢复和CHB治愈具有重要意义。
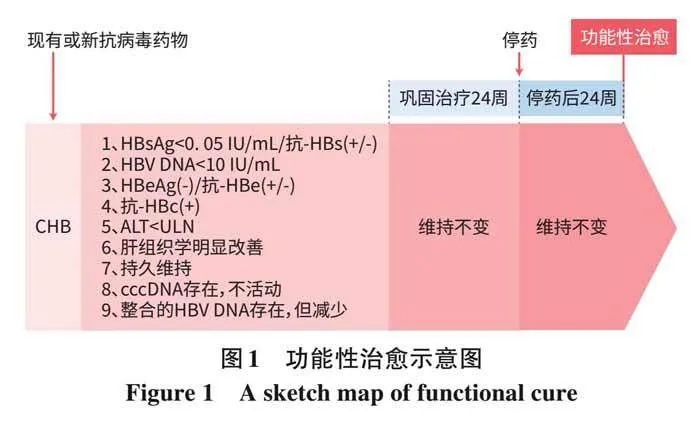
CHB完全治愈(彻底治愈)是指肝细胞中HBV cccDNA和整合的HBV DNA消失。但目前由于无消除cccDNA和整合HBV DNA的新药、无商品化和标准化检测cccDNA及整合的 HBV DNA试剂,很难达到这一治疗目标[8-9] 。目前,抗HBV的现有药物和新药临床试验的主要治疗终点是功能性治愈,其定义是: (1)HBsAglt;0. 05 IU/mL(伴或不伴抗-HBs 阳转);(2)HBV DNAlt;10 IU/mL;(3)HBeAg血清学转换(伴或不伴抗-HBe阳性);(4)抗-HBc阳性;(5)ALTlt;正常值上限(ULN:男30 U/L,女19 U/L); (6)肝组织学明显改善;(7)持久维持;(8)ccc DNA存在,不活动;(9)整合的HBV DNA存在,但减少;(10)巩固治疗24周;(11)停药后24周上述指标仍维持不变[8-14](图1)。
1 现行抗HBV药物的功能性治愈
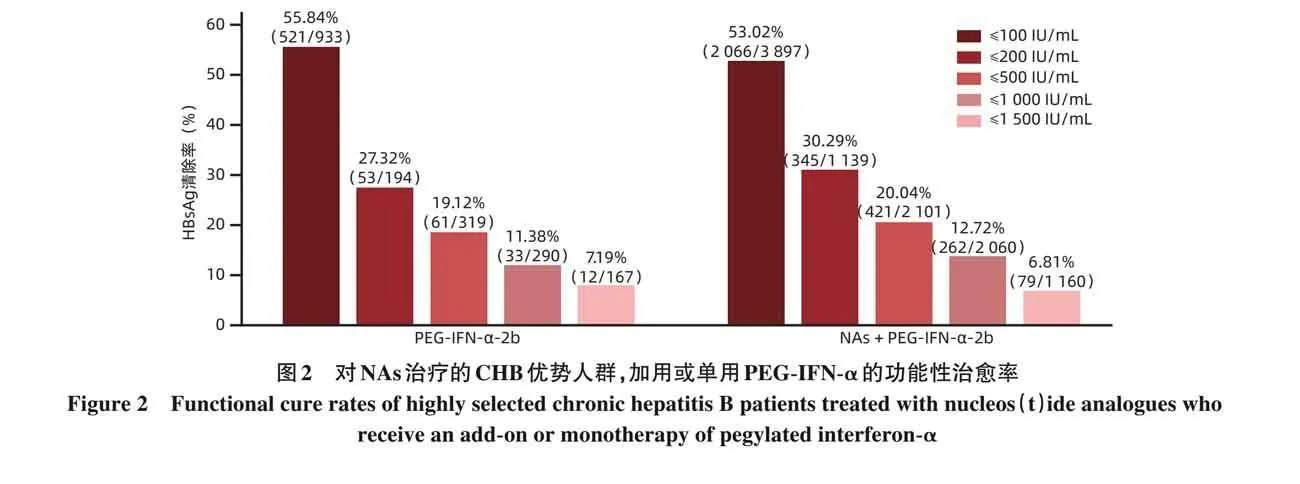
NAs治疗后,对优势人群加用或单用PEG-IFN-α治疗达到功能性治愈 NAs治疗后的所谓优势人群是指:(1)HBV DNAlt;10 IU/mL;(2)HBeAg血清学转换(抗-HBe阳转); (3)HBsAglt;500 IU/mL; (4)ALTlt;ULN。对该类CHB患者,加用 PEG-IFN-α 治疗,基线 HBsAg≤100 IU/mL、≤200 IU/mL 和≤500 IU/mL 的 CHB 功能性治愈率分别53. 02%、30. 29% 和 20. 04%;单用 PEG-IFN-α 治疗,基线HBsAg≤100 IU/mL、≤200 IU/mL和≤500 IU/mL功能性治愈率分别55. 84%、27. 32%和19. 12%[15](图2)。
Gao 等[16] 检测 47 例功能性治愈 CHB 患者的肝内HBV cccDNA和HBV DNA发现,23. 4%的患者上述2项指标均为阴性,提示已达到完全治愈(彻底治愈)。
1. 2 应用NAs有限疗程,获得功能性治愈 Hirode等[17]报道一项国际多中心、多人种队列研究(RETRACT-BStudy),对1 552例CHB患者于NAs停药后随访4年,停药时HBsAg水平低的患者HBsAg消失率较高。对于亚洲CHB患者,停药时HBsAglt;100 IU/mL和≥100 IU/mL患者的HBsAg消失率分别为33%和2%。对于白人CHB患者,停药时 HBsAglt;1 000 IU/mL 和≥1 000 IU/mL 患者的HBsAg消失率分别为 41%和 5%。因此,对于亚洲 CHB患者,NAs 治疗至 HBsAglt;100 IU/mL 可停药;对于白人CHB患者,NAs治疗至HBsAglt;1 000 IU/mL时可停药。但两者均须在确保密切监测的情况下,方可停药。
一项纳入24篇文献、3 732例CHB患者的荟萃分析结果显示,对于亚洲 CHB 患者,停药时 HBsAglt;100 IU/mL者,与停药时HBsAg≥100 IU/mL者比较,其HBsAg消失率高(28. 3% vs 2%)、病毒学复发率低(33. 4% vs 72. 1%)、生化学复发率低(17. 3% vs 48. 1%)。对于白人CHB患者,停药时HBsAglt;1 000 IU/mL患者的HBsAg消失率高于停药时HBsAg≥1 000 IU/mL者(38. 4% vs 6. 4%),但其病毒学复发率和生化学复发率均低于停药时HBsAg≥1 000 IU/mL者,分别为52. 7% vs 63. 8%和15. 9% vs 26. 4%[18]。
对于亚洲CHB患者,NAs有限疗程的优势人群是:(1)HBsAglt;100 IU/mL;(2)HBeAg 血清学转换(抗-HBe阳性);(3)HBV DNAlt;10 IU/mL;(4)ALT
NAs 治疗有限疗程停药后必须确保密切监测,停药后前3个月,每月检测1次ALT和HBV DNA,之后每2~3月检测1次ALT和HBV DNA,评价其是否需要再治疗。1年后每3~6个月监测1次[17]。
2 提高CHB功能性治愈率的新药
2. 1 反义寡核苷酸Bepirovirsen (BPV) Ⅱb期临床试验BPV Ⅱb期B-Clear多中心随机开放临床试验,将入组的CHB 患者随机分为 A、B、C、D 4 组,A 组每周皮下注射BPV 300 mg,连续治疗24周,该组进一步分为NAs治疗组(68例)和初治组(70例),停药时两组HBsAg消失率分别为26%和29%,停药后24周时,两组HBsAg消失率分别为12%和14%[21]。
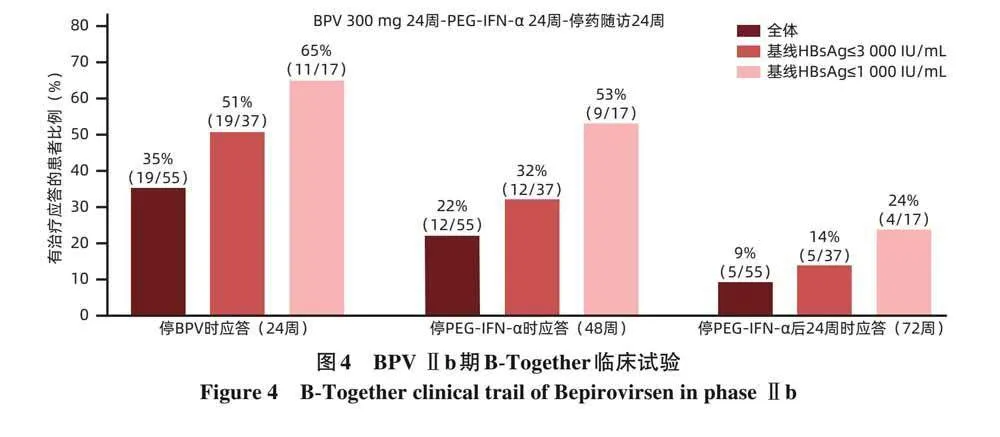
BPV Ⅱb期B-Together多中心随机开放临床试验中,CHB患者每周皮下注射BPV 300 mg,连续治疗24周,之后每周皮下注射PEG-IFN-α 180 μg,治疗24周,PEG-IFN-α停药后随访24周,所有入组患者的HBsAg和HBV DNA消失率为9%(5/55),基线HBsAg≤3 000 IU/mL患者的HBsAg和HBV DNA消失率为14%(5/37)(图4)。上述结果提示,BPV与PEG-IFN-α序贯治疗可提高HBsAg和HBV DNA消失率[22]。BPV Ⅲ期临床试验(B-Well)正在进行中[23-24]。
2. 2 siRNA Xalnesiran (RG6346)的Ⅱ期临床试验 罗氏公司研发的Xalnesiran联合PEG-IFN-α或Ruzotolimod(一种Toll样受体7激动剂)Ⅱ期临床试验的入组患者标准是:(1)CHB≥6个月;(2)NAs治疗≥12个月;(3) HBVDNAlt;定量下限(LLOQ)或lt;20 IU/mL;(4)ALTlt;1. 5×ULN;(5)无肝硬化。将入组的 CHB患者随机分为 5组:A组30 例,Xalnesiran (100 mg,每 4 周皮下注射)+ NAs,治疗48周;B组30例,Xalnesiran (200 mg,每4周皮下注射)+NAs,治疗48周;C组34例,Xalnesiran (200 mg,每4周皮下注射)+NAs,治疗 48 周,另于第 12~24 周和第 36~49周分别每日1次口服Ruzotolimod 150 mg;D组30例,Xalnesiran(200 mg,每 4 周皮下注射)+NAs+PEG-IFN-α(180 μg,每周皮下注射1次),治疗48周;E组36例,服用NAs作为对照组。但各组患者均持续服用NAs,直至符合停药标准。A、B、C、D和 E组于停药后均随访 24周,其HBsAg消失(lt;0. 05 IU/mL)率分别为7%、3%、12%、23%和 0%,提示 Xalnesiran联合 PEG-IFNα或 Ruzotolimod 可提高HBsAg消失率。但HBsAg消失只见于基线HBsAglt;1 000 IU/mL的CHB患者[25-26]。
2. 3 衣壳组装调节剂(capsid assembly modulator, CAM)+siRNA+NAs Ⅱb 期临床试验(REEF-2) CAM+siRNA+NAs Ⅱb期临床试验(REEF-2)为双盲、安慰剂对照随机研究,入组 130 例经 NAs 治疗 HBV DNA 完全抑制的HBeAg 阴性 CHB 患者,试验组 85 例,接受 JNJ-3989(siRNA, 200 mg,每4周皮下注射1次)+JNJ-6379 (CAM,250 mg,每日口服 1 次)+NAs(每日口服);安慰剂组用JNJ-3989 安慰剂+JNJ-6379 安慰剂+NAs,两组均治疗48周,停药后随访48周。
随访至停药后24周和48周,无1例患者获得功能性治愈(HBsAg消失),但治疗至48周,试验组HBsAg平均下降水平较安慰剂组显著(1. 89 log 10 IU/mL vs 0. 06 log 10 IU/mL,P=0. 001);随访至48周时,试验组HBsAg水平较基线下降gt;1 log 10 IU/mL 占 81. 5%,安慰剂组仅为 12. 5%;试验组HBsAglt;100 IU/mL患者占比大于安慰剂组(46. 9% vs15. 0%)。停药后,试验组HBV DNA复阳和ALT升高率(再治疗率)低于安慰剂组(9. 1% vs 26. 8%)。上述结果提示,CAM+siRNA+NAs联合治疗可明显降低HBsAg水平,但无1例获得功能性治愈[27]。
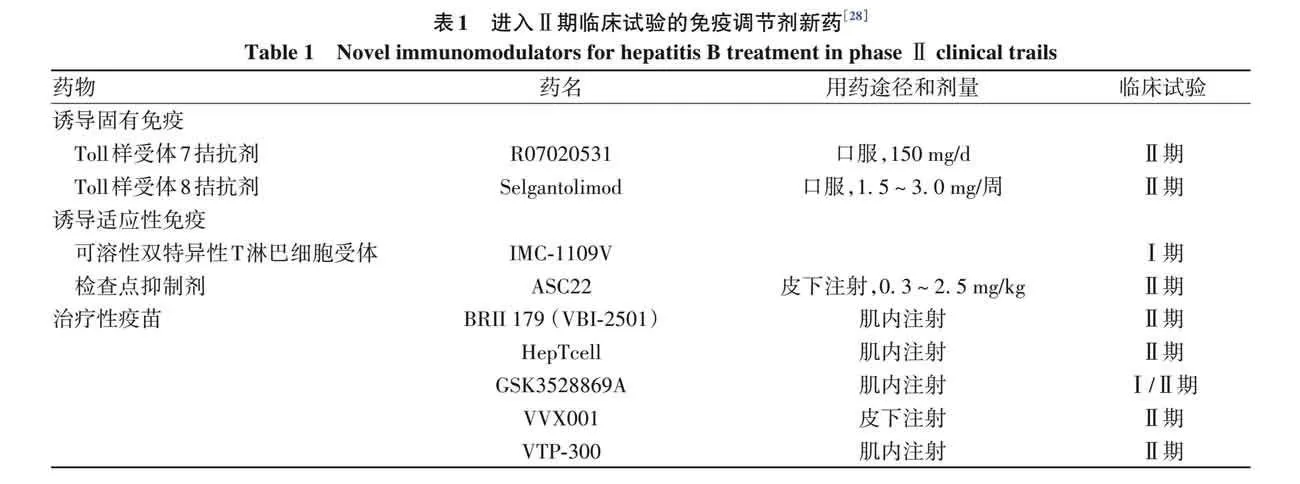
2. 4 Ⅱ期临床试验在研免疫调节剂新药 目前有多种CHB免疫调节剂进入Ⅱ期 临床试验(表1), 但至今尚无一种被证明可获得功能性治愈[28]。
3 联合治疗
其他感染性疾病如艾滋病和丙型肝炎药物联合治疗的成功经验表明,慢性HBV感染要达到功能性治愈也需要联合治疗。虽然目前已有NAs和PEG-IFN-α抗HBV药物,并有40余种新药正在研发中,但功能性治愈方案主要还是凭临床实践经验,缺乏对控制 HBV 复制和HBsAg消失机制的深入认识。目前功能性治愈最有希望的治疗策略是抑制病毒复制、降低HBV抗原产生、恢复对HBV的免疫应答(图5)[28]。
目前,联合治疗的研究焦点是何时联合、不同药物如何序贯治疗及其治疗的持续时间等。此外,可能还需要考虑如何基于CHB患者基线HBeAg状况、HBV DNA载量、HBsAg水平,以及有无肝硬化等因素,制订个体化的治疗方案。
道长且阻,行则将至!CHB功能性治愈不是梦,人类终将完全治愈HBV感染!
参考文献:
[1] CHAI N, CHANG HE, NICOLAS E, et al. Properties of subviral par⁃ticles of hepatitis B virus[J]. J Virol, 2008, 82(16): 7812-7817. DOI:10.1128/JVI.00561-08.
[2] GANEM D, PRINCE AM. Hepatitis B virus infection: Natural historyand clinical consequences[J]. N Engl J Med, 2004, 350(11): 1118-1129. DOI: 10.1056/NEJMra031087.
[3] REHERMANN B, LAU D, HOOFNAGLE JH, et al. Cytotoxic T lympho⁃cyte responsiveness after resolution of chronic hepatitis B virus infection
[J]. J Clin Invest, 1996, 97(7): 1655-1665. DOI: 10.1172/JCI118592.
[4] BONI C, LACCABUE D, LAMPERTICO P, et al. Restored function ofHBV-specific T cells after long-term effective therapy with nucleos(t)ideanalogues[J]. Gastroenterology, 2012, 143(4): 963-973. DOI: 10.1053/j.gastro.2012.07.014.
[5] KIM JH, GHOSH A, AYITHAN N, et al. Circulating serum HBsAglevel is a biomarker for HBV-specific T and B cell responses inchronic hepatitis B patients[J]. Sci Rep, 2020, 10(1): 1835. DOI: 10.1038/s41598-020-58870-2.
[6] RINKER F, ZIMMER CL, HÖNER ZU SIEDERDISSEN C, et al. Hepati⁃tis B virus-specific T cell responses after stopping nucleos(t)ideanalogue therapy in HBeAg-negative chronic hepatitis B[J]. J Hepa⁃tol, 2018, 69(3): 584-593. DOI: 10.1016/j.jhep.2018.05.004.
[7] van BÖMMEL F, BERG T. Risks and benefits of discontinuation ofnucleos(t)ide analogue treatment: A treatment concept for patientswith HBeAg-negative chronic hepatitis B[J]. Hepatol Commun, 2021,5(10): 1632-1648. DOI: 10.1002/hep4.1708.
[8] LOK ASF. Toward a functional cure for hepatitis B[J]. Gut Liver,2024, 18(4): 593-601. DOI: 10.5009/gnl240023.
[9] GHANY MG, BUTI M, LAMPERTICO P, et al. Guidance on treatmentendpoints and study design for clinical trials aiming to achieve curein chronic hepatitis B and D: Report from the 2022 AASLD-EASLHBV-HDV treatment endpoints conference[J]. J Hepatol, 2023, 79(5): 1254-1269. DOI: 10.1016/j.jhep.2023.06.002.
[10] LI MH, YI W, ZHANG L, et al. Predictors of sustained functional curein hepatitis B envelope antigen-negative patients achieving hepatitisB surface antigen seroclearance with interferon-alpha-based therapy
[J]. J Viral Hepat, 2019, 26(Suppl 1): 32-41. DOI: 10.1111/jvh.13151.
[11] LI MH, SUN FF, BI XY, et al. Consolidation treatment needed for sus⁃tained HBsAg-negative response induced by interferon-alpha inHBeAg positive chronic hepatitis B patients[J]. Virol Sin, 2022, 37(3): 390-397. DOI: 10.1016/j.virs.2022.03.001.
[12] YIP TCF, WONG GLH, WONG VWS, et al. Durability of hepatitis Bsurface antigen seroclearance in untreated and nucleos(t)ideanalogue-treated patients[J]. J Hepatol, 2018, 68(1): 63-72. DOI:10.1016/j.jhep.2017.09.018.
[13] LOK AS, ZOULIM F, DUSHEIKO G, et al. Durability of hepatitis Bsurface antigen loss with nucleotide analogue and peginterferontherapy in patients with chronic hepatitis B[J]. Hepatol Commun,2019, 4(1): 8-20. DOI: 10.1002/hep4.1436.
[14] YIP TCF, LOK ASF. How do we determine whether a functional curefor HBV infection has been achieved?[J]. Clin Gastroenterol Hepa⁃tol, 2020, 18(3): 548-550. DOI: 10.1016/j.cgh.2019.08.033.
[15] XIE C, LIN BL, XIE DY, et al. Peginterferon alpha-2b promoted HB⁃sAg loss in nucleos(t)ide analogue-treated patients: a large-scalereal-world study (Everest Project)[J]. J Hepatol, 2026 (Revised).
[16] GAO N, GUAN GW, XU GL, et al. Integrated HBV DNA and cccDNAmaintain transcriptional activity in intrahepatic HBsAg-positive pa⁃tients with functional cure following PEG-IFN-based therapy[J]. Ali⁃ment Pharmacol Ther, 2023, 58(10): 1086-1098. DOI: 10.1111/apt.17670.
[17] HIRODE G, CHOI HSJ, CHEN CH, et al. Off-therapy response afternucleos(t)ide analogue withdrawal in patients with chronic hepatitisB: An international, multicenter, multiethnic cohort (RETRACT-B study)
[J]. Gastroenterology, 2022, 162(3): 757-771. DOI: 10.1053/j.gas⁃tro.2021.11.002.
[18] LIM SG, TEO AE, CHAN ESY, et al. Stopping nucleos(t)ide ana⁃logues in chronic hepatitis B using HBsAg thresholds: A meta-analysis and meta-regression[J]. Clin Gastroenterol Hepatol, 2024.DOI: 10.1016/j.cgh.2024.05.040. [Online ahead of print]
[19] BLOCK PD, LIM JK. Unmet needs in the clinical management ofchronic hepatitis B infection[J]. J Formos Med Assoc, 2024. DOI:10.1016/j.jfma.2024.08.020. [Online ahead of print]
[20] PETERS MG, YUEN MF, TERRAULT N, et al. Chronic hepatitis B fi⁃nite treatment: Similar and different concerns with new drug classes
[J]. Clin Infect Dis, 2024, 78(4): 983-990. DOI: 10.1093/cid/ciad506.
[21] YUEN MF, LIM SG, PLESNIAK R, et al. Efficacy and safety of Bepiro⁃virsen in chronic hepatitis B infection[J]. N Engl J Med, 2022, 387(21): 1957-1968. DOI: 10.1056/NEJMoa2210027.
[22] BUTI M, HEO J, TANAKA Y, et al. Sequential Peg-IFN after bepiro⁃virsen may reduce post-treatment relapse in chronic hepatitis B[J].J Hepatol, 2024. DOI: 10.1016/j.jhep.2024.08.010. [Online ahead ofprint]
[23] Study of Bepirovirsen in nucleos(t)ide analogue-treated participantswith chronic hepatitis B (B-Well 1)[R/OL]. [2024-11-16]. http://Clini⁃calTrials.gov identifier NCT05630807.
[24] Study of Bepirovirsen in nucleos(t)ide analogue-treated participantswith chronic hepatitis B (B-Well 2)[R/OL]. [2024-11-16]. http://Clini⁃calTrials.gov identifier NCT05630820.
[25] HOU JL, XIE Q, ZHANG WH, et al. OS-030 efficacy and safety of xal⁃nesiran with and without an immunomodulator in virologically sup⁃pressed participants with chronic hepatitis B: end of study resultsfrom the phase 2, randomized, controlled, adaptive, open-label plat⁃form study (PIRANGA)[J]. J Hepatol, 2024, 80: S26. DOI: 10.1016/S0168-8278(24)00471-9.
[26] HOU JL, ZHANG WH, XIE Q, et al. Xalnesiran with or without an im⁃munodulator in chronic hepatitis B[J]. N Engl J Med, 2024, 391(22): 2098-2109. DOI: 10.1056/NEJMoa2405485.
[27] AGARWAL K, BUTI M, van BÖMMEL F, et al. JNJ-73763989 andbersacapavir treatment in nucleos(t)ide analogue-suppressed pa⁃tients with chronic hepatitis B: REEF-2[J]. J Hepatol, 2024, 81(3):404-414. DOI: 10.1016/j.jhep.2024.03.046.
[28] GOPALAKRISHNA H, GHANY MG. Perspective on emerging thera⁃pies to achieve functional cure of chronic hepatitis B[J]. Curr Hepa⁃tol Rep, 2024, 23(2): 241-252. DOI: 10.1007/s11901-024-00652-9.
收稿日期:2024-11-16;录用日期:2024-11-28本文编辑:刘晓红
