抗MDA5抗体阳性皮肌炎患者的临床特征和预后
2024-12-18陈燕烽钱捷达展云
[摘" "要]" "目的:探讨抗黑色素瘤分化相关基因5(melanoma differentiation associated gene 5, MDA5)抗体阳性皮肌炎(抗MDA5+皮肌炎)患者的临床特征及其与预后的关系。方法:将2018年4月—2021年12月南通大学附属医院风湿免疫科收治的33例抗MDA5+皮肌炎患者分为生存组和死亡组,比较两组患者临床特征(性别、发病年龄、病程、肌无力、典型皮肤损害、雷诺现象、技工手、关节炎)、并发间质性肺病(interstitial lung disease, ILD)情况及实验室检查结果尤其是抗体检测情况。Kaplan-Meier生存曲线比较眶周皮疹阳性和阴性生存结局的差异。结果:抗MDA5+皮肌炎33例患者中男14例,女19例;发病年龄(52.4±10.7)岁,其中生存组24例(72.7%),死亡组9例(27.3%)。生存组眶周皮疹的发生率高于死亡组(P=0.005)。12.1%的患者检测到肌炎特异性自身抗体,60.6%的患者检测到与肌炎相关性自身抗体,其中54.5%的患者检测到抗Ro52抗体,抗Ro52抗体阳性与并发ILD独立不相关(P=0.375)。剔除非因病死亡1例,抗MDA5+皮肌炎死亡率为25.0%,死亡组在5个月内死亡7例(87.5%),死因均为ILD引起的呼吸衰竭。眶周皮疹阳性与阴性患者的生存率差异有统计学意义(Plt;0.05)。结论:抗MDA5+皮肌炎5个月内死亡率极高,≥5个月的患者死亡率将大大下降。眶周皮疹是抗MDA5+皮肌炎患者生存的保护性因素,应重点关注无眶周皮疹的患者,早期积极强化免疫抑制治疗,以提高抗MDA5+皮肌炎患者生存率。同时应重视抗MDA5+皮肌炎患者的心理健康,及时疏导。
[关键词]" "皮肌炎;抗黑色素瘤分化相关基因5;间质性肺病
[中图分类号]" "R593.26" " " " " " " "[文献标志码]" "B" " " " " " " "[文章编号]" "1674-7887(2024)05-0460-05
皮肌炎是特发性炎性肌病(idiopathic inflammatory myopathies, IIMs)的一个亚组,其特征是特定的皮疹,包括眶周皮疹和Gottron征/丘疹[1]。肌炎特异性自身抗体(myositis-specific autoantibodies, MSAs)和与肌炎相关性自身抗体(myositis-associated autoantibodies, MAAs)对于皮肌炎的诊断、分类至关重要,与预后密切相关[2-3]。抗黑色素瘤分化相关基因5(melanoma differentiation associated gene 5, MDA5)抗体是代表性的MSA,它与临床上无肌病皮肌炎(amyopathic dermatomyositis, ADM)的迅速进展型间质性肺病(rapidly progressive interstitial lung disease, RP-ILD)有关[4-5]。尽管使用糖皮质激素、静脉注射环磷酰胺和钙调神经磷酸酶抑制剂的强化免疫抑制治疗,抗MDA5+皮肌炎的预后已有所改善,但仍有20%~30%的病例是难治且致命的。所以,难治性的抗MDA5+皮肌炎可能需要联合其他治疗,如Janus激酶抑制剂[6]、静脉免疫球蛋白[7]和血浆置换[8]。然而,这些难治性患者治疗反应差、进展迅速的病因和病理生理学尚不清楚,如果对所有抗MDA5+皮肌炎患者实施积极的强化免疫抑制治疗,机会性感染的风险将大大增加[9]。所以,改善抗MDA5+皮肌炎患者的预后,尤其对病情危重患者,是一项充满挑战且迫切需要的任务。本研究回顾性分析了抗MDA5+皮肌炎患者的临床特征及其与预后的关系,以期进一步提高对这种罕见疾病的认识,提高患者的生存率。
1" "资料与方法
1.1" "一般资料" "回顾性分析2018年4月—2021年12月在南通大学附属医院风湿免疫科就诊的33例符合皮肌炎诊断标准[10]的患者。抗MDA5+皮肌炎患者的临床特征(性别、发病年龄、病程、肌无力、典型皮肤损害、雷诺现象、技工手、关节炎)、并发ILD情况、实验室检查尤其是抗体检测情况)和生存情况。随访1~72个月,平均20个月。本研究经南通大学附属医院伦理委员会审查批准(2023-K107)。
1.2" "研究方法" "将所有患者根据生存情况分为生存组和死亡组,比较两组患者的临床特征、并发ILD情况及实验室检查结果,通过电话随访获取生存预后信息。抗MDA5抗体及其他MSAs和MAAs通过使用商业试剂盒(Euroimmun,德国吕贝克)的免疫印迹法检测[11]。“生存时间”定义为从皮肌炎初次诊断到死亡或最后随访日期的时间。肺部病变通过高分辨率计算机断层扫描(high-resolution computed tomography, HRCT)评估,RP-ILD定义为呼吸症状发作1个月内表现出快速进展的ILD,且存在以下情况之一:(1)呼吸困难急性发作且进行性恶化,需住院治疗或辅助供氧;(2)肺功能下降,包括用力肺活量(forced vital capacity, FVC)下降gt;10%或肺一氧化碳弥散能力降低≥15%且FVC下降;(3)胸部HRCT结果显示间质异常程度增加gt;20%;(4)动脉血气分析提示呼吸衰竭或氧分压降低gt;10 mmHg[12]。
1.3" "统计学方法" "使用SPSS 25.0软件(IBM,美国纽约阿蒙克)进行统计分析,正态分布的数据以x±s描述,偏态分布的数据以中位数(四分位间距)[M(Q1~Q3)]描述,组间差异通过Student's t检验或非参数检验(Mann-Whitney U检验)进行分析,分类变量以百分比表示,采用χ2检验或Fisher精确检验。使用Kaplan-Meier方法和对数秩检验评估相关因素,Plt;0.05为差异有统计学意义。
2" "结" " " 果
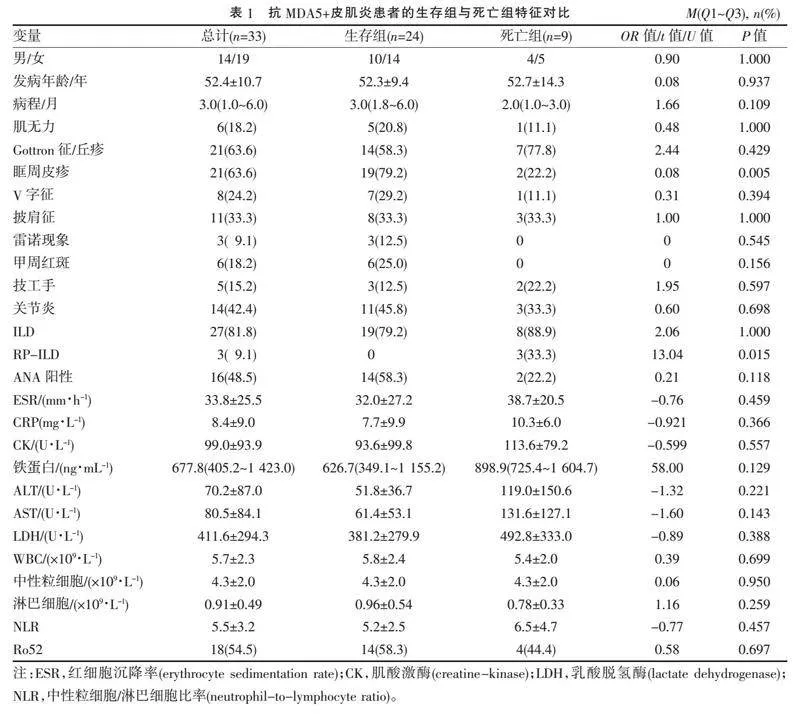
2.1" "抗MDA5+皮肌炎患者的临床特征" "共纳入抗MDA5+皮肌炎患者33例,男14例,女19例;发病年龄(52.4±10.7)岁,其中生存组24例(72.7%),死亡组9例(27.3%),生存组和死亡组间性别、发病年龄、病程、肌无力、典型皮肤损害、雷诺现象、技工手、关节炎、并发ILD情况、实验室检查结果比较见表1。生存组眶周皮疹的发生率较死亡组更高(Plt;0.05),生存组甲周红斑出现率和抗核抗体(antinuclear antibody, ANA)阳性率较死亡组高,但差异均无统计学意义(均Pgt;0.05)。其余各指标两组比较差异均无统计学意义(均Pgt;0.05)。
所有患者均接受了MSAs和MAAs检查,检测到MSAs共4例(12.1%),其中抗组氨酰tRNA合成酶(histidyl-tRNA synthetase, Jo-1)阳性、抗异亮氨酸tRNA合成酶(isoleucyl-tRNA synthetase, OJ)阳性、抗信号识别颗粒(signal recognition particle, SRP)阳性、抗Mi-2β阳性各1例;检测到MAAs共20例(60.6%),其中抗Ro52阳性18例,抗PM-Scl100阳性3例,抗PM-Scl75阳性2例。抗Ro52抗体阳性与并发ILD独立不相关(Fisher's精确检验,OR=2.81,P=0.375)。25例患者并发ILD,3例患者并发RP-ILD,其中死亡9例(32.1%)。
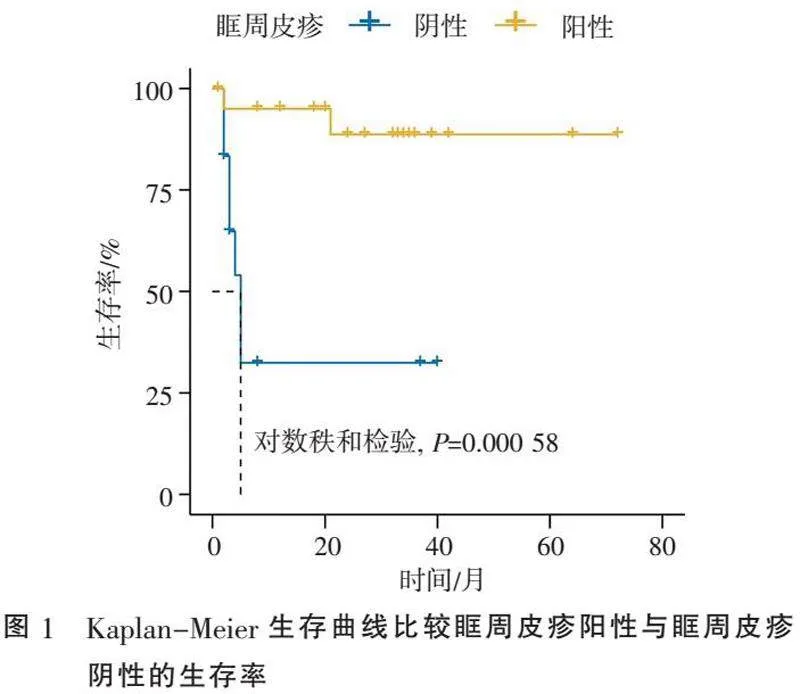
2.2" "预后" "死亡9例患者中自杀死亡1例,剔除非因病死亡的,抗MDA5+皮肌炎死亡率25.0%。根据随访情况,发病2个月死亡2例,3个月2例,4个月1例,5个月2例,21个月1例,其中5个月内死亡共7例(87.5%),死因均为ILD引起的呼吸衰竭。抗MDA5+皮肌炎前5个月死亡高发,病程≥5个月,死亡风险大大下降。Kaplan-Meier生存曲线比较眶周皮疹阳性与阴性的生存率,对数秩和检验显示差异有统计学意义(Plt;0.05)(图1)。
3" "讨" " " 论
MDA5是一种可以识别病毒双链RNA的胞浆视黄酸诱导基因-Ⅰ类受体,激活后可触发Ⅰ型干扰素和促炎细胞因子的表达[13]。早期识别抗MDA5+皮肌炎快速进展的疾病表型,提高患者的生存率。
抗MDA5抗体通常与典型的皮疹和ILD相关,但没有肌炎的临床表现[14]。本研究中,生存组眶周皮疹和甲周红斑的发生率更高,且Kaplan-Meier生存曲线比较结果显示眶周皮疹阳性生存率明显高于眶周皮疹阴性。提示眶周皮疹可能是抗MDA5+皮肌炎患者生存的保护性因素,无眶周皮疹患者可能短期内出现疾病快速进展,应早期积极强化免疫抑制治疗,以提高抗MDA5+皮肌炎患者的生存率。
根据MSAs可以将皮肌炎分为不同亚组[15],如抗Ro52抗体等MAAs在炎性肌病中经常出现。本研究中,12.1%的患者检测到MSAs,60.6%的患者检测到MAAs,54.5%的患者检测到抗Ro52抗体,其中检测到抗Jo-1阳性、抗OJ阳性、抗SRP阳性、抗Mi-2β阳性各1例,均纳入MDA5+皮肌炎进行分析,因合并其他MSAs比如抗Jo-1和抗OJ阳性均为低滴度阳性,抗Jo-1和抗OJ抗体均为抗合成酶抗体,MDA5+皮肌炎常表现出抗合成酶综合征的标志性特征[14]。研究[16-18]表明,抗Ro52阳性可能导致炎性肌病患者出现严重且进展迅速的ILD,并影响预后,但本研究中抗Ro52抗体阳性与并发ILD或RP-ILD无显著相关性,可能与样本量少有关;也有研究[19-20]表明单独抗Ro52抗体阳性的皮肌炎患者即使并发RP-ILD也预后良好,但抗MDA5抗体和抗Ro52抗体共存与RP-ILD的高发病率和ADM中高死亡率相关。本研究中,48.5%的抗MDA5+皮肌炎患者ANA阳性,生存组中ANA阳性的比例更高,但与死亡组相比差异无统计学意义。ANA阳性可能与恶性肿瘤风险增加相关[21],但皮肌炎患者中ANA阳性的临床意义尚未完全明确。
ILD是皮肌炎的常见并发症,不同ILD亚型的患者肺部症状的严重程度各不相同[22]。轻度ILD的患者往往病情稳定,对治疗反应良好,而一些RP-ILD患者病情更严重,预后较差[23-24]。抗MDA5+皮肌炎与RP-ILD相关,早期死亡率高[25],本研究中81.8%的抗MDA5+皮肌炎患者并发ILD,9.1%的患者并发RP-ILD,并发ILD或RP-ILD的患者有32.1%死亡;9例死亡患者中有1例为自杀死亡,该患者虽未因疾病直接导致死亡结局,但其病情危重,预计生存期较短,故本研究仍将该患者纳入死亡组分析,剔除该患者非因病死亡的,本研究中抗MDA5+皮肌炎死亡率25.0%,在发病5个月内死亡7例(87.5%),均因ILD引起的呼吸衰竭死亡。抗MDA5+皮肌炎患者前5个月死亡率高,病程gt;5个月者死亡风险大大下降,这与文献[26-28]报道的抗MDA5+皮肌炎死亡大多发生在6个月内相符。
一项前瞻性研究[26]表明,大剂量糖皮质激素、他克莫司和环磷酰胺联合治疗可以改善并发ILD皮肌炎患者的生存率,但这种方案增加了机会性感染的风险,从而导致ILD加重。并发ILD的皮肌炎患者血清中细胞因子水平增高,所以血浆置换也可考虑通过清除细胞因子来达到治疗的目的[8]。早期干预并发ILD(甚至在ILD出现之前)的抗MDA5+皮肌炎患者可以显著提高生存率[9]。尽管采取了积极的免疫抑制治疗,并发RP-ILD的抗MDA5+皮肌炎患者在6个月内的死亡率仍为5%~70%[15, 29-31]。同时,抗MDA5+皮肌炎对于风湿科医师和患者的心理都是一个挑战,应重视抗MDA5+皮肌炎患者的心理健康问题,并及时疏导。
综上所述,眶周皮疹是抗MDA5+皮肌炎患者生存的保护性因素,无眶周皮疹有助于识别快速进展的疾病表型。目前皮肌炎的治疗主要是经验性的而非基于证据的,所以早期识别可能快速进展的患者,进行积极强化免疫抑制治疗可以提高抗MDA5+皮肌炎患者的长期生存率。
[参考文献]
[1]" "LUNDBERG I E, TJÄRNLUND A, BOTTAI M, et al. 2017 European league against rheumatism/American college of rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups[J]. Arthritis Rheumatol, 2017, 69(12):2271-2282.
[2]" "RICHETTE P, DOHERTY M, PASCUAL E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout[J]. Ann Rheum Dis, 2017, 76(1):29-42.
[3]" "MIMORI T, IMURA Y, NAKASHIMA R, et al. Autoantibodies in idiopathic inflammatory myopathy: an update on clinical and pathophysiological significance[J]. Curr Opin Rhe-umatol, 2007, 19(6):523-529.
[4]" "SATO S, HOSHINO K, SATOH T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease[J]. Arthritis Rheum, 2009, 60(7):2193-2200.
[5]" "NAKASHIMA R, IMURA Y, KOBAYASHI S, et al. The RIG-I-like receptor IFIH1/MDA5 is a dermatomyositis-specific autoantigen identified by the anti-CADM-140 antibody[J]. Rheumatology, 2010, 49(3):433-440.
[6]" "CHEN Z, WANG X, YE S. Tofacitinib in amyopathic dermatomyositis-associated interstitial lung disease[J]. N Engl J Med, 2019, 381(3):291-293.
[7]" "KOGUCHI-YOSHIOKA H, OKIYAMA N, IWAMOTO K, et al. Intravenous immunoglobulin contributes to the control of antimelanoma differentiation-associated protein 5 antibody-associated dermatomyositis with palmar violaceous macules/papules[J]. Br J Dermatol, 2017, 177(5):1442-1446.
[8]" "ABE Y, KUSAOI M, TADA K, et al. Successful treatment of anti-MDA5 antibody-positive refractory interstitial lung disease with plasma exchange therapy[J]. Rheumatology, 2020, 59(4):767-771.
[9]" "MATSUDA K M, YOSHIZAKI A, KUZUMI A, et al. Combined immunosuppressive therapy provides favorable prognosis and increased risk of cytomegalovirus reactivation in anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis[J]. J Dermatol, 2020, 47(5):483-489.
[10]" "BOHAN A, PETER J B. Polymyositis and dermatomyositis(first of two parts)[J]. N Engl J Med, 1975, 292(7):344-347.
[11]" "SHI J L, LI S S, YANG H B, et al. Clinical profiles and prognosis of patients with distinct antisynthetase autoantibodies[J]. J Rheumatol, 2017, 44(7):1051-1057.
[12]" "SATO S, HIRAKATA M, KUWANA M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis[J]. Art-hritis Rheum, 2005, 52(5):1571-1576.
[13]" "KATO H, TAKEUCHI O, SATO S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses[J]. Nature, 2006, 441(7089):101-105.
[14]" "HALL J C, CASCIOLA-ROSEN L, SAMEDY L A, et al. Anti-melanoma differentiation-associated protein 5-associated dermatomyositis: expanding the clinical spectrum[J]. Arthritis Care Res, 2013, 65(8):1307-1315.
[15]" "ALLENBACH Y, UZUNHAN Y, TOQUET S, et al. Different phenotypes in dermatomyositis associated with anti-MDA5 antibody: study of 121 cases[J]. Neurology, 2020, 95(1):e70-e78.
[16]" "SABBAGH S, PINAL-FERNANDEZ I, KISHI T, et al. Anti-Ro52 autoantibodies are associated with interstitial lung disease and more severe disease in patients with juvenile myositis[J]. Ann Rheum Dis, 2019, 78(7):988-995.
[17]" "VOJINOVIC T, CAVAZZANA I, CERUTI P, et al. Predictive features and clinical presentation of interstitial lung disease in inflammatory myositis[J]. Clin Rev Allergy Immunol, 2021, 60(1):87-94.
[18]" "WANG L, LV C Y, YOU H X, et al. Rapidly progressive interstitial lung disease risk prediction in anti-MDA5 positive dermatomyositis: the CROSS model[J]. Front Immunol, 2024, 15:1286973.
[19]" "CHEN F, ZUO Y, LI S S, et al. Clinical characteristics of dermatomyositis patients with isolated anti-ro-52 antibody associated rapid progressive interstitial lung disease: data from the largest single Chinese center[J]. Respir Med, 2019, 155:127-132.
[20]" "XU A T, YE Y, FU Q, et al. Prognostic values of anti-Ro52 antibodies in anti-MDA5-positive clinically amyopathic dermatomyositis associated with interstitial lung disease[J]. Rheumatology, 2021, 60(7):3343-3351.
[21]" "HOESLY P M, SLUZEVICH J C, JAMBUSARIA-PAHLAJANI A, et al. Association of antinuclear antibody status with clinical features and malignancy risk in adult-onset dermatomyositis[J]. J Am Acad Dermatol, 2019, 80(5):1364-1370.
[22]" "FATHI M, LUNDBERG I E, TORNLING G. Pulmonary complications of polymyositis and dermatomyositis[J]. Semin Respir Crit Care Med, 2007, 28(4):451-458.
[23]" "SELVA-O'CALLAGHAN A, PINAL-FERNANDEZ I, TRALLERO-ARAGUÁS E, et al. Classification and management of adult inflammatory myopathies[J]. Lancet Neurol, 2018, 17(9):816-828.
[24]" "SATO S, KUWANA M. Clinically amyopathic dermatomyositis[J]. Curr Opin Rheumatol, 2010, 22(6):639-643.
[25]" "GONO T, SATO S, KAWAGUCHI Y, et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis[J]. Rheumatology, 2012, 51(9):1563-1570.
[26]" "TSUJI H, NAKASHIMA R, HOSONO Y, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis[J]. Arthritis Rheumatol, 2020, 72(3):488-498.
[27]" "YE S, CHEN X X, LU X Y, et al. Adult clinically amyopathic dermatomyositis with rapid progressive interstitial lung disease: a retrospective cohort study[J]. Clin Rheumatol, 2007, 26(10):1647-1654.
[28]" "CHEN Z Y, CAO M S, PLANA M N, et al. Utility of anti-melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis[J]. Arthritis Care Res, 2013, 65(8):1316-1324.
[29]" "WU W L, XU W W, SUN W J, et al. Forced vital capacity predicts the survival of interstitial lung disease in anti-MDA5 positive dermatomyositis: a multi-centre cohort study[J]. Rheumatology, 2021, 61(1):230-239.
[30]" "YANG Q H, LI T F, ZHANG X, et al. Initial predictors for short-term prognosis in anti-melanoma differentiation-associated protein-5 positive patients[J]. Orphanet J Rare Dis, 2021, 16(1):58.
[31]" "LI Y H, LI Y M, WU J, et al. Predictors of poor outcome of anti-MDA5-associated rapidly progressive interstitial lung disease in a Chinese cohort with dermatomyositis[J]. J Immunol Res, 2020, 2020:2024869.
[收稿日期] 2024-06-05
