单核细胞计数/高密度脂蛋白胆固醇及促甲状腺激素对绝经期女性急性冠脉综合征患者预测价值及与冠状动脉病变相关性研究
2024-08-07戴承晔邓毅凡何胜虎张晶



【摘要】 背景 既往研究发现单核细胞计数、高密度脂蛋白胆固醇(HDL-C)、促甲状腺激素(TSH)与急性冠脉综合征(ACS)相关,然而单核细胞计数/高密度脂蛋白胆固醇(MHR)与ACS发病的相关性研究较少,绝经期女性MHR、TSH与ACS发病是否存在关联仍不明确。目的 探讨MHR、TSH对绝经期女性ACS患者发病的预测价值,并研究上述指标与患者冠状动脉狭窄程度的相关性。方法 选择2020—2021年在苏北人民医院心血管内科住院诊治并行冠状动脉造影的325例绝经期女性作为研究对象。通过电子病历系统收集患者一般资料,研究对象入院后采集静脉血检测单核细胞计数、总胆固醇(TC)、三酰甘油(TG)、HDL-C、低密度脂蛋白胆固醇(LDL-C)、TSH等。以双平面Simpson法测量左心室射血分数(LVEF),通过冠状动脉造影术观察冠状动脉病变情况。采用Gensini评分标准统一衡量冠状动脉病变情况。符合ACS诊断标准的患者为ACS组(n=184),非ACS者为对照组(n=141)。同时依据Gensini评分三分位数将ACS组进行分组:≤36.5分为低危亚组(n=59),36.6~66.5分为中危亚组(n=64),>66.5分为高危亚组(n=61)。采用单因素及多因素Logistic回归分析探究ACS的影响因素。绘制受试者工作特征(ROC)曲线评估TSH、MHR及联合检测对ACS的诊断价值并计算ROC曲线下面积(AUC)。采用Spearman秩相关分析探究TSH、MHR及联合检测指标与Gensini积分的相关性。结果 ACS组与对照组患者基线资料结果示,ACS组BMI、吸烟比例、高血压、糖尿病、LDL-C、单核细胞计数、TSH、MHR高于对照组,LVEF、HDL-C低于对照组(P<0.05)。多因素Logistic回归分析结果示,吸烟、高血压、BMI≥24.0 kg/m2、LDL-C≥3.30 mmol/L、TSH≥2.1 mU/L、MHR≥0.25是绝经期女性发生ACS的危险因素,HDL-C≥1.2 mmol/L为保护因素(P<0.05)。ROC曲线结果显示,MHR、TSH及联合预测指标诊断绝经期女性ACS的AUC分别为0.777(95%CI=0.725~0.830,P<0.001)、0.747(95%CI=0.694~0.800,P<0.001)、0.810(95%CI=0.764~0.857,P<0.001)。中危亚组、高危亚组MHR、TSH均高于低危亚组,高危亚组MHR、TSH高于中危亚组(P<0.05)。Spearman秩相关分析结果示,ACS组患者MHR(rs=0.497,P<0.01)、TSH(rs=0.498,P<0.01)及联合预测指标与Gensini评分均呈正相关(rs=0.600,P<0.001)。结论 TSH及MHR升高是绝经期女性发生ACS的独立危险因素,两指标及联合对病情预测具有较高的灵敏度和特异度,并与患者冠状动脉病变程度相关,对绝经期女性ACS的早期识别及风险评估具有一定的临床应用价值。
【关键词】 急性冠脉综合征;绝经期;单核细胞计数/高密度脂蛋白胆固醇比值;促甲状腺激素;风险评估
【中图分类号】 R 542.22 【文献标识码】 A DOI:10.12114/j.issn.1007-9572.2024.0094
Clinical Study on the Predictive Value of Monocyte Count to High-Density Lipoprotein Cholesterol Ratio and Thyroid-stimulating Hormone for Acute Coronary Syndrome in Postmenopausal Women and Their Correlation with Coronary Artery Lesions
DAI Chengye1,2,3,DENG Yifan3,4,HE Shenghu3,4,ZHANG Jing3,4*
1.The Yangzhou School of Clinical Medicine of Dalian Medical University,Yangzhou 225001,China
2.Ningbo NO.2 Hospital,Ningbo 315010,China
3.Northern Jiangsu People's Hospital,Yangzhou 225001,China
4.Northern Jiangsu People's Hospital Affiliated to Yangzhou University,Yangzhou 225001,China
*Corresponding author:ZHANG Jing,Chief physician;E-mail:zhangjingyjs@163.com
【Abstract】 Background Previous studies have found associations between monocyte count,high-density lipoprotein cholesterol(HDL-C),and thyroid-stimulating hormone(TSH)with acute coronary syndrome(ACS). However,research on the correlation between the monocyte count to high-density lipoprotein cholesterol ratio(MHR)and the onset of ACS is limited. The association between MHR,TSH,and the onset of ACS in postmenopausal women remains unclear. Objective To explore the predictive value of MHR and TSH for the onset of ACS in postmenopausal women and to investigate the correlation between these indicators and the degree of coronary artery stenosis in patients. Methods A total of 325 postmenopausal women hospitalized in the Department of Cardiology at Northern Jiangsu People's Hospital,from 2020 to 2021 and who underwent coronary angiography were selected as the study subjects. Patient general information was collected through the electronic medical record system. Venous blood was collected upon admission to measure monocyte count,total cholesterol(TC),triglycerides(TG),HDL-C,low-density lipoprotein cholesterol(LDL-C),and TSH. Left ventricular ejection fraction(LVEF)was measured using the biplane Simpson method,and coronary artery lesions were observed through coronary angiography. The Gensini scoring system was used to uniformly measure the extent of coronary artery lesions. Patients meeting the diagnostic criteria for ACS were classified as the ACS group(n=184),and non-ACS patients as the control group(n=141). The ACS group was further divided into subgroups based on the tertiles of the Gensini score:≤36.5 as the low-risk subgroup(n=59),36.6-66.5 as the moderate-risk subgroup(n=64),and &gQl3cvamcgmcUCfFIwKJXXHPZ5TYN+okbZbUCDloxsO8=t;66.5 as the high-risk subgroup(n=61). Univariate and multivariate Logistic regression analyses were used to explore the influencing factors of ACS. The receiver operating characteristic(ROC)curve was plotted to evaluate the diagnostic value of TSH,MHR,and combined detection for ACS and to calculate the area under the curve(AUC). Spearman's rank correlation analysis was used to explore the correlation between TSH,MHR,and combined detection indicators with the Gensini score. Results The baseline data of patients in the ACS and control groups showed that BMI,smoking rate,hypertension,diabetes,LDL-C,monocytes,TSH,and MHR in the ACS group were higher than in the control group,while LVEF and HDL-C were lower(P<0.05). Multivariate Logistic regression analysis showed that smoking,hypertension,BMI≥24.0 kg/m2,LDL-C≥3.30 mmol/L,TSH≥2.1 mU/L,and MHR≥0.25 were risk factors for the occurrence of ACS in postmenopausal elderly women,and HDL-C≥1.2 mmol/L was a protective factor(P<0.05). The ROC curve analysis demonstrated that the AUC for MHR,TSH,and the combined predictive index in diagnosing ACS in postmenopausal women were 0.777(95%CI=0.725-0.830,P<0.001),0.747(95%CI=0.694-0.800,P<0.001),and 0.810(95%CI=0.764-0.857,P<0.001),respectively. In the moderate and high-risk subgroups,MHR and TSH were higher than in the low-risk subgroup,and the high-risk subgroup had higher MHR and TSH than the moderate-risk subgroup(P<0.05). Spearman's rank correlation analysis showed that in the ACS group,MHR(rs=0.497,P<0.01),TSH(rs=0.498,P<0.01),and the combined predictive indicators were positively correlated with the Gensini score(rs=0.600,P<0.001). Conclusion Elevated TSH and MHR are independent risk factors for the occurrence of ACS in postmenopausal women. Both indicators and their combination have certain sensitivity and specificity for disease prediction and are correlated with the extent of coronary artery lesions in patients,which has certain clinical application value for the early identification and risk assessment of ACS in postmenopausal women.
【Key words】 Acute coronary syndrome;Menopause;Monocyte count to high-density lipoprotein cholesterol ratio;Thyroid-stimulating hormone;Risk assessment
由于人口老龄化、饮食质量提高、生活压力增大等多种原因,中国的急性冠脉综合征(ACS)发病率持续增高,其中绝经后女性ACS患者发病后短期的死亡率比同年龄段男性患者高20%[1],这种死亡率差异可能与绝经后激素水平变化有关[2]。ACS发病机制涉及炎症反应和脂质代谢等多方面,而绝经期女性激素水平改变明显,易出现以代谢障碍为特征的一系列生理变化,影响了激素的血管保护作用[3]。在这一过程中炎症诱导单核细胞迁移到炎症组织[4]、血清促甲状腺激素(TSH)水平升高、高密度脂蛋白胆固醇(HDL-C)功能失调诱导胆固醇逆向转运能力受损均与ACS发病密切相关。考虑用单一种炎症细胞或炎症因子来评估其病情,容易受感染、药物、创伤等因素的影响而有所偏差,综合性指标可更全面地反映机体炎症水平及应激状态[5],如单核细胞计数/高密度脂蛋白胆固醇(MHR)与心血管疾病的发生、发展密切相关[6]。据此,本研究以绝经期女性ACS患者为对象,通过观察其MHR、TSH水平,探讨两者及联合对ACS发生的预测价值,并探讨其与冠状动脉病变的相关性,为临床上此类患者早期风险评估、靶点干预、改善预后提供指导。
1 对象与方法
1.1 研究对象
选择2020—2021年在苏北人民医院心血管内科住院诊治并行冠状动脉造影的325例绝经期女性作为研究对象。纳入标准:(1)年龄50~88岁,停经1年及以上;(2)首次接受冠状动脉造影术。排除标准:(1)严重心肺功能不全者;(2)甲状腺肿瘤、垂体促甲状腺激素腺瘤等内分泌疾病者;(3)服用可能影响甲状腺功能的药物(治疗甲状腺功能亢进或甲状腺功能减退),曾行甲状腺切除手术或曾行甲状腺放射性核素治疗;(4)2周内有感染、发热等疾病者;(5)患周围血管性疾病者;(6)慢性消耗性疾病、风湿免疫性疾病等慢性疾病;(7)严重肝肾功能不全;(8)肿瘤、血液病等影响血细胞计数的疾病;(9)临床资料不全者。本研究符合《赫尔辛基宣言》并通过了苏北人民医院医学伦理委员会审批(审核批件号:2022ky326),入选患者均自愿参加本研究并签署知情同意书。
1.2 研究方法
1.2.1 一般资料:通过电子病历系统收集患者年龄、身高、体质量、吸烟史、饮酒史、糖尿病、高血压病史等一般资料。患者非同日3次测量血压,收缩压≥140 mmHg(1 mmHg=0.133 kPa)和/或舒张压≥90 mmHg诊断为高血压。依据《中国2型糖尿病防治指南(2020年版)》[7]诊断糖尿病。吸烟定义为患者吸烟数量>1支/d,吸烟时间≥6个月。饮酒定义为患者饮酒量≥50 mL/次,>3次/周,连续饮酒6个月以上[8]。
1.2.2 临床检验结果:研究对象入院后尽快采集静脉血,检测单核细胞计数;空腹12 h后于第2天早晨采集静脉血,检测总胆固醇(TC)、三酰甘油(TG)、HDL-C、低密度脂蛋白胆固醇(LDL-C)、TSH等。以双平面Simpson法测量左心室射血分数(left ventricular ejection fractions,LVEF)。患者冠状动脉造影术在右冠状动脉至少投照2个体位,左冠状动脉至少投照4个体位,由2名心血管内科介入医师对图像进行分析和结果判读,判断冠状动脉狭窄情况,结合胸痛、胸闷等临床症状,心电图、心肌坏死标志物等辅助检查,判定血管情况。ACS诊断参照《急性冠脉综合征急诊快速诊治指南(2019)》[9],并记录冠状动脉狭窄1e9671fe32573dcc2c8c48b38820c06f2dfa75a37432d324ee72616b98b83868程度及病变的支数。
1.2.3 冠状动脉病变程度评估:采用Gensini评分标准[10]统一衡量冠状动脉病变情况,血管无狭窄基本评分0分,狭窄程度≤25%基本评分1分,狭窄程度26%~50%基本评分2分,狭窄程度51%~75%基本评分4分,狭窄程度76%~90%基本评分8分,狭窄程度91%~99%基本评分16分,狭窄程度100%基本评分32分。不同病变血管对应的权重系数如下,左主干系数为5,左前降支近段和左回旋支近段系数为2.5,左前降支中段和左回旋支中段系数为1.5,左前降支远段、左回旋支远段、左回旋支后降支、第一对角支及右冠状动脉系数为1。根据每一条冠状动脉其狭窄程度所对应的基本评分值,及该病变血管对应的权重系数,将二者相乘即为Gensini评分,对于多处病变者,将各处病变的Gensini评分相加,即为该患者Gensini总评分。
1.2.4 分组:符合ACS诊断标准的患者为ACS组(n=184),非ACS者为对照组(n=141)。同时参考文献[1 1],依据Gensini评分三分位数将ACS组进行分组:≤36.5分为低危亚组(n=59),36.6~66.5分为中危亚组(n=64),>66.5分为高危亚组(n=61)。
1.3 统计学方法
采用SPSS 20.0统计学软件进行数据分析。计数资料采用例(%)表示,两组间比较采用χ2检验;采用Kolmogorov-Smirnov检验计量资料数据是否符合正态分布,符合正态分布的计量资料以(x-±s)表示,两组间比较用独立样本t检验,多组间比较采用单因素方差分析,组间两两比较采用LSD-t检验;不符合正态分布的计量资料以M(P25,P75)表示,两组比较采用Mann-Whitney U检验。采用单因素及多因素Logistic回归分析探究ACS的影响因素。绘制受试者工作特征(ROC)曲线评估TSH、MHR及联合检测对ACS的诊断价值并计算曲线下面积(AUC)。采用Spearman秩相关分析探究TSH、MHR及联合检测指标与Gensini积分的相关性。以P<0.05为差异有统计学意义。
2 结果
2.1 ACS组与对照组患者基线资料
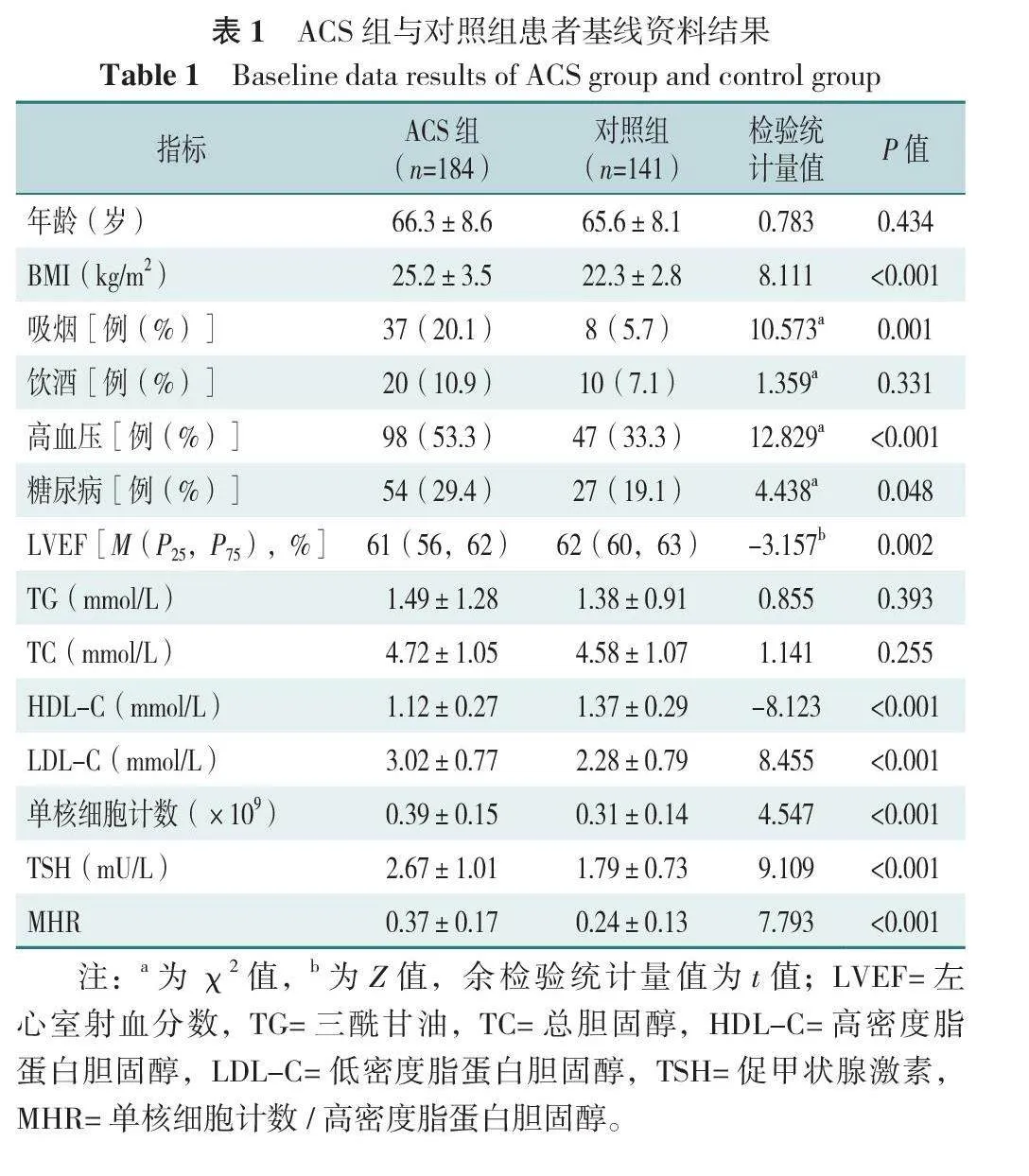
两组患者基线资料比较结果示,ACS组BMI、吸烟比例、高血压、糖尿病、LDL-C、单核细胞计数、TSH、MHR高于对照组,LVEF、HDL-C低于对照组,差异有统计学意义(P<0.05);两组患者年龄、饮酒比例、TG、TC比较,差异无统计学意义(P>0.05),见表1。
2.2 绝经期女性发生ACS影响因素的单因素和多因素Logistic回归分析
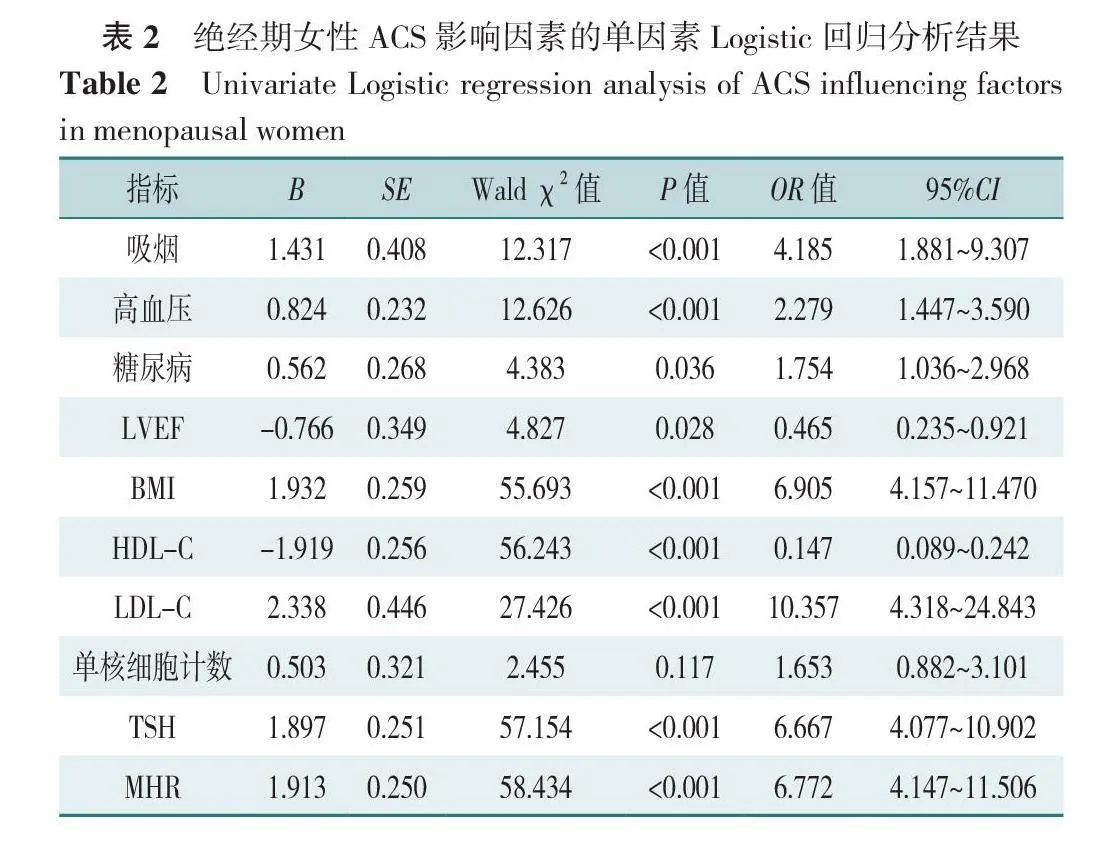
以绝经期女性是否发生ACS(赋值:是=1,否=0)为因变量,以表1中差异有统计学意义变量[吸烟(赋值:是=1,否=0)、高血压(赋值:是=1,否=0)、糖尿病(赋值:是=1,否=0)、LVEF(赋值:≤50%=0,>50%=1)、BMI(赋值:<24.0 kg/m2=0,≥24.0 kg/m2=1)、单核细胞计数(赋值:<0.5×109=0,≥0.5×109=1)、HDL-C(赋值:<1.2 mmol/L=0,≥1.2 mmol/L=1)、LDL-C(赋值:<3.30 mmol/L=0,≥3.30 mmol/L=1)、TSH(赋值:<2.1 mU/L=0,≥2.1 mU/L=1)、MHR(赋值:<0.25=0,≥0.25=1)]为自变量进行单因素Logistic回归分析,结果显示吸烟、高血压、糖尿病、LVEF、BMI、HDL-C、LDL-C、TSH、MHR为绝经期女性发生ACS的影响因素(P<0.05),见表2。
将单因素Logistic回归分析中P<0.05的指标纳入多因素Logistic回归分析(赋值同上),结果显示,吸烟、高血压、BMI≥24.0 kg/m2、LDL-C≥3.30 mmol/L、TSH≥2.1 mU/L、MHR≥0.25是绝经期女性发生ACS的危险因素,HDL-C≥1.2 mmol/L为保护因素(P<0.05),见表3。
2.3 MHR、TSH及联合预测指标对绝经期女性ACS的诊断价值
ROC曲线结果显示,MHR、TSH及联合预测指标诊断绝经期女性ACS的AUC分别为0.777(95%CI=0.725~0.830)、0.747(95%CI=0.694~0.800)、0.810(95%CI=0.764~0.857),见表4、图1。
2.4 低危亚组、中危亚组、高危亚组MHR、TSH水平比较
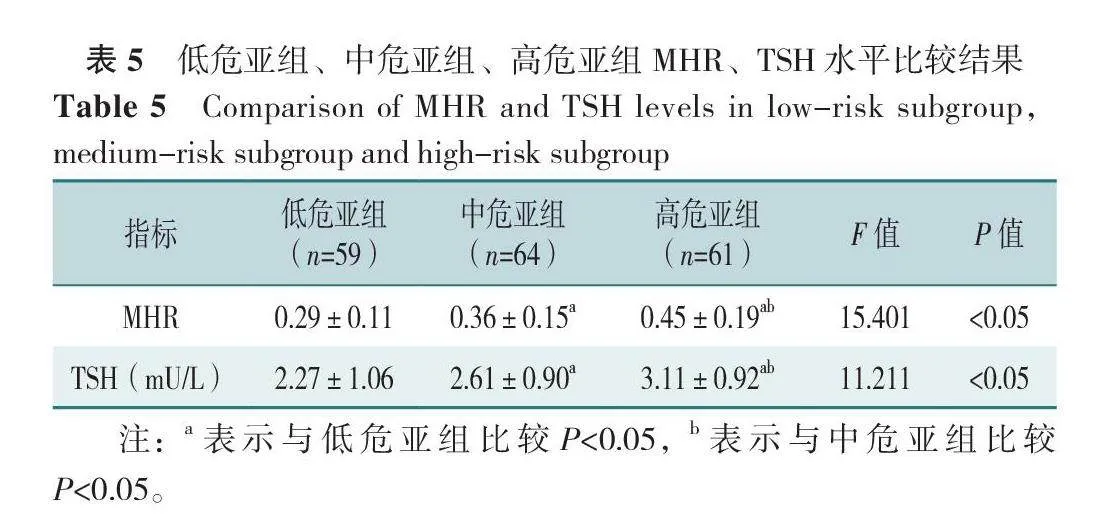
低危亚组、中危亚组、高危亚组MHR、TSH比较,差异有统计学意义(P<0.05)。组间两两比较结果示,中危亚组、高危亚组MHR、TSH均高于低危亚组,高危亚组MHR、TSH高于中危亚组,差异有统计学意义(P<0.05),见表5。
2.5 MHR、TSH及联合预测与Gensini评分相关性分析
Spearman秩相关分析结果示,ACS组患者MHR(rs=0.497,P<0.01)、TSH(rs=0.498,P<0.01)及联合预测指标与Gensini评分均呈正相关(rs=0.600,P<0.001)。
3 讨论
本研究通过对比研究绝经期女性ACS患者与非ACS患者的基线资料和临床指标资料发现,ACS组伴有高血压、糖尿病、吸烟的比例及BMI明显升高,而饮酒对绝经期女性患者有无ACS影响不大;吸烟、高血压、糖尿病、BMI升高均为绝经期女性发生ACS的独立危险因素,这与SALTIKI等[12]发现绝经期妇女高脂血症、糖尿病、冠心病家族史以及胰岛素抵抗等多种危险因素与冠心病严重程度密切相关的结果相符。
TSH通过调控甲状腺激素的循环浓度影响心血管系统[13],如患有亚临床甲状腺功能减退心血管疾病患者的全因死亡率增加[14]。同时,TSH参与脂肪生成和分解[15-16],可以影响血清TC和LDL-C水平[17]。DELITALA等[18]发现女性绝经状态显著改变了TSH和血脂之间的相关性。本研究发现绝经期女性ACS组患者TSH较对照组明显升高,且多因素Logistic回归分析结果显示TSH水平升高是此类人群罹患ACS的独立危险因素,相关性分析结果示TSH与Gensini评分呈正相关,表明高TSH与绝经期女性发生ACS及冠状动脉严重病变密切相关。
HDL-C在延缓动脉粥样硬化进展中起到了重要的作用,与抗炎和抗氧化有关,而LDL-C可以在氧自由基的控制下,运载胆酸,形成脂质和相关氧化物,导致动脉粥样硬化,控制LDL-C水平是防治心血管疾病有效的策略之一[19]。在绝经期女性体内,随着雌激素水平的下降,载脂蛋白基因和低密度脂蛋白(LDL)受体的表达减少[20],对绝经期女性加用雌激素补充治疗,可以降低LDL-C和脂蛋白(a)水平,增加HDL-C水平,改善绝经期女性的脂质分布[21]。本研究发现HDL-C水平升高是绝经期女性发生ACS的独立保护因素,ACS组LDL-C水平明显升高,HDL-C水平明显降低,这与高LDL-C、低HDL-C是总人群冠心病危险因素的认识较为一致。但在达到目标LDL-C水平后,部分患者仍有罹患心血管疾病的风险[22],已有文献表明,炎症指标在绝经期女性冠心病的发生和发展中起主导作用,BJÖRNSTRÖM等[23]发现在动脉粥样硬化过程的早期阶段,绝经期女性的血管内皮更易捕获LDL分子,触发局部炎症反应,单核细胞在活化内皮上滚动,从而让外周循环单核细胞动员到病变组织[24]。在进入组织后,活化的单核细胞与受损的内皮细胞相互作用,诱导促炎细胞因子和黏附分子的分泌[25]。本研究的临床指标比较中可以看出,ACS组单核细胞水平高于对照组,考虑可能与不同程度心肌细胞损伤有关。因此,控制患者LDL-C水平的同时降低炎症水平在ACS的发生、发展中起着重要作用。目前研究发现,在胸痛患者中,即使LDL-C<1.8 mmol/L,新型炎症指标MHR以及HDL-C仍然与ACS的发生和严重程度相关[26]。其中,MHR代表了炎症和脂质代谢之间的平衡状态,在一项针对ACS患者的研究中,其被发现是冠心病严重程度和未来心脏事件发展的独立预测因子[27]。单核细胞转变为炎症性巨噬细胞,通过摄取氧化LDL颗粒形成泡沫细胞来协调动脉粥样硬化的进展[28]。HDL-C通过逆转巨噬细胞胆固醇的转运,改善内皮功能障碍,抑制黏附分子的表达,抑制LDL-C的氧化。HDL-C还可以抑制单核祖细胞的增殖分化和单核细胞的活化[29]。本研究中ACS组患者MHR水平高于对照组,将单因素分析中有意义的指标纳入多因素Logistic回归分析中,发现MHR升高是此类人群罹患ACS的独立危险因素,与既往研究是相符的。同时本研究发现随着患者血MHR指标的升高,冠状动脉病变程度加重,MHR与Gensini评分呈正相关。这与女性绝经过渡期及以后,卵巢分泌激素能力改变,雌激素水平下降,炎症风险增加,抑制早期动脉粥样硬化能力减弱相关[30]。
本研究存在以下局限性:本研究作为单中心回顾性研究存在样本量较少、纳入影响因素不足和选择偏倚等局限性,后续可进一步增加人群,扩展试验,进行更多中心大样本的前瞻性研究以丰富及完善本研究结果。
4 小结
MHR和TSH可协同传统心血管危险因素,一定程度上更精确、全面评估绝经期女性ACS患者的发病风险。ROC曲线可见TSH、MHR以及两指标联合对绝经期女性ACS的发生均具有较高预测价值,其中两指标联合预测的灵敏度最高,三者特异度类似。MHR与TSH均是临床易获取的生物标志物,本研究通过探究TSH、MHR与冠状动脉病变的相关性,并探讨了TSH、MHR及其联合预测指标在绝经期女性冠心病患者中的意义,发现在绝经期女性患者中,ACS组患者血清MHR、TSH水平显著高于对照组患者,并且随着MHR、TSH水平增高,冠状动脉病变逐渐加重,这意味着在绝经期女性临床诊疗中通过MHR、TSH指标预测ACS的风险,为扩展绝经期女性的疾病风险评估及预测指标,优化此类人群冠心病一、二级预防具有重大价值。
作者贡献:戴承晔进行研究的构思、设计以及文章撰写;邓毅凡进行数据的收集、整理与统计学分析;何胜虎、张晶负责文章的修订;张晶负责文章的质量控制及审校,对文章整体负责,监督管理。
本文无利益冲突。
参考文献
POTTS J,SIRKER A,MARTINEZ S C,et al. Persistent sex disparities in clinical outcomes with percutaneous coronary intervention:insights from 6.6 million PCI procedures in the United States[J]. PLoS One,2018,13(9):e0203325. DOI:10.1371/journal.pone.0203325.
U1ak73jxWlWobqxJosQJB8H3PwcWMjR02bbwhqTqmpY=SAVONITTO S,COLOMBO D,FRANCO N,et al. Age at menopause and extent of coronary artery disease among postmenopausal women with acute coronary syndromes[J]. Am J Med,2016,129(11):1205-1212. DOI:10.1016/j.amjmed.2016.05.031.
DAMA,BAGGIO C,BOSCARO C,et al. Estrogen receptor functions and pathways at the vascular immune interface[J]. Int J Mol Sci,2021,22(8):4254. DOI:10.3390/ijms22084254.
KRATOFIL R M,KUBES P,DENISET J F. Monocyte conversion during inflammation and injury[J]. Arterioscler Thromb Vasc Biol,2017,37(1):35-42. DOI:10.1161/ATVBAHA.116.308198.
LI Q X,MA X T,SHAO Q Y,et al. Prognostic impact of multiple lymphocyte-based inflammatory indices in acute coronary syndrome patients[J]. Front Cardiovasc Med,2022,9:811790. DOI:10.3389/fcvm.2022.811790.
KARATAŞ M B,ÇANGA Y,ÖZCAN K S,et al. Monocyte to high-density lipoprotein ratio as a new prognostic marker in patients with STEMI undergoing primary percutaneous coronary intervention[J]. Am J Emerg Med,2016,34(2):240-244. DOI:10.1016/j.ajem.2015.10.049.
中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2020年版)[J]. 中华糖尿病杂志,2021,13(4):315-409. DOI:10.3760/cma.j.cn115791-20210221-00095.
刘强. 血尿酸水平与冠心病及其严重程度相关性的研究[D]. 唐山:唐山华北理工大学,2023. DOI:10.27108/d.cnki.ghelu.2023.001356.
张新超,于学忠,陈凤英,等. 急性冠脉综合征急诊快速诊治指南(2019)[J]. 临床急诊杂志,2019,20(4):253-262. DOI:10.13201/j.issn.1009-5918.2019.04.001.
RAMPIDIS G P,BENETOS G,BENZ D C,et al. A guide for Gensini Score calculation[J]. Atherosclerosis,2019,287:181-183. DOI:10.1016/j.atherosclerosis.2019.05.012.
DEGER M,OZMEN C,AKDOGAN N,et al. The relationship between gensini score and erectile dysfunction in patients with chronic coronary syndrome[J]. Sex Med,2021,9(3):100376. DOI:10.1016/j.esxm.2021.100376.
SALTIKI K,DOUKAS C,KANAKAKIS J,et al. Severity of cardiovascular disease in women:relation with exposure to endogenous estrogen[J]. Maturitas,2006,55(1):51-57. DOI:10.1016/j.maturitas.2005.12.008.
GÜRDOĞAN M,ALTAY S,KORKMAZ S,et al. The effect of thyroid stimulating hormone level within the reference range on in-hospital and short-term prognosis in acute coronary syndrome patients[J]. Medicina,2019,55(5):175. DOI:10.3390/medicina55050175.
SUN Y,TENG D,ZHAO L,et al. Impaired sensitivity to thyroid hormones is associated with hyperuricemia,obesity,and cardiovascular disease risk in subjects with subclinical hypothyroidism[J]. Thyroid,2022,32(4):376-384. DOI:10.1089/thy.2021.0500.
ZHANG J M,WU H X,MA S Z,et al. TSH promotes adiposity by inhibiting the browning of white fat[J]. Adipocyte,2020,9(1):264-278. DOI:10.1080/21623945.2020.1783101.
LI J X,KONG D X,GAO X Y,et al. TSH attenuates fatty acid oxidation in hepatocytes by reducing the mitochondrial distribution of miR-449a/449b-5p/5194[J]. Mol Cell Endocrinol,2021,530:111280. DOI:10.1016/j.mce.2021.111280.
SU X,PENG H,CHEN X,et al. Hyperlipidemia and hypothyroidism[J]. Clin Chim Acta,2022,527:iB878J0kJPdAz404UKWLFQ==61-70. DOI:10.1016/j.cca.2022.01.006.
DELITALA A P,SCUTERI A,FIORILLO E,et al. Role of adipokines in the association between thyroid hormone and components of the metabolic syndrome[J]. J Clin Med,2019,
8(6):764. DOI:10.3390/jcm8060764.
BREATHETT K,SIMS M,GROSS M,et al. Cardiovascular health in American indians and Alaska natives:a scientific statement from the American heart association[J]. Circulation,2020,141(25):e948-959. DOI:10.1161/CIR.0000000000000773.
SHIFFLER J A,GOERGER K A,GORRES-MARTENS B K. Estrogen receptor α activation modulates the gut microbiome and type 2 diabetes risk factors[J]. Physiol Rep,2022,10(11):e15344. DOI:10.14814/phy2.15344.
HODIS H N,MACK W J. Menopausal hormone replacement therapy and reduction of all-cause mortality and cardiovascular disease:it is about time and timing[J]. Cancer J,2022,28(3):208-223. DOI:10.1097/PPO.0000000000000591.
KOENIG W. Low-grade inflammation modifies cardiovascular risk even at very low LDL-C levels:are we aiming for a dual target concept?[J]. Circulation,2018,138(2):150-153. DOI:10.1161/CIRCULATIONAHA.118.035107.
BJÖRNSTRÖM L,SJÖBERG M. Signal transducers and activators of transcription as downstream targets of nongenomic estrogen receptor actions[J]. Mol Endocrinol,2002,16(10):2202-2214. DOI:10.1210/me.2002-0072.
BAJPAI G,BREDEMEYER A,LI W J,et al. Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury[J]. Circ Res,2019,124(2):263-278. DOI:10.1161/CIRCRESAHA.118.314028.
GHATTAS A,GRIFFITHS H R,DEVITT A,et al. Monocytes in coronary artery disease and atherosclerosis:where are we now?[J]. J Am Coll Cardiol,2013,62(17):1541-1551. DOI:10.1016/j.jacc.2013.07.043.
LIU M P,LIU X J,WEI Z,et al. MHR and NHR but not LHR were associated with coronary artery disease in patients with chest pain with controlled LDL-C[J]. J Investig Med,2022,70(7):1501-1507. DOI:10.1136/jim-2021-002314.
CETIN M S,OZCAN CETIN E H,KALENDER E,et al. Monocyte to HDL cholesterol ratio predicts coronary artery disease severity and future major cardiovascular adverse events in acute coronary syndrome[J]. Heart Lung Circ,2016,25(11):1077-1086. DOI:10.1016/j.hlc.2016.02.023.
KIM K W,IVANOV S,WILLIAMS J W. Monocyte recruitment,specification,and function in atherosclerosis[J]. Cells,2020,10(1):15. DOI:10.3390/cells10010015.
NAZIR S,JANKOWSKI V,BENDER G,et al. Interaction between high-density lipoproteins and inflammation:function matters more than concentration![J]. Adv Drug Deliv Rev,2020,159:94-119. DOI:10.1016/j.addr.2020.10.006.
NEWSON L. Menopause and cardiovascular disease[J]. Post Reprod Health,2018,24(1):44-49. DOI:10.1177/2053369117749675.
(收稿日期:2024-04-08;修回日期:2024-06-10)
(本文编辑:邹琳)
