基于代谢组学比较分析5个栽培种梨叶片代谢物的差异
2024-06-30焦慧君冉昆董冉董肖昌关秋竹王宏伟魏树伟
焦慧君 冉昆 董冉 董肖昌 关秋竹 王宏伟 魏树伟


摘 要:【目的】为加强对梨叶片资源的药用价值以及农副产品的开发利用,全面分析梨叶片中次生代谢物质的种类。【方法】通过代谢组学分析库尔勒香梨、超红、南红、鸭梨、翠冠5个品种与福鼎大白茶的成熟期叶片的代谢物差异。【结果】PCA分析表明,代谢物种内聚类较为集中,种间聚类较远;5个比较组特异性差异代谢物分别有55、155、54、59和28种;Heatmap分析表明,排名前50的差异代谢物在5个主栽梨叶片中的表达量具有显著差异;黄酮和黄酮醇生物合成、ABC转运和类黄酮生物合成等途径是差异代谢物富集的主要通路,其中山梨醇、果糖、甘露醇、柠檬酸、水杨酸、马来酸等含量显著上调。【结论】不同栽培种梨叶片的代谢物在种类和表达量上均具有显著差异,研究结果为开发利用梨叶片资源提供了理论基础。
关键词:梨;叶片;次生代谢物质
中图分类号:S661.2 文献标志码:A 文章编号:1009-9980(2024)06-1111-14
Comparative analysis of metabolites in leaves of five pear cultivars based on metabonomics
JIAO Huijun, RAN Kun#, DONG Ran, DONG Xiaochang, GUAN Qiuzhu, WANG Hongwei, WEI Shuwei*
(Shandong Institute of Pomology, Taian 271000, Shandong, China)
Abstract: 【Objective】 Plant leaves are rich in secondary metabolites, mainly including phenols, terpenoids, alkaloids and other secondary substances, which have physiological functions in regulating plant growth, biological defense, anti-abiotic stress and so on. Pear is one of the important economic fruits of Rosaceae, and widespread in the world. China is one of the origins of pears, and has a long history of cultivation and rich variety resources. Pyrus pyrifolia, P. ussuriensis, P. sinkiangensis, P. communis and P. bretschnrideri are the main cultivated species of pear in China. Pears contain a wide variety of phenolic compounds, which would be beneficial for human health. Therefore, there have been many researches on the metabolite content of pear fruit. There are also abundant secondary metabolites in pear leaves, which are very important for the growth and development of fruit trees. However, there is a lack of systematic analysis of the metabolites in pear leaves. 【Methods】 In this study, we collected mature, healthy pear leaves in September, and carried them into lab. The leaves of Fuding Dabai tea rich in secondary metabolites were selected as control, and the differences of metabolites in the mature leaves of five cultivars Kuerle, Chaohong, Nanhong, Yali and Cuiguan were compared and analyzed by metabolomics. PCA analysis, Heatmap analysis, KEGG analysis and K-means analysis were used to analysis differential metabolites. 【Results】 A total of 9011 metabolites were detected under positive and negative ion mode. 11 747 peaks and 4987 metabolites were identified in the positive ion model, and 11 575 peaks and 4024 metabolites were identified in the negative ion model. The principal component analysis (PCA) showed that the detected intra-species clustering of metabolic species was relatively concentrated, while the interspecific clustering was relatively distant. The metabolites of the leaves of Chaohong were clustered far apart from those of the other four kinds of pear, which indicated the difference of metabolites in the leaves of occidental pear and oriental pear. In the oriental pear varieties, the metabolites of Kuerle fragrant pear were far from those of Yali, Cuiguan and Nanhong, and there were some differences. The number of differential metabolites of Kuerle, Chaohong, Nanhong, Yali, Cuiguan and Fuding Dabai was 1194, 1153, 1176, 1153 and 1164, respectively. There were 456, 468, 469, 481 and 446 kinds of significantly up-regulated differential metabolites in Kuerle, Chaohong, Nanhong, Yali and Cuiguan compared with Fuding Dabai, respectively, and 728, 685, 707, 672 and 718 kinds of significantly down-regulated differential metabolites. The number of common differential metabolites was 747, and the specific differential metabolites were 55, 155, 54, 59 and 28, respectively. The heatmap analysis showed that the expression levels of the top 50 differential metabolites were significantly different in the leaves of 5 cultivated pears. Among the top 50 differential metabolites, the number of metabolites in Yali leaves was the largest, with about 24 kinds, mainly including chrysanthemin, genistin, swertiajaponin, quercetin 3-lathyroside, picein, swertiajaponin, 6alpha-Hydroxycastasterone, glycyrrhetinic acid and protobassic acid. The correlation analysis could measure the degree of correlation between different metabolites, and further understand the interrelationship between metabolites in the process of biological state change. Therefore, we used Pearson correlation coefficient to measure the correlation between the top 50 differential metabolites with metabolite expression. It was found that 642 pairs of metabolites were positively correlated, and 633 pairs of metabolites were negatively correlated. We divided all the differential metabolites into 12 subclusters using K-mean analysis, and the results showed that the variation trend of differential metabolites in these 12 subclusters was basically the same. The number of differential metabolites in subclusters 1, 2, 6, 8, 10 and 11 was the most distributed, and the number was 229, 200, 104, 304, 256 and 81, respectively. The variation trend of differential metabolites in cluster 1, 2 and 8 was basically the same. The expression of metabolites in Chaohong leaves was quite different in subgroups 3, 5 and 7, and the contents of metabolites in Nanhong leaves was quite different in subgroup 12. The KEGG analysis were used to analyze the differential enrichment of the metabolic pathways between the leaves of Kuerle, Chaohong, Nanhong, Yali and Cuiguan and the leaves of Fuding Dabai. The 5 comparison groups were enriched in 78 KEGG pathways, there were 76, 71, 73, 70 and 74 KEGG pathways in the 5 cultivars, respectively. The flavone and flavonol biosynthesis (pxb00944), ABC transport (pxb02010) and flavonoid biosynthesis (pxb00941) were the main pathways for the enrichment of differential metabolite, among them, the contents of sorbitol, fructose, mannitol, citric acid, salicylic acid, maleic acid and methylmalonic acid were significantly up-regulated. Additionally, the zeatin synthesis (pxb00908) and monobacterial biosynthesis (pxb00261) were also metabolic pathways in which metabolites were significantly enriched in the leaves of Chaohong. 【Conclusion】To sum up, there are obvious differences in kinds and expressions of metabolites in the leaves of different pear cultivars, and this study could provide theoretical basis for the development and utilization of the leaf resources of pear.
Key words: Pear; Leaves; Secondary metabolites
植物次生代谢物是由植物次生代谢产生的非必需低分子有机化合物,来源广泛、结构复杂,主要包括酚类、类萜、生物碱类和其他次生物质。酚类是芳香族环上的氢原子被羟基或功能衍生物取代后生成的化合物,以糖苷或糖脂状态积存于液泡中,在植物体内广泛分布,是重要的次生代谢物质之一,主要有莽草酸和乙酸-丙二酸等两条合成途径;类萜又称萜烯类化合物,是以异戊二烯为单元组成,由乙酰CoA经甲羟戊酸和异戊二烯焦磷酸转变而来。生物碱是存在于自然界中的一大类含氮杂环化合物,通常具有碱性,广泛存在于植物体内,是氮素代谢的中间产物,由不同氨基酸衍生而来[1]。次生代谢产物广泛参与植物的生长、发育和防御等生理过程。武予清等[2]报道黄酮类化合物是棉花植株中重要的抗病虫害的物质,陆地棉的黄酮类化合物以异槲皮苷和芸香苷为主。生物碱是含氮化合物的一种,大多数生物碱是植物的防御物质,对动物具有毒性。另外,许多生物碱具有药用价值,如吗啡、奎宁、长春花碱、利血平等。吴雅琼等[3]采用代谢组学系统分析了黑莓、黑树莓成熟果实中主要代谢物的差异,为高效利用果实中黄酮、花色苷等次生代谢物提供理论依据。张琴等[4]采用UPLC-MS/MS分析不同产地的蜂糖李果实中氨基酸等初生代谢物质的差异。鲁忠富等[5]采用UPLC-MS/MS分析了瓠瓜果实鲜味的代谢物质差异,发现氨基酸及其衍生物和黄酮类等代谢物可能共同影响瓠瓜风味。另外,LC-MS技术在柑橘[6]、李[7]、猕猴桃[8]等果树,番茄[9]、青花菜[10]和平菇[11]等蔬菜,以及荞麦[12]、玉米[13]、棉花[14]等作物研究中应用广泛。
梨属于蔷薇科苹果亚科梨属多年生落叶果树,是我国重要的经济水果之一。我国是梨起源中心之一,种质资源丰富,在长期的栽培驯化过程中逐渐形成了五大栽培种,分别是白梨(Pyrus bretschneideri)、砂梨(P. pyrifolia)、秋子梨(P. ussuriensis)、新疆梨(P. sinkiangensis)和西洋梨(P. communis)[15]。梨果实富含多种矿质元素、维生素、酚类、糖类和有机酸等营养成分。王宏等[16]采用代谢组和转录组关联分析苏翠1号与其亲本翠冠和华酥果实成熟过程中的差异代谢物以及差异基因,发现调控糖代谢、山梨醇代谢、氨基酸代谢和脂肪酸代谢的基因在苏翠1号中高表达,解析了苏翠1号果实品质优良的分子基础。赵芫[17]通过代谢组学从红早酥果皮中筛选出211种黄酮类物质,发现乙烯处理后可以抑制异黄酮的积累和提高花青苷的含量,从而诱导果皮变黄。解敏等[18]采用GC-MS技术测定砀山酥梨成熟期果实品质,分析糖、有机酸和芳香物质的组分和含量。另外,高静怡等[19]采用非靶向代谢组学分析砂梨品系水心病发病潜在的分子机制。代谢组学多用于分析果实品质和抗病性研究,尚未有系统分析梨叶片代谢物成分及含量的相关报道。
梨叶片是梨树重要的组成部分之一,是进行光合作用的场所,输送营养物质支持梨树的生长发育过程。梨叶片中含有不同种类的次生代谢物质,影响梨树抗性以及果实品质等。梨中关于次生代谢物质的研究大多集中于果实品质方面,缺乏系统全面鉴定叶片次生代谢物质的研究。为系统分析成熟叶片代谢产物种类和组分含量差异,笔者通过非靶向代谢组学技术,以茶叶片为对照,比较分析5个主栽品种梨叶片代谢物种类和含量的差异。库尔勒香梨、超红、南红、鸭梨和翠冠5个梨品种,分别属于新疆梨、西方梨、秋子梨、白梨和砂梨系统。5个主栽品种不论果实品质特征还是栽培区域等方面均存在明显差异,间接说明在生长发育过程中叶片合成的次生代谢物在向果实转化时存在差异,最终导致果实品质的差异。因此,笔者在本研究中的结果不仅为合理开发利用梨叶片资源、加工梨叶农副产品提供理论基础依据,同时也为探究果实品质、抗性调控研究提供理论参考。
1 材料和方法
1.1 梨叶片采集
5个主栽品种(库尔勒香梨,KEL;超红,RC;南红,NH;鸭梨,YL;翠冠,CG)的梨叶片采自山东省果树研究所大河种质资源圃。于9月份采集成熟期的梨叶片,每个品种设置6组生物学重复,将采集的叶片放置于液氮罐中,带回实验室后放入-80 ℃冰箱中保存。
1.2 代谢物提取
首先,配置代谢物提取所需的溶液,包括内标溶液(L-2-氯苯丙氨酸,0.3 mg·mL-1;Lyso PC17:0,0.01 mg·mL-1,均由甲醇配置),70%和20%的甲醇溶液。提前在-20 ℃预冷提取液;分别称取80 mg梨和茶叶片于2 mL的无酶离心管中,然后分别加入20 μL的内标溶液和1 mL 70%的甲醇溶液;加入2个小钢珠,放置于-20 ℃条件下预冷2 min,然后放入研磨机(60 Hz)研磨2 min;超声提取30 min后,置于-20 ℃条件下静置20 min;在4 ℃、13 000 r·min-1条件下离心10 min,取300 μL上清液,挥发干净后用400 μL 20%的甲醇复溶,涡旋30 s,超声2 min。在相同条件下,离心10 min,然后用注射器吸取150 μL的上清液,使用0.22 μm有机相针孔过滤器过滤后,转移到LC进样小瓶,-80 ℃保存,直到进行LC-MS分析。另外,质控样本(QC)由所有样本的提取液等体积混合制备而成,每个QC体积与样本相同。分析仪器为AB ExionLC超高效液相串联AB TripleTOF 6600高分辨质谱仪组成的液质联用系统。
1.3 差异代谢物的多元分析
删除QC样本中相对标准偏差>0.4的离子峰,PCA统计分析不同组样本之间的差异,绘制质控样本的代谢物强度;采用UNIFI 1.8.1 软件采集原始数据,用Progenesis QI v2.3 软件(Nonlinear Dynamics,Newcastle,UK)处理和鉴定原始数据,使用The Human Metabolome Database(HMDB)和Lipidmaps(v2.3)以及METLIN数据库进行定性分析;然后基于有监督的降维方法,采用偏最小二乘判别分析(Partial least squares discriminant analysis,PLS-DA)法和正交偏最小二乘判别分析(Orthogonal projection to latent structures-discriminant analysis,OPLS-DA)法来更好地区分2组样品的代谢组学特征和类别。通过OPLS-DA分析得到比较组之间差异代谢物的变量权重值(variable important in projection,VIP)。
1.4 差异代谢物分析
依据代谢物的多元分析和单变量分析的结果,筛选出5个比较组的差异代谢物。在OPLS-DA分析中,VIP值可用来衡量各代谢物的表达模式对各组样本分类判别的影响强度和解释能力。筛选的标准为OPLS-DA模型第一主成分VIP>1,t检验p<0.05。结合迈维云平台(https://cloud.metware.cn/#/home)在线软件对筛选出的差异代谢物进行火山图、韦恩图、Heatmap分析和相关性分析。具体参数为FC筛选阈值为1、p-value筛选阈值是0.05,VIP筛选阈值是1。依据 KEGG 数据库(https://www.kegg.jp/)对差异代谢物进行代谢通路富集分析。
1.5 K-means分析
对筛选到的1319种代谢物在6种叶片中的含量趋势进行聚类分析。使用迈维云平台(https://cloud.metware.cn/#/home)在线软件对筛选出的差异代谢物含量进行K-means聚类分析。由于差异代谢物含量差异范围较大,因此对代谢物含量的数值进行数值转换,对其分别取对数(log2),选取手动指定聚类方法,分类数目设置为12。
2 结果与分析
2.1 代谢组样本质量控制和定性分析
采用PCA方法对所有质控样本的离散程度进行分析,结果如图1-A所示,QC样本和6种叶片的组内样本分别聚类紧密,稳定性和重复性较好。另外,对每组样品的6个生物学重复进行组内相关性分析,结果显示每组样品的6组生物学重复均高度相关,相关系数均大于0.8;5组梨叶片的代谢物与茶叶叶片的代谢物的相关系数较小,为0.03~0.24(图1-B)。在PLS-DA分析中,R2X(cum)均大于0.942、R2Y(cum)均是1,Q2(cum)均是1或0.999,说明该方法可以有效地区分种间和种内叶片代谢物的差异,并且可靠性较高。结果显示,每组样品的代谢物组内紧密聚集在一起,梨叶片与茶叶片的代谢物聚类相聚较远,具有明显差异。其中,超红叶片的代谢物与其他4种梨叶片的代谢物聚类较远,说明西洋梨和东方梨两大种群梨叶片代谢物成分的差异。在东方梨品种中库尔勒香梨与鸭梨、翠冠、南红代谢物聚类分布较远,具有一定的差异(图1-C)。在正负离子模式下共鉴定出23 322个物质峰,相应的代谢物总共9011个。在正离子模式下鉴定出11 747个物质峰,4987种代谢物;在负离子模式下鉴定出11 575个物质峰,4024种代谢物(图1-D)。
2.2 差异代谢物筛选及火山图、韦恩图分析
对检测出的代谢物进行分析,库尔勒香梨、超红、南红、鸭梨、翠冠分别与福鼎大白茶相比较,每个比较组分别检测出1194、1153、1176、1153和1164种显著差异的代谢物。依据差异代谢物的VIP、FC和p值绘制火山聚类图。结果如图3所示,库尔勒香梨、超红、南红、鸭梨、翠冠与福鼎大白茶相比显著上调的差异代谢物分别是466、468、469、481和446种,显著下调的差异代谢物有728、685、707、672和718种(图2-A~E)。韦恩图结果表明,库尔勒香梨、超红、南红、鸭梨、翠冠与福鼎大白茶相比,特异性的差异代谢物分别有55、155、54、59和28种,其中5个比较组共有的差异代谢物是747种(图2-F)。
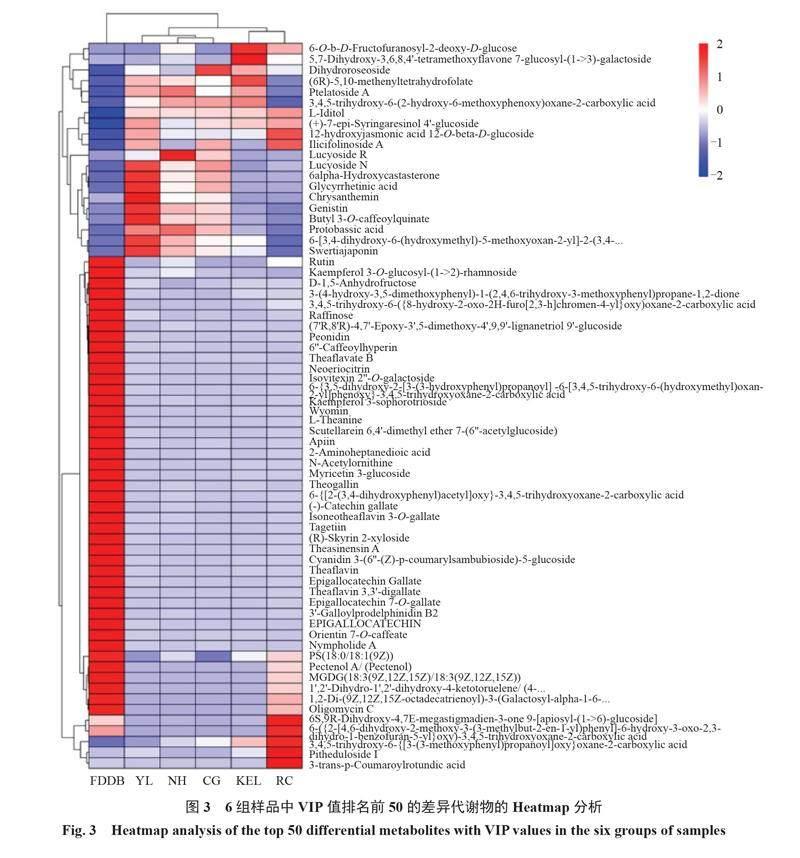
2.3 差异代谢物的定量Heatmap分析和相关性分析
为了更直观地展示样本之间代谢物的表达差异,对所有显著差异代谢物表达量进行层次聚类分析,根据VIP值对top 50差异代谢物表达量进行分析。筛选出的差异代谢物,大多数属于脂类和类脂分子、生物碱及其衍生物、苯环型化合物、木酚素、新木酚素及其相关化合物、有机氧化合物和糖类多酮类化合物。结果如图3所示,6种叶片的代谢物在表达丰度上具有明显差异,福鼎大白茶叶片中含有的差异代谢物数目居多,但是在5个栽培种的梨叶片中同时也具有特异性的差异代谢物。相较于其他组代谢物质,在鸭梨叶片中,矢车菊苷(chrysanthemin)、三羟异黄酮(genistin)、獐牙菜红素(swertiajaponin)、日当药黄素(swertiajaponin)、6α-羟基蓖麻酮(6alpha-hydroxycastasterone )、甘草次酸(glycyrrhetinic acid)和原碱草酸(protobassic acid)等的含量相对较高;在南红叶片中含量较高的差异代谢物主要有丝瓜皂苷R(lucyoside R)、3,4,5-三羟基-6-(2-羟基-6-甲氧基苯氧基)恶烷-2-羧酸[3,4,5-trihydroxy-6-(2-hydroxy-6-methoxyphenoxy)oxane-2-carboxylic acid]和原碱草酸(protobassic acid);在翠冠叶片中含量较高的差异代谢物主要有二氢蔷薇苷(dihydroroseoside);在库尔勒香梨叶片中含量较高的差异代谢物主要有6-O-b-D-呋喃果糖基-2-脱氧-D-葡萄糖(6-O-b-D-fructofuranosyl-2-deoxy-D-glucose)、5,7-二羟基-3,6,8,4'-四甲氧基黄酮 7-葡萄糖基-(1->3)-半乳糖苷[5,7-dihydroxy-3,6,8,4'-tetramethoxyflavone 7-glucosyl-(1->3)-galactoside]、二氢蔷薇苷(dihydroroseoside)和(6R)-5,10-亚甲基四氢叶酸(6R)-5,10-methenyltetrahydrofolate等;在超红叶片中含量较高的代谢物主要有3-反式-香豆酰圆环酸(3-trans-p-coumaroylrotundic acid)、三萜皂苷Ⅰ(pitheduloside Ⅰ)和6S,9R-二羟基-4,7E-大扁豆烯-3-酮9-[芹菜糖基-(1->6)-葡萄糖苷]{6S,9R-dihydroxy-4,7E-megastigmadien-3-one 9-[apiosyl-(1->6)-glucoside]})等(图3)。以上结果表明,5个栽培种梨叶片中的代谢物存在显著差异,也暗示着叶片的功能存在差异。
采用Pearson相关系数来衡量VIP值位于前50的差异代谢物含量的相关性。结果表明(图4),1275对代谢物含量有642对(r>0)呈正相关,633对差异代谢物含量呈负相关(r<0)。与福鼎大白茶相比,5种梨叶片中排名位于前50的差异代谢物含量呈显著正相关,如椴树甙A(ptelatoside A)与(6R)-5,10-亚甲基四氢叶酸[(6R)-5,10-methenyltetrahydrofolate]、3-O-咖啡酰喹酸丁酯(butyl 3-O-caffeoylquinate)、原碱基酸(protobassic acid)、獐牙菜苷(swertiajaponin)、环西番莲苷Ⅵ(cyclopassifloside Ⅵ)和葫芦巴碱(trigonelline),其多数属于脂类和类脂分子。相比其他组,甘草酸(glycyrrhetinic acid)是在鸭梨叶片中含量较高的代谢物,与其正相关代谢物主要有表没食子儿茶素7-O-没食子酸酯(epigallocatechin 7-O-gallate)、东方素7-O-咖啡酸酯(orientin 7-O-caffeate)、矢车菊苷(chrysanthemin)、三羟异黄酮(genistin)、槲皮素3-板蓝根苷(quercetin 3-lathyroside)、3-O-咖啡酰喹酸丁酯(butyl 3-O-caffeoylquinate)、6α-羟基蓖麻酮(6alpha-hydroxycastasterone)、云杉苷(picein)、乙酸苯酚酯(phenyl acetate)和日当药黄素(swertiajaponin),其正相关系数均大于0.9。相关性分析的结果表明,在叶片生长发育过程中,正相关的代谢物可能位于相同的代谢通路上,参与相同的发育过程。
2.4 差异代谢物的K-mean分析
采用K-mean对差异代谢物的表达趋势进行聚类分析,将差异代谢物共划分为12个亚组,在这12个组中,差异代谢物的变化趋势基本一致,其中1、2、6、8、10和11组中差异代谢物的数目较多,分别是229、200、104、304、256和81个,其中1、2和8组中差异代谢物的含量变化趋势基本一致。在3、6、7和9组中,福鼎大白茶叶片中代谢物的含量明显高于梨叶片中代谢物的含量。在4组中总共含有29种代谢物,且其在梨叶片中的含量明显高于在茶叶片中的含量,主要包括勒皮啶、吡咯黄质、艾格利诺,其分属于苯环型化合物、糖类、多酮类化合物、脂类和类脂类分子。在5组中,超红叶片中代谢物的含量显著低于其他梨叶片中该代谢物的含量,主要代谢物有γ-西红花酸(gamma-crocetin)、阿尔巴呋喃素A(albafuran A)、甲基三烯炔诺酮(gestrinone)、槲皮素3-(6''-丙二酰半乳糖苷)[quercetin 3-(6''-malonylgalactoside)]、1-十六烷基-2-[(2'-α-葡糖基)-β-葡糖基]-3-β-木糖基-sn-甘油{1-hexadecanyl-2-[(2'-alpha-glucosyl)-beta-glucosyl]-3-beta-xylosyl-sn-glycerol}、异肝素 2''-[6-(E)-阿魏酰葡糖苷] 4'-葡糖苷{isoscoparin 2''-[6-(E)-feruloylglucoside] 4'-glucoside},多数属于脂类和类脂类分子,该结果也表明西方梨与东方梨叶片的代谢物含量具有明显的差异(图5)。
2.5 差异代谢物的代谢通路分析
对库尔勒香梨、超红、南红、鸭梨、翠冠与福鼎大白茶叶片的差异代谢物进行KEGG富集分析,结果表明,5个比较组的差异代谢物共富集到78个KEGG通路上,每个比较组分别富集在76、71、73、70和74通路上。对5个不同的比较组分别分析发现,不同比较组的差异代谢物排名前3位的KEGG通路一致,分别是ABC转运(pxb02010)、黄酮和黄酮醇生物合成(pxb00944)及类黄酮生物合成(pxb00941),分别有13~14、9~10和7~8种代谢物富集在这3个通路上。与福鼎大白茶相比,梨叶片中主要上调的代谢物有胆碱、D-核糖、D-甘露醇、山梨醇、木二糖和槲皮素3-(6''-丙二酰-葡萄糖苷);梨叶片中下调的差异代谢物含量主要有邻苯二甲酸、阿洛糖、槲皮苷、异牧荆素、芹菜苷、异槲皮苷、芸香苷、山柰酚、白藜芦素、叶酮醇和表儿茶素。相较于茶树叶片中的黄酮和黄酮醇生物合成途径中代谢物的含量,梨叶片中检测出该途径所涉及VIP值排名靠前的差异代谢物含量呈现下调的趋势,间接说明梨叶片中黄酮和黄酮醇次生代谢物的含量低于茶叶中该类代谢物的含量。梨中黄酮类物质主要存在于叶片和果皮中,在果树应对非生物胁迫、调节花色、药用、调控激素等方面具有重要作用。黄酮和黄酮醇生物合成途径中包含的代谢物主要有槲皮苷、异牡荆素、芹菜苷、异槲皮苷、芦丁、黄芪苷、漆叶苷、山柰酚-3-槐糖苷、槲皮素3-(6''-丙二酰基-糖苷)等,是植物体内重要的次生代谢物质,参与调节梨生长发育过程[20-21]。另外,VIP值排名前20的差异代谢物的富集通路基本一致,富集在每个通路上的差异代谢物的数目也基本一致,主要有甲硫腺苷、顺-玉米素-O-葡萄糖苷、顺-玉米素-7-N-葡萄糖苷、二氢玉米素-O-葡萄糖苷和二氢玉米素核苷。在单杆菌生物合成途径中富集的代谢物主要有天门冬氨酸、精氨酸和四氢吡啶二羧酸(图6)。
3 讨 论
库尔勒香梨、超红、南红、鸭梨、翠冠5个主栽品种代谢物数量和含量具有明显差异,其中超红梨叶片的代谢物明显区别于其他4个梨品种,这可能是超红梨属于西方梨,而翠冠、鸭梨、南红、库尔勒香梨属于东方梨所导致。另外,库尔勒香梨叶片的代谢物在PCA聚类分析中与翠冠、鸭梨和南红梨叶片代谢物聚类较远,这也进一步说明起源不同的梨可能导致其代谢物差异。库尔勒香梨属于新疆梨系统,其是西方梨和东方梨杂交形成的品种[15]。因此,库尔勒香梨叶片代谢物PCA聚类分析介于西方梨和东方梨之间。
叶片作为植物重要的营养器官,不仅支持果树生长发育,也为果实品质发育提供营养成分。在超红梨叶片代谢物的KEGG富集分析时发现,玉米素合成途径(pxb00908)和单杆菌生物合成(pxb00261)途径是超红叶片代谢物显著富集的两个通路,该通路包含的代谢物主要有顺-玉米素-O-葡萄糖苷、顺-玉米素-7-N-葡萄糖苷和二氢玉米素-O-葡萄糖苷、天门冬氨酸、精氨酸和四氢吡啶二羧酸等,分属于有机氧化合物和羧酸及其衍生物,主要参与植物抗性调控和果实品质前体物质的合成。梨叶片代谢物与果实品质的形成和调控具有相关性,代谢物的差异也可能是果实品质差异的原因之一。另外,果实中可溶性糖含量是评价果实品质和风味的重要因素,其主要包括葡萄糖、果糖、蔗糖、山梨醇、木糖和甘露醇等。果实中可溶性糖主要来源于叶片光合作用的产物,以蔗糖和山梨醇的形式存在于叶片中,经过韧皮部的长距离运输最终到达果实,经过不同酶的催化反应,形成不同种类的糖,在梨中糖的运输形式则以山梨醇为主[22-23]。叶片和果实中的糖代谢过程可按照产物的不同划分为3种,即山梨醇代谢型、蔗糖代谢型和己糖代谢型。另外,果实获取山梨醇的能力和对其转化速率决定着果实的生长速度,调控果实的生长发育。山梨醇在山梨醇脱氢酶的催化下转化成葡萄糖和果糖,在山梨醇氧化酶的作用下氧化成葡萄糖[24-25]。梨果实在生长发育的后期,叶片中山梨醇、果糖、甘露醇等代谢物的含量显著上调是果实成熟时期合成和转运糖分必要条件之一。此外,山梨醇还参与提高植物的抗逆性,促进养分吸收、提高愈伤诱导率、参与其他糖类物质的合成转化等[26]。温带植物越冬期间,植物体内通过代谢糖醇(山梨醇、甘露醇等)、可溶性糖(蔗糖、海藻糖等)等冷冻保护剂的合成,来提高自身的耐寒性[27]。
福鼎大白茶叶片包含着丰富的营养成分与药效成分,氨基酸、维生素、矿物质、多酚、咖啡碱和茶色素等均是茶叶中的重要组分,不仅可以满足人们对营养物质的需求,同时具有抗氧化、抗炎、抗菌、抗癌及降血糖、降血压、降血脂等功效[28-29]。梨叶片中也含有丰富的氨基酸类与黄酮类代谢物,具有抗菌消炎、抗氧化、防癌和抗癌的作用,如甘草次酸、三羟异黄酮、反式绿原酸等。笔者在本研究中也鉴定出具有开发利用价值的次生代谢物质,如在鸭梨叶片中的矢车菊苷、三羟异黄酮、獐牙菜红素和日当药黄素等;在南红梨叶片中含量较高的丝瓜皂苷R、原碱草酸;在库尔勒香梨叶片中含量较高的二氢蔷薇苷;在超红梨叶片中含量较高的3-反式-香豆酰圆环酸、三萜皂苷Ⅰ等。梨叶片中含有的可开发利用的次生代谢物质成分大多具有药用价值,抗氧化性较强,在抗炎、抗菌等方面具有潜在的应用价值。这也间接增加了梨叶片的开发利用途径,梨叶片可以作为活性物质的提取原料之一。
4 结 论
基于代谢组比较分析库尔勒香梨、超红、南红、鸭梨、翠冠与对照福鼎大白茶的叶片代谢物含量和种类的差异,共检测出9011种代谢物,代谢物种内聚类较为集中,种间聚类相对较远。库尔勒香梨、超红、南红、鸭梨、翠冠与福鼎大白茶比较组,差异代谢物的数目分别是1194、1153、1176、1153和1164种,共有的差异代谢物的数目为747种,特异性差异代谢物分别有55、155、54、59和28种。排名前50的差异代谢物在5个主栽梨叶片中的表达丰度上具有明显差异,并且642对代谢物含量存在正相关,633对代谢物含量存在负相关。超红叶片中代谢物在3、5和7组中含量差异较大,南红梨在12组中代谢物含量相对较少。黄酮和黄酮醇生物合成(pxb00944)、ABC转运(pxb02010)和类黄酮生物合成(pxb00941)等途径是差异代谢物富集的主要通路,其中山梨醇、果糖、甘露醇、柠檬酸、水杨酸、马来酸、甲基丙二酸等代谢物的含量显著上调。玉米素合成(pxb00908)和单杆菌生物合成(pxb00261)途径也是超红叶片中代谢物显著富集的代谢通路。因此,不同栽培种的梨叶片的代谢物在种类和含量上具有显著差异,研究结果可为开发利用梨叶片资源提供理论基础。
参考文献 References:
[1] ZHAO J,DAVIS L C,VERPOORTE R. Elicitor signal transduction leading to production of plant secondary metabolites[J]. Biotechnology Advances,2005,23(4):283-333.
[2] 武予清,郭予元,杨舰. 棉株中抗虫物质黄酮类化合物的高效液相色谱分析[J]. 植物保护,2000,26(5):1-3.
WU Yuqing,GUO Yuyuan,YANG Jian. Analysis of flavonoid substance in cotton plants for resistance to pests by HPLC[J]. Plant Protection,2000,26(5):1-3.
[3] 吴雅琼,张春红,杨海燕,闾连飞,李维林,吴文龙. 基于代谢组学分析黑莓、黑树莓果实代谢物的差异[J]. 江苏农业学报,2022,38(3):790-797.
WU Yaqiong,ZHANG Chunhong,YANG Haiyan,L? Lianfei,LI Weilin,WU Wenlong. Metabolomics analysis of differentially expressed metabolites in blackberry and black raspberry fruits[J]. Jiangsu Journal of Agricultural Sciences,2022,38(3):790-797.
[4] 张琴,董晓庆,林欣,彭俊森,罗登灿,黄世安,朱守亮. 基于UPLC-MS/MS的不同产地蜂糖李果实初生代谢差异分析[J]. 食品科学,2023,44(8):284-292.
ZHANG Qin,DONG Xiaoqing,LIN Xin,PENG Junsen,LUO Dengcan,HUANG Shian,ZHU Shouliang. Ultra-high performance liquid chromatography-tandem mass spectrometry-based metabolomic analysis of primary metabolites in ‘Fengtang plum fruits from different geographical origins[J]. Food Science,2023,44(8):284-292.
[5] 鲁忠富,李艳伟,汪颖,吴晓花,吴新义,王尖,汪宝根,李国景. 基于广泛靶向代谢组学的瓠瓜果实鲜味差异代谢物分析[J]. 中国蔬菜,2021(6):34-41.
LU Zhongfu,LI Yanwei,WANG Ying,WU Xiaohua,WU Xinyi,WANG Jian,WANG Baogen,LI Guojing. Analysis of fruit umami differential metabolites of bottle gourd based on widely targeted metabolomics[J]. China Vegetables,2021(6):34-41.
[6] 祝爱艳,梁露,侯金雪,孙雪枫,杨延峰,王远兴. 赣南脐橙代谢组学研究[J]. 中国食品学报,2020,20(3):276-281.
ZHU Aiyan,LIANG Lu,HOU Jinxue,SUN Xuefeng,YANG Yanfeng,WANG Yuanxing. Studies on metabonomics of Gannan navel orange[J]. Journal of Chinese Institute of Food Science and Technology,2020,20(3):276-281.
[7] 林欣,黄世安,张琴,彭俊森,罗登灿,朱守亮,陈红,董晓庆. 采用UPLC-MS/MS分析低温贮藏期间‘空心李果实初生代谢物[J]. 植物生理学报,2022,58(10):1982-1994.
LIN Xin,HUANG Shian,ZHANG Qin,PENG Junsen,LUO Dengcan,ZHU Shouliang,CHEN Hong,DONG Xiaoqing. Primary metabolites analysis of ‘Kongxin plum fruits during storage by UPLC-MS/MS[J]. Plant Physiology Journal,2022,58(10):1982-1994.
[8] 赵燕妮,张坤,许牡丹,雷靖,刘欢,刘宁,陈雪峰,张迪. 基于GC-MS代谢组学技术的不同品种猕猴桃果实化学成分差异性研究[J]. 陕西科技大学学报,2022,40(1):45-50.
ZHAO Yanni,ZHANG Kun,XU Mudan,LEI Jing,LIU Huan,LIU Ning,CHEN Xuefeng,ZHANG Di. Study on the chemical constituents of different kiwifruit cultivars based on GC-MS metabolomics[J]. Journal of Shaanxi University of Science & Technology,2022,40(1):45-50.
[9] 潘铜华. CO2富集与光强互作对番茄光合碳同化的影响及代谢组研究[D]. 杨凌:西北农林科技大学,2019.
PAN Tonghua. Interactive effects of elevated CO2 and light intensity on photosynthetic carbon assimilation and metabolomics of tomato[D]. Yangling:Northwest A & F University,2019.
[10] 唐晨晨,张文霞,陈芳珍,武志健,黄科,王军伟. 代谢组和转录组联合解析青花菜芽苗黄酮类物质对外源ABA的响应机制[J]. 中国瓜菜,2024,37(3):35-44.
TANG Chenchen,ZHANG Wenxia,CHEN Fangzhen,WU Zhijian,HUANG Ke,WANG Junwei. The metabolome and transcriptome jointly resolve the response mechanism of flavonoids in broccoli sprouts to exogenous ABA[J]. China Cucurbits and Vegetables,2024,37(3):35-44.
[11] 刘芹,黄保,胡素娟,牛森园,吴杰,周奥硕,孔维丽. 基于非靶向代谢组学的平菇子实体发育过程中菌丝体差异代谢物分析[J]. 中国瓜菜,2024,37(1):45-55.
LIU Qin,HUANG Bao,HU Sujuan,NIU Senyuan,WU Jie,ZHOU Aoshuo,KONG Weili. Analysis of differential metabolites during fruiting body development of Pleurotus ostreatus based on untargeted metabolomics[J]. China Cucurbits and Vegetables,2024,37(1):45-55.
[12] LI H Y,LV Q Y,LIU A K,WANG J R,SUN X Q,DENG J,CHEN Q F,WU Q. Comparative metabolomics study of Tartary [Fagopyrum tataricum (L.) Gaertn] and common (Fagopyrum esculentum Moench) buckwheat seeds[J]. Food Chemistry,2022,371:131125.
[13] 李川,乔江方,黄璐,张美微,张盼盼,牛军,刘京宝. 转录组及代谢组联合解析玉米响应花粒期高温胁迫机制[J]. 华北农学报,2020,35(1):8-21.
LI Chuan,QIAO Jiangfang,HUANG Lu,ZHANG Meiwei,ZHANG Panpan,NIU Jun,LIU Jingbao. Transcriptome and metabolome analysis to reveal the mechanisms responding to high temperature stress in anthesis stage of maize[J]. Acta Agriculturae Boreali-Sinica,2020,35(1):8-21.
[14] 郭家鑫,鲁晓宇,陶一凡,郭慧娟,闵伟. 棉花在盐碱胁迫下代谢产物及通路的分析[J]. 作物学报,2022,48(8):2100-2114.
GUO Jiaxin,LU Xiaoyu,TAO Yifan,GUO Huijuan,MIN Wei. Analysis of metabolites and pathways in cotton under salt and alkali stresses[J]. Acta Agronomica Sinica,2022,48(8):2100-2114.
[15] WU J,WANG Y T,XU J B,KORBAN S S,FEI Z J,TAO S T,MING R,TAI S S,KHAN A M,POSTMAN J D,GU C,YIN H,ZHENG D M,QI K J,LI Y,WANG R Z,DENG C H,KUMAR S,CHAGN? D,LI X L,WU J Y,HUANG X S,ZHANG H P,XIE Z H,LI X,ZHANG M Y,LI Y H,YUE Z,FANG X D,LI J M,LI L T,JIN C,QIN M F,ZHANG J Y,WU X,KE Y Q,WANG J,YANG H,ZHANG S L. Diversification and independent domestication of Asian and European pears[J]. Genome Biology,2018,19:77.
[16] 王宏,杨王莉,蔺经,杨青松,李晓刚,盛宝龙,常有宏. 早熟砂梨‘苏翠1号与其亲本‘翠冠‘华酥成熟果实差异代谢产物及差异基因比较分析[J]. 园艺学报,2022,49(3):493-508.
WANG Hong,YANG Wangli,LIN Jing,YANG Qingsong,LI Xiaogang,SHENG Baolong,CHANG Youhong. Comparative metabolic and transcriptomic analysis of ripening fruit in pear cultivars of ‘Sucui 1 ‘Cuiguan and ‘Huasu[J]. Acta Horticulturae Sinica,2022,49(3):493-508.
[17] 赵芫. 乙烯利协同茉莉酸甲酯调控‘红早酥梨果皮着色的代谢组学研究[D]. 杭州:浙江大学,2019.
ZHAO Yuan. Metabonomics study on ethephon and methyl jasmonate synergistically regulating peel coloration of ‘Hongzaosu pear[D]. Hangzhou:Zhejiang University,2019.
[18] 解敏,黄瑛,王馨,王秀梅,唐娟娟,方从兵. 基于GC-MS技术测定砀山酥梨果实主要品质特征成分的代谢物谱[J]. 安徽农业大学学报,2012,39(5):692-696.
XIE Min,HUANG Ying,WANG Xin,WANG Xiumei,TANG Juanjuan,FANG Congbing. Metabolomic analysis on the major quality components in pear (Pyrus bretschneideri Rehd. cv. Dangshansuli) fruits by gas chromatography-mass spectrometry[J]. Journal of Anhui Agricultural University,2012,39(5):692-696.
[19] 高静怡,马浩,刘东贺,方啸宇,刘晓. 非靶向代谢组学对砂梨水心病不同代谢物质的差异分析[J]. 分子植物育种,2021,19(24):8297-8304.
GAO Jingyi,MA Hao,LIU Donghe,FANG Xiaoyu,LIU Xiao. The difference analysis of non targeted metabonomics on different metabolites in watercore Pyrus pyrifolia[J]. Molecular Plant Breeding,2021,19(24):8297-8304.
[20] 曹运琳,邢梦云,徐昌杰,李鲜. 植物黄酮醇生物合成及其调控研究进展[J]. 园艺学报,2018,45(1):177-192.
CAO Yunlin,XING Mengyun,XU Changjie,LI Xian. Biosynthesis of flavonol and its regulation in plants[J]. Acta Horticulturae Sinica,2018,45(1):177-192.
[21] 张小双,郑迎春,曹玉芬,田路明,董星光,张莹,齐丹,霍宏亮. ‘早酥和‘南果梨16个部位多酚物质组成及含量分析[J]. 中国农业科学,2017,50(3):545-555.
ZHANG Xiaoshuang,ZHENG Yingchun,CAO Yufen,TIAN Luming,DONG Xingguang,ZHANG Ying,QI Dan,HUO Hongliang. The composition and content of polyphenols in 16 parts of ‘Zaosu and ‘Nanguoli[J]. Scientia Agricultura Sinica,2017,50(3):545-555.
[22] 张虎平. 梨果实内糖的转运及积累特性研究[D]. 南京:南京农业大学,2011.
ZHANG Huping. Study on characteristic of transport and accumulation of sugar in developing Pyrus ssp. fruit[D]. Nanjing:Nanjing Agricultural University,2011.
[23] LIU D F,NI J B,WU R Y,TENG Y W. High temperature alters sorbitol metabolism in pyrus pyrifolia leaves and fruit flesh during late stages of fruit enlargement[J]. Journal of the American Society for Horticultural Science,2013,138(6):443-451.
[24] 李甲明. 梨糖转运蛋白基因家族分析及PbtMT4和PbSWEET基因功能研究[D]. 南京:南京农业大学,2017.
LI Jiaming. Identification of pear sugar transporter genes and PbtMT4 and PbSWEET genes function analysis[D]. Nanjing:Nanjing Agricultural University,2017.
[25] OURA Y,YAMADA K,SHIRATAKE K,YAMAKI S. Purification and characterization of a NAD+-dependent sorbitol dehydrogenase from Japanese pear fruit[J]. Phytochemistry,2000,54(6):567-572.
[26] HOEKSTRA F A,GOLOVINA E A,BUITINK J. Mechanisms of plant desiccation tolerance[J]. Trends in Plant Science,2001,6(9):431-438.
[27] JANSK? A,MARS?K P,ZELENKOV? S,OVESN? J. Cold stress and acclimation - what is important for metabolic adjustment?[J]. Plant Biology,2010,12(3):395-405.
[28] 戴伟东,解东超,林智. 白茶功能性成分及保健功效研究进展[J]. 中国茶叶,2021,43(4):1-8.
DAI Weidong,XIE Dongchao,LIN Zhi. Research progress of white teas functional ingredients and health benefits[J]. China Tea,2021,43(4):1-8.
[29] 黄彪,刘文静,吴建鸿,吴建衍. 福鼎大白茶树鲜叶不同茶制品活性成分比较[J]. 食品安全质量检测学报,2021,12(8):3219-3223.
HUANG Biao,LIU Wenjing,WU Jianhong,WU Jianyan. Comparison of active components of different tea products from fresh leaves of Camellia sinensis Fuding Dabai[J]. Journal of Food Safety & Quality,2021,12(8):3219-3223.
收稿日期:2024-02-02 接受日期:2024-04-20
基金项目:山东省农科院创新工程项目(CXGC2023F21、CXGC2023A13);山东省果树研究所青年基金(GSS2022QN11);国家梨产业技术体系(CARS-28-37);山东省自然科学基金(ZR2020MC141、ZR2021MC177、ZR2023MC061);农业农村部园艺作物种质资源利用重点实验室开放基金(NYZS202206)
作者简介:焦慧君,女,助理研究员,博士,研究方向为果实品质调控。Tel:0538-8806302,E-mail:jiaohj_njau@163.com。#为共同第一作者。冉昆,男,研究员,博士,研究方向为果树遗传育种与栽培管理。Tel:0538-8207123,E-mail:rkrl001@126.com
*通信作者 Author for correspondence. Tel:0538-8207123,E-mail:weisw2007@163.com
