中药纳米制剂研究进展
2024-05-10杨靖柳炜佳李英曾莉闫芳
杨靖 柳炜佳 李英 曾莉 闫芳
本文引用: 杨 靖, 柳炜佳, 李 英, 曾 莉, 闫 芳. 中药纳米制剂研究进展[J]. 湖南中医药大学学报, 2024, 44(4): 706-712.
〔摘要〕 纳米制剂是目前药剂学领域的研究热点。传统中药“纳米化”为中药创新研究打开了新的大门,不仅改变了中药应用的“尺度”,更是拓展了中药治疗的范围。中药纳米制剂既能延续传统中药治疗“多靶点、多层次”的特点,又能改善活性成分口服生物利用度低、组织靶向性不足等问题。通过总结中药外泌体、碳点、纳米凝胶、聚合物胶束、自微乳和纳米脂质体相关研究,分析不同中药纳米制剂的作用特点,为创新中药研究提供思路。
〔关键词〕 中药;纳米制剂;研究进展;构成;生物活性
〔中图分类号〕R283 〔文献标志码〕A 〔文章编号〕doi:10.3969/j.issn.1674-070X.2024.04.029
Research progress on nanoagents of Chinese medicine
YANG Jing1, LIU Weijia2, LI Ying1, ZENG Li1, YAN Fang3*
1. Department of Pharmacy, Chengdu Fifth People's Hospital (The Fifth People's Hospital of Chengdu University of Chinese Medicine), Chengdu, Sichuan 611130, China; 2. Department of Rehabilitation Medicine, Chengdu Fifth People's Hospital (The Fifth People's Hospital of Chengdu University of Chinese Medicine), Chengdu, Sichuan 611130, China; 3. Medical Research and Transformation Center, Chengdu Fifth People's Hospital (The Fifth People's Hospital of Chengdu University of Chinese Medicine), Chengdu, Sichuan 611130, China
〔Abstract〕 Recently, nanoagents have become a hot topic in the field of pharmaceutics. The "nanotization" of Chinese medicine has opened up new avenues for the innovative research of Chinese medicine, not only changing the "scale" of its application but also expanding the scope of Chinese Medicine treatment. Nanoagents of Chinese medicine can maintain the characteristics of "multi-target and multi-level" of Chinese medicine treatment and improve low oral bioavailability and inadequate tissue targeting of active ingredients. By summarizing the relevant research on exosomes, carbon dots, nanogels, polymeric micelles, self-microemulsion, and nano-liposomes of Chinese medicine, and analyzing the functional characteristics of different nanoagents of Chinese medicine, this study provides ideas for innovative research on Chinese medicine.
〔Keywords〕 Chinese medicine; nanoagent; research progress; composition; biological activity
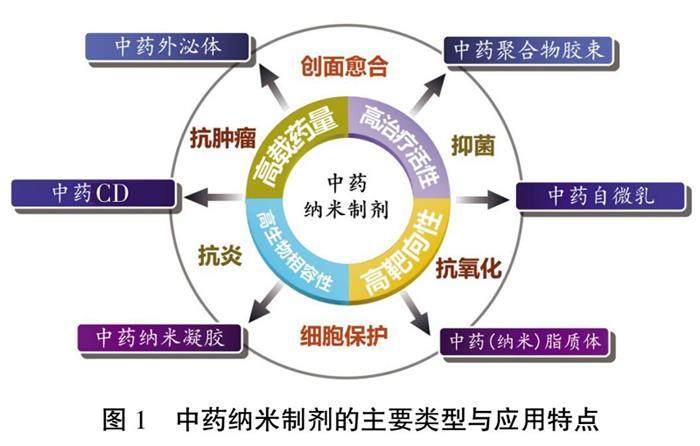
近年来,伴随材料科学、药物制剂学、药物代谢动力学等学科的创新发展,药物剂型不断丰富,新剂型的涌现刷新了人们对药物作用特点的认识,也打破了传统药物应用的固有边界。“纳米药物”,即通过“纳米化”,将药物与载体材料制成粒径在10~1 000 nm范围内的药物晶体或载药微粒,从而获得有别于常规药物的靶向性和作用强度[1]。在中医药领域,外泌体、碳点(carbon dot,CD)、纳米凝胶、聚合物胶束、自微乳、脂质体等纳米技术,已广泛应用于中药新药研发,并衍生出多种生物学活性(图1),旨在从细胞、亚细胞、分子层面赋予传统中药理论新的内涵[2]。
1 中藥负载纳米材料的主要类型和特点
1.1 中药来源的外泌体
外泌体是由细胞内多泡体与细胞膜融合后释放到细胞外基质中的脂质包裹体,直径30~100 nm,内含蛋白、核酸、脂质和代谢物。几乎所有类型的细胞都可产生并释放外泌体。不同细胞通过分泌不同组分的外泌体实现细胞间通信,进而实现细胞功能的调节。诸多研究发现,来源于药用植物的外泌体或外泌体样纳米囊泡富含各种具有生物活性的脂质、蛋白、RNA等成分[3],是天然的纳米制剂,在创伤修复与再生[4]、抗炎与免疫调节[5]、抗肿瘤[6]、肠转运蛋白[7]等方面均有显著调控作用。此外,鉴于植物外泌体或外泌体样纳米囊泡体积小、穿透性强,且一定程度耐酸碱、耐高温的特点,这些微囊泡成为药物递送的理想载体[8]。
1.2 中药CD
CD是一类具有显著荧光性能的零维碳纳米材料,由超细、分散、准球形且直径小于10 nm的碳纳米颗粒组成[9],根据碳源的不同可分为碳量子点(car?bon quantum dot,CQD)、石墨烯量子点(graphene quan?tum dot,GQD)、碳化聚合物点(carbonized polymer dot,CPD)和碳纳米点(carbon nanodot,CND)[10]。因其良好的光学性质、水溶性、低毒性和生物相容性,CD广泛应用于医学成像[11]、光热治疗[12]、抗肿瘤治疗[13]等医药领域。
1.3 中药纳米凝胶
纳米凝胶,即直径小于200 nm的水凝胶,是一种三维网状的聚合物。与其他尺寸的凝胶相比,纳米凝胶容易被细胞吞噬,易于透过血脑屏障实现脑部给药,且载药效率高[14],目前在促进药物经皮吸收、控制药物释放、靶向给药等方面发挥重要作用。当传统中药具备纳米凝胶特性(即形成中药纳米凝胶)时,中药的治疗潜能被极大开发。
1.4 中药聚合物胶束
胶束是一种有序排列的热力学稳定胶状团聚体,当水溶液中的表面活性剂达到一定浓度时,分子自组装形成[15]。由两亲性嵌段共聚物自组装形成的热力学稳定胶体溶液称为聚合物胶束[16]。聚合物胶束由于其粒径小、内部疏水不含水、外表面链段保护作用、增溶性、低毒性等特点,已成为目前药物递送系统的重要选择之一[17]。
1.5 中药自微乳
微乳是一种透明或半透明、低黏度、各向同性且热力学稳定的纳米级油水混合体系,由水相、油相、表面活性剂和助表面活性剂在适当比例下自发形成[18]。微乳因增溶能力强、透明度高、热力学稳定性强、扩散快、吸收率高、便于制备等特点被视为强大的替代载药系统[19]。与微乳相比,自微乳(self-microemulsifying,SME)型药物递送系统由相同的组分构成,在水中自动分散形成微乳,属于微乳的浓缩液。
1.6 中药脂质体
“脂质体”一词可追溯至二十世纪六十年代,最初用于描述“磷脂在水中可自发形成双分子层囊泡”的结构[20]。脂质体由一个或几个脂质双层组成,直径20~1 000 nm,包含磷脂或鞘脂尾部(亲脂性)聚集形成的疏水区域和头部(亲水性)暴露的亲水区域。因该结构与细胞膜成分非常相似,可与细胞膜融合,进而释放内容物进入细胞,调节细胞功能,故可用于靶向细胞内部。为提高药物对细胞的选择性,避免非目的性吞噬细胞摄取,载药脂质体直径一般不超过100 nm[21]。由此,脂质纳米颗粒[或称纳米脂质体(lipid nanoparticle, LNP)作为脂质体的优化剂型出现在制药行业。除粒径大小差异外,LNP与传统脂质体在组成成分、内部结构方面亦有显著不同,如LNP中胆固醇比例明显高于传统脂质体;传统脂质体内部有亲水空腔,而LNP内部因内容物(如核酸)与磷脂头部的电荷差异而形成多层核心[22]。目前,传统脂质体已广泛用于小分子药物递送[23];LNP多用于核酸[如mRNA[24]、小干扰RNA(small interfering RNA,siRNA)[25]等]递送。随着脂质体/LNP制备组装技术的不断完善,源自中药的天然化合物逐渐成为脂质体/LNP药物递送体系的主角。
2 中药纳米制剂的生物学活性
2.1 抗炎
源自生姜的外泌体样纳米囊泡直径为50~200 nm,内含脂质(如磷脂酸、磷脂酰乙醇胺、磷脂酰胆碱、磷脂酰丝氨酸、二半乳糖二酰基甘油)、蛋白(如酶、肌动蛋白、通道蛋白/转运体)、微小RNA(microRNA,miRNA)、活性化合物(如6-姜酚、8-姜酚、10-姜酚、6-姜烯酚)等生物活性组分。其中,姜酚和姜烯酚类化合物具有抗炎活性,如6-姜酚能抑制核因子κB(nuclear factor-κB,NF-κB)活化与蛋白激酶C(protein kinase C,PKC)易位,抑制细胞因子生成和T细胞激活,从而发挥抗炎作用[26]。大蒜衍生的外泌体样纳米囊泡(ginger-derived exosome-like nanoparticle,GEN)含有26种脂质、61种蛋白质和127种已知的miRNA。GEN可显著下调Toll样受体4(Toll-like rec?eptor 4,TLR4)、髓细胞分化初级反应基因88(myeloid differentiation factor 88,MyD88)和NF-κB的表达,减少葡聚糖硫酸钠(dextran sulfate sodium,DSS)诱导的促炎细胞因子分泌;GEN所含Han-miR3630-5p可结合TLR4的3非翻译区,从而抑制TLR4表达;此外,GEN通过恢复毛螺杆菌科的相对丰度和降低幽门螺杆菌的相对丰度,改变结肠炎小鼠肠道微生物群[27]。可见,抑制TLR4/MyD88/NF-κB信号通路和调节肠道微生物群是GEN减轻DSS诱导肠道炎症损伤的潜在机制[27]。LIU等[28]制备鱼腥草精油(Houttuynia essential oil,HEO)自微乳(SME-HEO)研究该制剂对脂多糖(lipopolysaccharide, LPS)诱导小鼠乳腺炎及血乳屏障(blood-milk barrier, BMB)的影响,结果发现,SME-HEO可显著下调促炎因子肿瘤坏死因子-α(tumor necrosis factor-α, TNF-α)和白细胞介素-1β(interleukin-1β, IL-1β),上调抗炎因子白细胞介素-10(interleukin-10, IL-10),抑制髓過氧化物酶(myeloperoxidase, MPO)表达,减轻乳腺组织病理损伤;此外,SME-HEO通过上调闭锁小带蛋白(zonula occludens protein 1, ZO-1)、紧密连接蛋白-1(claudin-1)、紧密连接蛋白-3(claudin-3)和紧密连接组分闭合蛋白(occludin)表达保护BMB的完整性。可见,SME-HEO具有良好的抗炎和乳腺保护作用。一种装载紫草素的透明质酸-玉米醇溶蛋白纳米凝胶能选择性抑制巨噬细胞炎症小体,同时减少促炎细胞因子的释放,作用途径与抑制胱天蛋白酶-1(cysteine aspartic acid specific protease-1,Caspase-1)活化和IL-1β生成有关[29]。
2.2 抗肿瘤
人参来源的微囊泡结构[细胞外纳米囊泡(ginseng-derived extracellular nanovesicle,GsNV)或外泌体样纳米颗粒(ginseng-derived exosome-like nanop?articles,GsEN)]因其广泛的生物活性备受关注。近期研究发现,GsNV在体外能抑制破骨细胞的分化,并维持骨髓源性巨噬细胞(bone marrow-derived macroph?age,BMM)的活性;在LPS诱导的骨吸收小鼠模型中,GsNV同样能抑制破骨细胞分化[30]。该效应与抑制NF-κB受体活化因子配体(receptor activator of NF-κB ligand,RANKL)诱导的NF-κB抑制蛋白(inhibitor of NF-κB,IκBα)、c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)、细胞外信号调节激酶(extracellular signal-regulated kinase,ERK)信号通路和调节破骨细胞成熟的基因有关[30]。此外,GsEN在体外、体内被证明是胶质瘤治疗的候选制剂,能通过血脑屏障靶向胶质瘤,并在肿瘤微环境(tumor microenvironment,TME)中招募M1巨噬细胞,表明GsEN在抑制胶质瘤进展和调节肿瘤相关巨噬细胞(tumor-associated macrophage,TAM)方面具有良好的潜力[31]。类似地,由RES合成的CD(RES-CD)能提高RES的肿瘤靶向性,癌细胞毒性作用更显著[32]。损伤肿瘤细胞线粒体功能是RES-CD抗肿瘤作用的途径之一,包括细胞内钙释放、细胞色素C氧化酶活性抑制和线粒体膜扰动。这一特性在多种癌细胞模型中被发现,预示该途径是RES-CD抗肿瘤效应优于单独RES或其他CD制剂的重要基础[32]。海藻酸是源自海带、巨藻等褐藻细胞壁的一种天然多糖醛酸,在抗肿瘤[33]、抗氧化[34]、抑菌[35]、抗炎[36]、免疫调节[37]等领域发挥重要作用。来源于金银花的外泌体含有活性组分miR-2911,该物质富集于金银花不同组织中;miR-2911可靶向人乳头瘤病毒(human papillomavirus,HPV)16/18的E6和E7致癌基因,抑制HPV16/18阳性子宫颈癌细胞增殖并诱导其凋亡;E6/E7-p53/Caspase3轴是miR-2911抗肿瘤效应的关键分子途径[38]。源自郁金、姜黄、莪术等中药的活性成分姜黄素因其广泛的药理活性而备受关注,然而,低口服生物利用度的问题一直限制其应用。基于聚合物胶束的姜黄素递送系统能显著提高姜黄素的口服生物利用度,阻止网状内皮系统摄取姜黄素,且可增强姜黄素的肿瘤渗透和保留效应,表现出强于游离姜黄素的抗癌活性[39]。紫草素是源自中药紫草的脂溶性萘醌类化合物。已有研究发现,紫草素具有抗肿瘤活性[40]。然而,低口服生物利用度、不良反应和非选择性毒性限制了该化合物的临床应用[41]。与单纯紫草素比较,紫草素纳米凝胶能显著提高紫草素释放靶向性和细胞凋亡率[紫草素纳米凝胶干预的7-氨基放线菌素D阳性率(50.33%±2.60%)显著高于单纯紫草素作用(30.33%±0.88%)][42]。另外,中药活性化合物纳米联合用药策略为耐药肿瘤的治疗提供新的机遇。一种双氢青蒿素(dihydroartemisinin,DHA)-粉防己碱(tetrandrine,TET)联合的pH敏感脂质体(DT-pH-LP)治疗多柔比星(doxorubicin,DOX)耐药乳腺癌表现出良好的肿瘤抑制作用;该脂质体具有良好的球形结构、均匀的分散结构以及长期稳定性;随着DOX耐药逆转能力的增强,DT-pH-LP对MCF-7/ADR细胞和MCF-7细胞的抑制作用均显著增强,且对心肌细胞H9c2的毒性低[43]。机制研究提示,该脂质体中DHA能增强细胞内活性氧(reactive oxygen species,ROS)生成和脂质过氧化反应,表明通过负载的纳米载体可促进TET抗肿瘤作用,实现二者协同效应[43]。
2.3 抗氧化
从西蓝花水提取物(broccoli water extract,BWE)中获得的CD(BWE-CD)具有突出的抗氧化性能,能有效清除A549细胞、293T细胞和斑马鱼体内的ROS,并减轻LPS诱导的斑马鱼体内炎症[44]。这些效应依赖于BWE-CD与自由基的直接反应,可调节一氧化氮水平,上调超氧化物歧化酶和谷胱甘肽过氧化物酶-4的表达[44]。由于氧化应激与炎症反应存在关联性,通过抗氧化(清除ROS)增强抗炎效应也是中药纳米凝胶作用特点之一。YEO等[45]设计了一种生物相容性聚苯硼酸(polymeric phenylboronic acid,PPBA)-单宁酸(tannic acid,TA)纳米凝胶(PPBA-TA nanogel,PTNG),能有效清除外源性過氧化氢和体内ROS;对于体外和体内诱导性炎症模型,PTNG均表现出抗炎作用,能显著减少中性粒细胞募集和促炎细胞因子表达。在植物界广泛分布的槲皮素(quercetin,QCT)因其优异的抗氧化和抗炎特性而得到广泛认可,在急性肺损伤(acute lung injury,ALI)治疗中显示出巨大潜力,然而低溶解度和口服生物利用度降低其应用价值。一种可吸入的槲皮素-海藻酸纳米凝胶(QCT-alginate nanogel,QUNG)能显著提高QCT的溶解度和口服生物利用度;超声雾化吸入QUNG可明显逆转ALI大鼠氧化应激损伤,并下调炎症细胞因子表达[46],提示超声雾化吸入QUNG是一种可行的肺靶向给药方法。
2.4 抑菌
纳米技术在抗菌药物传递应用中具有提高抗菌效果的潜力。由静电作用和疏水作用控制的小檗碱(berberine,BBR)-黄酮苷自组装纳米粒子(nanoparticle,NP)和纳米纤维(nanofiber,NFib)展示了与BBR不同的抗菌特性。三者抑菌活性比较:NP远大于BBR,BBR远大于NFib。该活性差异可归因于NP和NFib不同的空间配置和自组装过程[47]。黄酮苷和BBR首先形成一维复合单元,随后自组装成三维纳米结构;随着亲水性的葡萄糖醛酸朝向外部,NP展现出更强的细菌亲和力,导致菌群锐减和生物膜减少;体外溶血试验、细胞毒性试验和体内斑马鱼毒性评估表明,NP与NFib自组装体具有良好的生物相容性,为自释放药物用于细菌感染治疗提供了重要参考[47]。一种抑菌纳米传递系统由来源于中药黄连和大黄的抗菌化合物BBR和大黄素通过自主装构成。大黄素作为分层的骨架,BBR嵌入其中。体外抑菌实验表明,纳米颗粒的最小杀菌浓度为0.1 μmol/mL,低于BBR和大黄素;纳米颗粒对金黄色葡萄球菌生物膜有强烈的抑制作用;同时,纳米颗粒具有良好的生物相容性和安全性[48]。该研究揭示了源自传统中药组合的小分子自组装设计模式。
2.5 促创面愈合
一种由壳聚糖/蚕丝水凝胶海绵、血小板血浆外泌体(platelet-rich plasma exosome,PRP-Exos)和莪术均质多糖(Curcuma zedoaria polysaccharide,ZWP)组成的药物递送系统用于治疗糖尿病大鼠创面愈合,表现出优于单用PRP-Exos或ZWP的創面闭合性能,且未见不良反应;该治疗效应可能与促进胶原合成与沉积以及伤口部位血管生成有关[49]。XU等[50]开发了一种木质素纳米凝胶(lignin nanogel,LNG),该纳米凝胶由聚乙二醇、聚丙烯乙二醇、聚二甲基硅氧烷三者的聚氨酯共聚物加入木质素制成。LNG能降低ROS水平,保护人正常肝细胞免受氧化应激引起的凋亡;体内实验结果提示,LNG能提高Ki67表达,加速小鼠烧伤创面的愈合[50]。表明抗氧化促进创面愈合是LNG生物活性特征之一。
2.6 细胞保护
近年来,来源于植物的天然化合物显示出美容的应用潜力,包括防晒、保湿、抗衰老、烧烫伤修复和皮肤疾病的治疗。“纳米化”植物化合物能增强药妆产品的无菌体验,实现持续递送并增强皮肤保护活性。姜黄素[51]、白藜芦醇[52]等天然产物借助脂质体/LNP载体的高渗透性和高稳定性成功实现皮肤各层次护理,加速烧烫伤愈合[53]。纳米递送技术为增强化妆品中植物衍生物的生物活性提供了理想载体,为皮肤科患者与化妆品用户提供了许多临床益处[54]。番茄红素(lycopene,LYC)是常见类胡萝卜素之一,具有抗氧化[55]、抗炎[56]、抗衰老[57]、免疫调节[58]、护肝[59]等生物学活性。一项研究发现,LYC的纳米脂质体(LNP-LYC)比单纯LYC能更有效地减少脑缺血再灌注损伤大鼠的脑梗死病变,并改善大鼠的神经行为。分子层面,LNP-LYC能降低一氧化氮合酶、NADPH氧化酶-2等氧化酶蛋白与Caspase-3水平,升高B淋巴细胞瘤-2表达,并能通过下调丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)-JNK信号抑制细胞凋亡[60]。此外,LNP-LYC能抑制铁调素介导的铁转运蛋白-1的减少,并使铁水平正常化,提示LNP-LYC通过调节铁代谢对脑缺血再灌注损伤大鼠发挥神经保护作用[60]。作为一种抗疲劳、抗抑郁的经典中药,肉桂油(cinnamon oil,CO)常因低口服生物利用度而受限。MA等[61]设计CO固体自微乳化给药系统(CO solid SME drug delivery system,CO-S-SME),该系统能有效改善慢性不可预知温和刺激模型小鼠的抑郁样行为,增加模型小鼠神经递质水平,降低皮质酮和炎症因子表达;CO-S-SME能改变肠道菌群组成,降低厚壁菌/拟杆菌比例以及乳酸杆菌的相对丰度,调节α多样性和β多样性。综上,CO-S-SME通过单胺类神经递质、皮质酮、炎症细胞因子、肠道菌群等途径发挥抗抑郁作用。
2.7 止血
常见临床止血炭药包括黄柏炭、丝路蓟炭、芝麻炭等。源自这些炭化药用植物的CD具有止血作用。在动物出血模型中,接受黄柏炭CD治疗的动物的出血时间明显短于对照组,表现出止血活性;黄柏炭CD干预后,纤维蛋白原和血小板水平显著增加;于低剂量黄柏炭CD干预后,凝血酶时间缩短,而凝血酶原时间和活化部分凝血活酶时间在黄柏炭CD治疗组与对照组之间差异无统计学意义,提示黄柏炭CD止血效能与激活纤维蛋白系统有关[62]。
3 总结
中药外泌体、CD、纳米凝胶、聚合物胶束、自微乳、脂质体/LNP等制剂充分发挥了纳米剂型的优势,将中药作用原理从整体、宏观层面引向分子、微观层面,可增强中药治疗的靶向性,提高活性成分的利用率,弥补中药应用时的不足。中药“纳米化”是创新中药研究的重要内容之一,不仅衍生出中药作用的新理论,而且有助于发现中药治疗的新适应证。虽然中药纳米制剂的基础研究已有突破性进展,但缺少足够的证据支持中药纳米化的临床推广。后续研究可筛选潜在中药纳米制剂作为临床转化的候选药物,借助临床研究证据为复杂疾病的治疗提供新的思路。
参考文献
[1] 姚 硕, 刘鼎鑫, 韩 戈, 等. 纳米药物在癌症诊疗中的应用进展[J]. 中国细胞生物学学报, 2023, 45(12): 1945-1960.
[2] 李昀姝, 彭 鹏, 张茜玥, 等. 载中药纳米材料促进骨与软骨修复研究进展[J]. 中国中医骨伤科杂志, 2024, 32(2): 92-96.
[3] DAD H A, GU T W, ZHU A Q, et al. Plant exosome-like nanovesicles: Emerging therapeutics and drug delivery nanoplatforms[J]. Molecular Therapy, 2021, 29(1): 13-31.
[4] HWANG J H, PARK Y S, KIM H S, et al. Yam-derived exosome-like nanovesicles stimulate osteoblast formation and prevent osteoporosis in mice[J]. Journal of Controlled Release, 2023, 355: 184-198.
[5] CHEN X Y, LIU B L, LI X Z, et al. Identification of anti-inflammatory vesicle-like nanoparticles in honey[J]. Journal of Extracellular Vesicles, 2021, 10(4): e12069.
[6] SHEN Y X, ZHANG N, TIAN J L, et al. Advanced approaches for improving bioavailability and controlled release of anthocyanins[J]. Journal of Controlled Release, 2022, 341: 285-299.
[7] LI D F, TANG Q, YANG M F, et al. Plant-derived exosomal nanoparticles: Potential therapeutic for inflammatory bowel disease[J]. Nanoscale Advances, 2023, 5(14): 3575-3588.
[8] KIM J, LI S Y, ZHANG S Y, et al. Plant-derived exosome-like nanoparticles and their therapeutic activities[J]. Asian Journal of Pharmaceutical Sciences, 2022, 17(1): 53-69.
[9] BO?VER R D, TOWN J R, LI X, et al. Carbon dots for carbon dummies: The quantum and the molecular questions among some others[J]. Chemistry, 2022, 28(47): e202200748.
[10] GARCIA-MILLAN T, SWIFT T A, MORGAN D J, et al. Small variations in reaction conditions tune carbon dot fluorescence[J]. Nanoscale, 2022, 14(18): 6930-6940.
[11] MOHAMMADI R, NADERI-MANESH H, FARZIN L, et al. Fluorescence sensing and imaging with carbon-based quantum dots for early diagnosis of cancer: A review[J]. Journal of Pharmaceutical and Biomedical Analysis, 2022, 212: 114628.
[12] BALOU S, SHANDILYA P, PRIYE A. Carbon dots for photothermal applications[J]. Frontiers in Chemistry, 2022, 10: 1023602.
[13] XU J X, NING J, WANG Y, et al. Carbon dots as a promising therapeutic approach for combating cancer[J]. Bioorganic & Medicinal Chemistry, 2022, 72: 116987.
[14] 楊小瑜, 姜一平, 冯浩维, 等. 紫草外用传统制剂与新型纳米制剂的研究进展[J]. 中国药房, 2023, 34(15): 1909-1914.
[15] 王 燕, 周应学. 单分子胶束的制备及应用研究进展[J]. 化学研究与应用, 2024, 36(1): 10-27.
[16] 赵健清, 唐昭敏. 交联星型聚合物胶束的制备及其抗肿瘤应用[J]. 工业微生物, 2023, 53(4): 10-12.
[17] GHEZZI M, PESCINA S, PADULA C, et al. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions[J]. Journal of Controlled Release, 2021, 332: 312-336.
[18] 钟文嘉, 黄益穗, 刘灼波. 基于含水量-电导率拟合曲线模型优化丹参酮提取物微乳的研究[J]. 中国药学杂志, 2024, 59(3): 256-262.
[19] MADHAVI N, BATTU H. Enhanced in vitro and ex vivo transdermal permeation of microemulsion gel of tapentadol hydrochloride[J]. International Microencapsulation Society, 2024, 41(2): 127-139.
[20] 王兴芝, 代英辉, 王东凯. 脂质体的制备方法及应用的研究进展[J]. 中国药剂学杂志(网络版), 2024, 22(1): 14-24.
[21] 彭佩纯, 潘姿蕗, 邓 鑫. 靶向载药脂质体在肿瘤治疗中的应用研究进展[J]. 山东医药, 2023, 63(17): 91-96.
[22] EYGERIS Y, GUPTA M, KIM J, et al. Chemistry of lipid nanoparticles for RNA delivery[J]. Accounts of Chemical Research, 2022, 55(1): 2-12.
[23] LUO D H, LI X Y, GUO S S, et al. Paclitaxel liposome, cisplatin and 5-fluorouracil-based induction chemotherapy followed by de-escalated intensity-modulated radiotherapy with concurrent cisplatin in stage IVA-IVB childhood nasopharyngeal carcinoma in endemic area: A phase II, single-arm trial[J]. The Lancet Regional Health Western Pacific, 2023, 40: 100895.
[24] GOLUBOVSKAYA V, SIENKIEWICZ J, SUN J Y, et al. CAR-NK cells generated with mRNA-LNPs kill tumor target cells in vitro and in vivo[J]. International Journal of Molecular Sciences, 2023, 24(17): 13364.
[25] CHATTERJEE K, LAKDAWALA S, QUADIR S S, et al. siRNA-based novel therapeutic strategies to improve effectiveness of antivirals: An insight[J]. AAPS PharmSciTech, 2023, 24(6): 170.
[26] ZHU H, HE W X. Ginger: A representative material of herb-derived exosome-like nanoparticles[J]. Frontiers in Nutrition, 2023, 10: 1223349.
[27] ZHU Z Z, LIAO L Y, GAO M W, et al. Garlic-derived exosome-like nanovesicles alleviate dextran sulphate sodium-induced mouse colitis via the TLR4/MyD88/NF-κB pathway and gut microbiota modulation[J]. Food & Function, 2023, 14(16): 7520-7534.
[28] LIU Y Y, JIANG Y, YANG Y F, et al. Houttuynia essential oil and its self-microemulsion preparation protect against LPS-induced murine mastitis by restoring the blood-milk barrier and inhibiting inflammation[J]. Frontiers in Immunology, 2022, 13: 842189.
[29] CARDOSO M, GASPAR V M, FERREIRA C, et al. Macrophage-targeted shikonin-loaded nanogels for modulation of inflammasome activation[J]. Nanomedicine, 2022, 42: 102548.
[30] SEO K, YOO J H, KIM J, et al. Ginseng-derived exosome-like nanovesicles extracted by sucrose gradient ultracentrifugation to inhibit osteoclast differentiation[J]. Nanoscale, 2023, 15(12): 5798-5808.
[31] KIM J, ZHU Y, CHEN S H, et al. Anti-glioma effect of ginseng-derived exosomes-like nanoparticles by active blood-brain-barrier penetration and tumor microenvironment modulation[J]. Journal of Nanobiotechnology, 2023, 21(1): 253.
[32] BEN-ZICHRI S, RAJENDRAN S, BHUNIA S K, et al. Resveratrol carbon dots disrupt mitochondrial function in cancer cells[J]. Bioconjugate Chemistry, 2022, 33(9): 1663-1671.
[33] MONDAL A, NAYAK A K, CHAKRABORTY P, et al. Natural polymeric nanobiocomposites for anti-cancer drug delivery therapeutics: A recent update[J]. Pharmaceutics, 2023, 15(8): 2064.
[34] MAO L, ZUO J, LIU Y J, et al. Alginate based films integrated with nitrogen-functionalized carbon dots and layered clay for active food packaging applications[J]. International Journal of Biological Macromolecules, 2023, 253(Pt 1): 126653.
[35] DUDUN A A, CHESNOKOVA D V, VOINOVA V V, et al. Changes in the gut microbiota composition during implantation of composite scaffolds based on poly(3-hydroxybutyrate) and alginate on the large-intestine wall[J]. Polymers, 2023, 15(17): 3649.
[36] LI J Y, PU Y J, LI S, et al. Orally administrated olsalazine-loaded multilayer pectin/chitosan/alginate composite microspheres for ulcerative colitis treatment[J]. Biomacromolecules, 2023, 24(5): 2250-2263.
[37] CHEN Y M, WONG C C, WENG P W, et al. Bioinspired and self-restorable alginate-tyramine hydrogels with plasma reinforcement for arthritis treatment[J]. International Journal of Biological Macromolecules, 2023, 250: 126105.
[38] CHI Y H, SHI L, LU S, et al. Inhibitory effect of Lonicera japonica-derived exosomal miR2911 on human papilloma virus[J]. Journal of Ethnopharmacology, 2024, 318(Pt B): 116969.
[39] FARHOUDI L, KESHARWANI P, MAJEED M, et al. Polymeric nanomicelles of curcumin: Potential applications in cancer[J]. International Journal of Pharmaceutics, 2022, 617: 121622.
[40] SHI W, MEN L T, PI X, et al. Shikonin suppresses colon cancer cell growth and exerts synergistic effects by regulating ADAM17 and the IL-6/STAT3 signaling pathway[J]. International Journal of Oncology, 2021, 59(6): 99.
[41] YAN C M, LI Q X, SUN Q, et al. Promising nanomedicines of shikonin for cancer therapy[J]. International Journal of Nanom?edicine, 2023, 18: 1195-1218.
[42] LI S Y, ZHANG T, XU W G, et al. Sarcoma-targeting peptide-decorated polypeptide nanogel intracellularly delivers shikonin for upregulated osteosarcoma necroptosis and diminished pulmonary metastasis[J]. Theranostics, 2018, 8(5): 1361-1375.
[43] ZHANG X Y, LIU H, LI N, et al. A (traditional Chinese medicine) TCM-inspired doxorubicin resistance reversing strategy: Preparation, characterization, and application of a co-loaded pH-sensitive liposome[J]. AAPS PharmSciTech, 2023, 24(7): 181.
[44] DENG W W, ZANG C R, LI Q C, et al. Hydrothermally derived green carbon dots from broccoli water extracts: Decreased toxicity, enhanced free-radical scavenging, and anti-inflammatory performance[J]. ACS Biomaterials Science & Engineering, 2023, 9(3): 1307-1319.
[45] YEO J, LEE J, YOON S, et al. Tannic acid-based nanogel as an efficient anti-inflammatory agent[J]. Biomaterials Science, 2020, 8(4): 1148-1159.
[46] CHEN Y B, ZHANG Y B, WANG Y L, et al. A novel inhalable quercetin-alginate nanogel as a promising therapy for acute lung injury[J]. Journal of Nanobiotechnology, 2022, 20(1): 272.
[47] LI T, WANG P L, GUO W B, et al. Natural berberine-based Chinese herb medicine assembled nanostructures with modified antibacterial application[J]. ACS Nano, 2019, 13(6): 6770-6781.
[48] TIAN X H, WANG P L, LI T, et al. Self-assembled natural phytochemicals for synergistically antibacterial application from the enlightenment of traditional Chinese medicine combination[J]. Acta Pharmaceutica Sinica B, 2020, 10(9): 1784-1795.
[49] XU N, WANG L L, GUAN J J, et al. Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model[J]. International Journal of Biological Macromolecules, 2018, 117: 102-107.
[50] XU J, XU J J, LIN Q Y, et al. Lignin-incorporated nanogel serving As an antioxidant biomaterial for wound healing[J]. ACS Applied Bio Materials, 2021, 4(1): 3-13.
[51] SHARMA A, KUHAD A, BHANDARI R. Novel nanotechnological approaches for treatment of skin-aging[J]. Journal of Tissue Viability, 2022, 31(3): 374-386.
[52] CHEN J, WEI N, LOPEZ-GARCIA M, et al. Development and evaluation of resveratrol, Vitamin E, and epigallocatechin gallate loaded lipid nanoparticles for skin care applications[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2017, 117: 286-291.
[53] QADIR A, AHMAD U, ALI A, et al. Lipid engineered nanoparticle therapy for burn wound treatment[J]. Current Pharmaceutical Biotechnology, 2022, 23(12): 1449-1459.
[54] ESPOSITO E, NASTRUZZI C, SGUIZZATO M, et al. Nanomedi?cines to treat skin pathologies with natural molecules[J]. Current Pharmaceutical Design, 2019, 25(21): 2323-2337.
[55] KULAWIK A, CIELECKA-PIONTEK J, ZALEWSKI P. The importance of antioxidant activity for the health-promoting effect of lycopene[J]. Nutrients, 2023, 15(17): 3821.
[56] SILVA MENEGUELLI T, DUARTE VILLAS MISHIMA M, HERM?SDORFF HHM, et al. Effect of carotenoids on gut health and inflammatory status: A systematic review of in vivo animal studies[J]. Critical Reviews in Food Science and Nutrition, 2023: 1-16.
[57] CRUPI P, FAIENZA M F, NAEEM M Y, et al. Overview of the potential beneficial effects of carotenoids on consumer health and well-being[J]. Antioxidants, 2023, 12(5): 1069.
[58] LUO H, BAO Y H, ZHU P. Development of a novel functional yogurt rich in lycopene by Bacillus subtilis[J]. Food Chemistry, 2023, 407: 135142.
[59] ABDEL-NAIM A B, HASSANEIN E H M, BINMAHFOUZ L S, et al. Lycopene attenuates chlorpyrifos-induced hepatotoxicity in rats via activation of Nrf2/HO-1 axis[J]. Ecotoxicology and Environmental Safety, 2023, 262: 115122.
[60] ZHAO Y S, XIN Z, LI N N, et al. Nano-liposomes of lycopene reduces ischemic brain damage in rodents by regulating iron metabolism[J]. Free Radical Biology & Medicine, 2018, 124: 1-11.
[61] MA T Y, TANG B J, WANG Y, et al. Cinnamon oil solid self-microemulsion mediates chronic mild stress-induced depression in mice by modulating monoamine neurotransmitters, corticosterone, inflammation cytokines, and intestinal flora[J]. Heliyon, 2023, 9(6): e17125.
[62] LIU X M, WANG Y Z, YAN X, et al. Novel Phellodendri Cortex (Huang Bo)-derived carbon dots and their hemostatic effect[J]. Nanomedicine, 2018, 13(4): 391-405.
〔基金項目〕四川省医院协会青年药师科研专项资金项目(22007);成都市医学科研课题(2023459)。
〔通信作者〕*闫 芳,女,博士,副主任医师,E-mail:fangyan@cdutcm.edu.cn。
