Construction and validation of a neovascular glaucoma nomogram in patients with diabetic retinopathy after pars plana vitrectomy
2024-04-22YiShiYanXinZhangMingFeiJiaoXinJunRenBoJieHuAiHuaLiuXiaoRongLi
Yi Shi,Yan-Xin Zhang,Ming-Fei Jiao,Xin-Jun Ren,Bo-Jie Hu,Ai-Hua Liu,Xiao-Rong Li
Abstract BACKGROUND Neovascular glaucoma (NVG) is likely to occur after pars plana vitrectomy (PPV) for diabetic retinopathy (DR) in some patients,thus reducing the expected benefit.Understanding the risk factors for NVG occurrence and building effective risk prediction models are currently required for clinical research.AIM To develop a visual risk profile model to explore factors influencing DR after surgery.METHODS We retrospectively selected 151 patients with DR undergoing PPV.The patients were divided into the NVG (NVG occurrence) and No-NVG (No NVG occurrence) groups according to the occurrence of NVG within 6 months after surgery.Independent risk factors for postoperative NVG were screened by logistic regression.A nomogram prediction model was established using R software,and the model’s prediction accuracy was verified internally and externally,involving the receiver operator characteristic curve and correction curve.RESULTS After importing the data into a logistic regression model,we concluded that a posterior capsular defect,preoperative vascular endothelial growth factor ≥ 302.90 pg/mL,glycosylated hemoglobin ≥ 9.05%,aqueous fluid interleukin 6 (IL-6) ≥ 53.27 pg/mL,and aqueous fluid IL-10 ≥ 9.11 pg/mL were independent risk factors for postoperative NVG in patients with DR (P < 0.05).A nomogram model was established based on the aforementioned independent risk factors,and a computer simulation repeated sampling method was used to internally and externally verify the nomogram model.The area under the curve (AUC),sensitivity,and specificity of the model were 0.962 [95% confidence interval (95%CI): 0.932-0.991],91.5%,and 82.3%,respectively.The AUC,sensitivity,and specificity of the external validation were 0.878 (95%CI: 0.746-0.982),66.7%,and 95.7%,respectively.CONCLUSION A nomogram constructed based on the risk factors for postoperative NVG in patients with DR has a high prediction accuracy.This study can help formulate relevant preventive and treatment measures.
Key Words: Diabetic retinopathy;Retinopathy;Neovascular;Glaucoma;Risk factors;Nomogram
INTRODUCTION
Diabetic retinopathy (DR) is a clinical complication of diabetes,and its fundus manifestations include retinal exudation,edema,angiogenesis,hemorrhage,and proliferative membrane formation[1].The primary treatment is pars plana vitrectomy (PPV),which effectively controls disease progression[2].However,some patients develop postoperative neovascular glaucoma (NVG).NVG is a secondary glaucoma that can lead to severe visual impairment or even complete blindness,seriously affecting the postoperative recovery and prognosis of patients[3,4].Medical researchers widely believe that NVG is closely related to the release of various cytokines induced by retinal ischemia and hypoxia,which promotes extensive neovascularization and causes ocular hypertension by blocking the anterior chamber horn[5].Relevant reports have shown that inflammatory regulators are closely related to DR neovascularization[6].There is an urgent need to explore the relevant factors of postoperative NVG in DR and improve the postoperative recovery and prognostic effects of patients with DR.Currently,there are few reports on integrating the relevant factors of postoperative NVG in patients with DR and building risk screening tools based on this[7-9].A nomogram model can visualize the results of multifactor analysis and be intuitively used to predict individual risk factors[10].In this study,a nomogram was constructed to predict postoperative NVG in DR,providing a theoretical basis for the clinical screening of high-risk groups and the formulation of relevant preventive measures.
MATERlALS AND METHODS
Patients
We retrospectively selected 151 patients with DR who had undergone PPV at the Tianjin Medical University Eye Hospital.The treatment period for the selected patients was between January 2019 and December 2020.Patients were enrolled in the NVG group (with NVG) and the No-NVG group (without NVG) according to the occurrence of NVG within 6 months after surgery.The included patients met the following conditions: (1) Clinical diagnosis of DR and PPV treatment;and (2) complete information at the 6-month follow-up.The exclusion criteria were as follows: (1) Prior history of glaucoma or ocular hypertension;and (2) recurrent vitreous hemorrhage.
Method
The clinical data of the patients were collected.They included demographic data (age,sex,and body mass) at admission,clinical information [type of diabetes,DR severity,duration of retinopathy,hypertensive or not,whether hyperlipidemic,preoperative intraocular pressure,duration of surgery,number of vitrectomies performed,intraocular fillings,whether combined cataract surgery was performed,whether ipsilateral carotid artery stenosis was ≤ 25.0%,residual in retinal nonperfusion area,preoperative anti-vascular endothelial growth factor (VEGF) therapy],and preoperative laboratory data [serum VEGF,glycosylated hemoglobin (HbAlc),interleukin (IL)-6] and IL-10 in serum and aqueous humor).
Carotid artery stenosis assessment was as follows: Carotid intima-media thickness detected by color Doppler ultrasound of < 1.0 mm,11.2 mm,1.2-1.4 mm,and > 1.4 mm were categorized as normal,intimal thickening,plaque formation,and carotid artery stenosis,respectively.The degree of carotid artery stenosis with reference to blood flow velocity and whether the carotid artery stenosis of patients was ≤ 25.0% was assessed.
Fundus fluorescein angiography and optical coherence tomography angiography were used to determine the boundary of the non-perfusion area.After correcting the scale in the fundus fluorescein angiography image,Image J software version 1.48 measured the non-perfusion area.
Five mL of fasting venous blood was extracted from the patient and stored in a -70 °C refrigerator for examination.Serum VEGF was detected by enzyme-linked immunosorbent assay (ELISA).The kit was purchased from Shanghai Renjie Biotechnology Co.,LTD.HbA1C was determined by high-performance liquid chromatography using Gimp LC-4000 high-performance liquid chromatography.Serum IL-6,IL-10,and tumor necrosis factor (TNF-α) were detected by biotin-avidin double antibody sandwich ELISA.
Establishment and verification of the risk nomogram model
Demographic,clinical,and preoperative laboratory data of patients with and without NVG were compared.We incorporated variables with statistically significant differences into the logistic regression model to identify the risk factors for NVG.The rms package of the R language (R 4.0.3) software was used to establish a nomogram model for the risk of postoperative NVG in patients with DR.The line diagram model was internally verified using bootstrap sampling 500 times.The clinical and related laboratory data of another 72 patients (including 12 patients with NVG;the incidence of NVG was 16.67%) who underwent PPV (between January 2021 and December 2021) were used as an external validation cohort based on the same inclusion and exclusion criteria.After internal and external validation,we evaluated the differentiation using the receiver operator characteristic curve (ROC).A calibration curve was used to evaluate the degree of nomogram calibration.
Statistical processing
Statistical software (SPSS 23.0) was used to analyze the data.Qualitative data are presented as frequencies and percentages,and the two groups were compared using the chi-square test.The continuous correction chi-square was adopted when 1 ≤ theoretical frequency < 5,and the total sample size was ≥ 40.Quantitative normal distribution data are presented as means ± SD,and we compared the groups using at-test.Additionally,quantitative non-normal distribution data were described as M (P25,P75) and analyzed using the Mann-WhitneyUtest.Risk factors were analyzed using binary logistic regression.The test level is α=0.05.
RESULTS
Differences in clinical baseline indicators
Among the 151 patients with DR who underwent PPV,21 (13.91%) developed NVG (NVG group),and 130 (No-NVG group) did not develop NVG within 6 months after surgery.Compared with the No-NVG group,the ratio of posterior capsular defect,ipsilateral carotid artery stenosis ≤ 25.0%,residual retinal non-perfusion area,and the levels of preoperative VEGF,HbAlc,IL-6,IL-10,and TNF-α in aqueous humor were higher in the NVG group during cataract surgery (P< 0.05) (Table 1).
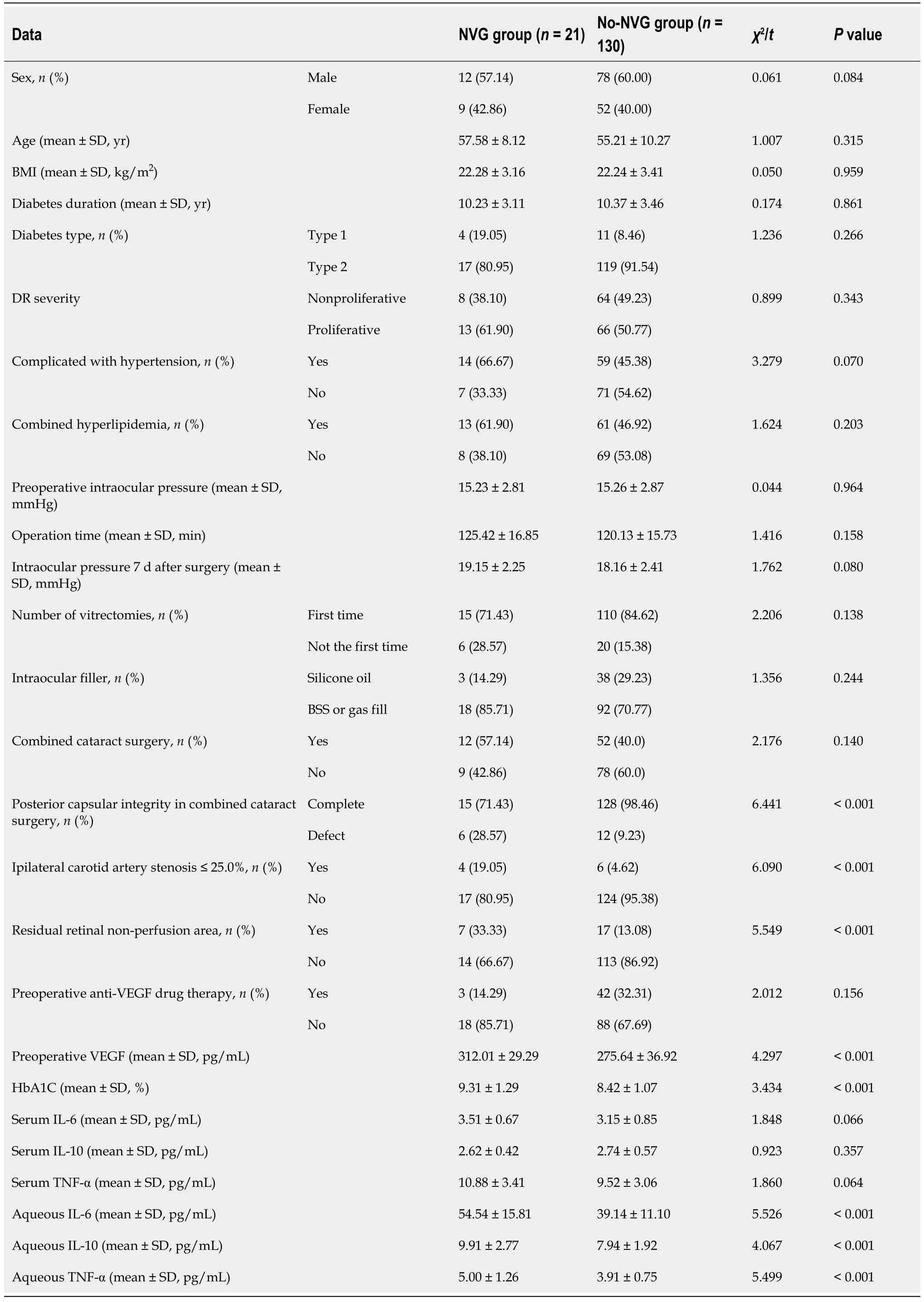
Table 1 Differences in clinical data between the neovascular glaucoma and No-neovascular glaucoma-groups
Analysis of factors affecting postoperative NVG in patients with DR
With the occurrence of NVG (1=occurrence,0=non-occurrence) in patients with DR after surgery as the dependent variable,eight factors with statistical significance in univariate analysis (posterior capsular integrity in combined cataract surgery,ipsilateral carotid artery stenosis ≤ 25.0%,residual retinal non-perfusion area,preoperative VEGF,HbAlc,aqueous humor IL-6,aqueous humor IL-10,and aqueous humor TNF-α) were used as independent variables.Original values of measurement data were entered and assigned to classified data [integrity of posterior capsule (1=defect,0=integrity),ipsilateral carotid artery stenosis ≤ 25.0% (1=yes,0=no),and residual retinal non-perfusion area (1=yes,0=no)].Multivariate logistic regression analysis showed that a posterior capsular defect,preoperative VEGF ≥ 302.90 pg/mL,HbAlc ≥ 9.05%,aqueous fluid IL-6 ≥ 53.27 pg/mL,and aqueous fluid IL-10 ≥ 9.11 pg/mL in combined cataract surgery were independent risk factors for postoperative NVG in patients with DR (P< 0.05;Table 2).
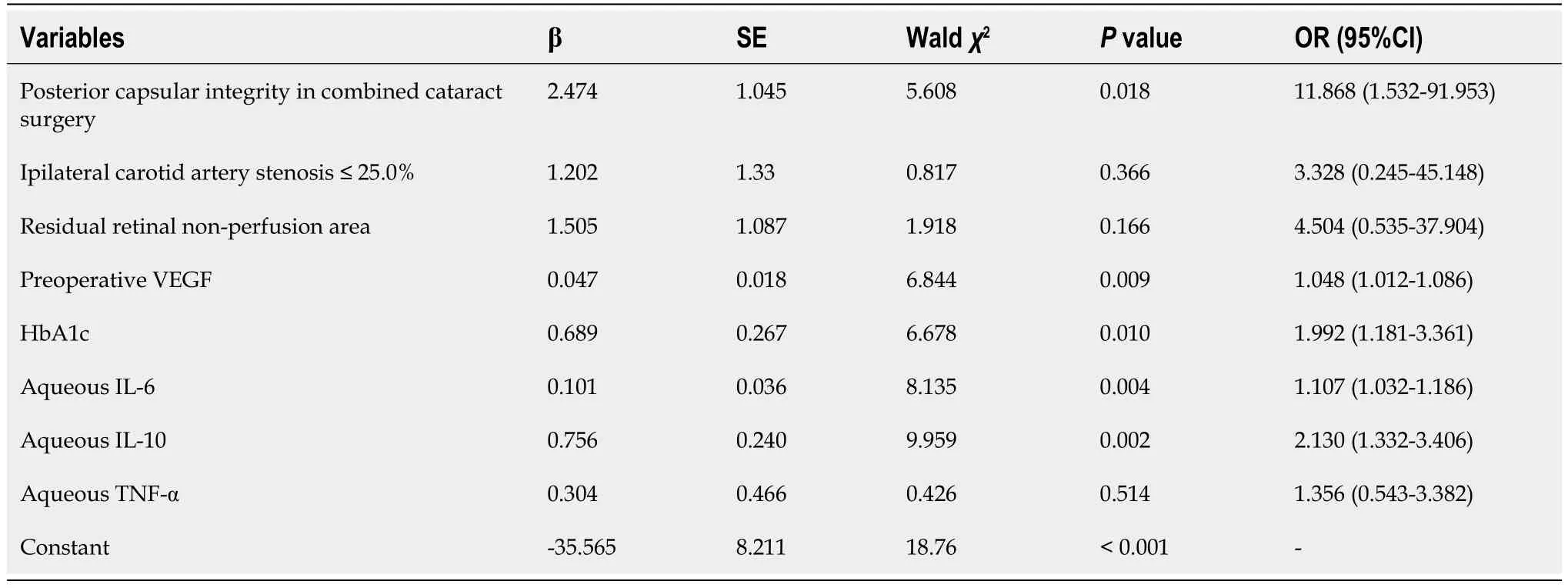
Table 2 Logistic regression analysis of postoperative neovascular glaucoma in patients with diabetic retinopathy
Establishment of a risk model for postoperative NVG profile in patients with DR
Based on the results of logistic regression analysis,R software was used to construct a nomogram model for predicting NVG risk (Figure 1).Based on the column nomogram,NVG risk can be quickly predicted.A patient with a posterior capsular defect and preoperative VEGF=250 pg/mL,HbAlc=10%,aqueous fluid IL-6=60 pg/mL,and IL-10=13.5 pg/mL,during combined cataract surgery had a total score of 133.5 (11.0+42.0+15.0+27.5+40.0),suggesting a 90% risk of postoperative NVG.
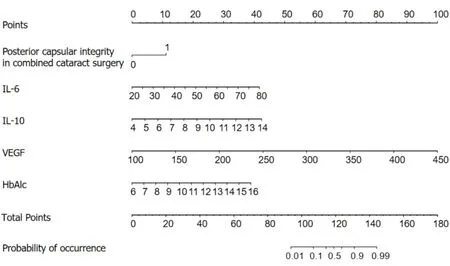
Figure 1 A nomogram model predicting the risk of neovascular glaucoma in patients with diabetic retinopathy After surgery. IL-6: Interleukin-6;IL-10: Interleukin-10;VEGF: Vascular endothelial growth factor;HbAlc: Glycosylated hemoglobin;NVG: Neovascular glaucoma;DR: Diabetic retinopathy.
Internal validation of the nomogram model
The area under the ROC curve (AUC) of the nomogram model for predicting the risk of postoperative NVG in DR was 0.962 [95% confidence interval (95%CI): 0.932-0.991],and the sensitivity and specificity were 95.2% and 89.2%,respectively,suggesting that the nomogram model had good differentiation ability (Figure 2A).After 500 repeated samples of the original data,a calibration curve was constructed (Figure 2B).The average absolute error of the calibration curve was 0.024,indicating that the degree of calibration and prediction consistency of the nomogram was high.In the Hosmer-Lemeshow goodness of fit testχ2=2.854 (P=0.943 > 0.05),the difference between the predicted risk and the observed risk was small;therefore,the NVG predicted by the model was in good agreement with the actual risk.
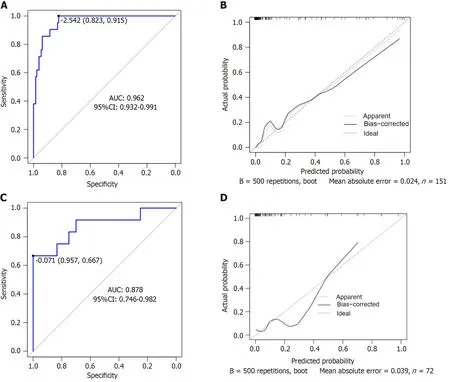
Figure 2 The prediction performance of the model was evaluated on the internal and external validation set. A: Receiver operating characteristic (ROC) curve of the internal validation set;B: Calibration curves of the internal validation set;C: ROC curve of the external validation set;D: Calibration curves for the external validation set.AUC: Area under the curve;CI: Confidence interval;ROC: Receiver operating characteristic.
External validation performance of the nomogram
The input validation set data for external verification showed that the AUC,sensitivity,and specificity were 0.878 (95%CI: 0.746-0.982),66.7%,and 95.7%,respectively,indicating high prediction accuracy (Figure 2C).The correction curve was close to the ideal value (the average absolute error was 0.039),showing a prediction probability consistent with the measured value (Figure 2D).
DISCUSSION
NVG is a common and refractory complication in patients with DR.Previous studies have shown that young age,coronary heart disease or cerebral infarction,cataract phacoemulsification surgery,ipsilateral carotid artery stenosis,residual non-perfusion area of the retina after PPV,poor intraoperative retinal photocoagulation effect,postoperative retinal redetachment,multiple operations,and perioperative blood glucose instability are independent risk factors for postoperative NVG in patients with DR[11].However,evidence on the relationship between serum and aqueous humor inflammatory factors and postoperative NVG in patients with DR is insufficient,and further research is needed.
NVG is caused by the release of various cytokines (such as VEGF) induced by retinal ischemia and hypoxia,thus promoting neovascularization,obstructing aqueous humor circulation,and increasing intraocular pressure[12].The results of this study showed that the risk factors for postoperative NVG in patients with DR were posterior capsule defects during cataract surgery,preoperative VEGF ≥ 302.90 pg/mL,HbAlc ≥ 9.05%,aqueous fluid IL-6 ≥ 53.27 pg/mL,and aqueous fluid IL-10 ≥ 9.11 pg/mL.Posterior capsular defects during cataract surgery are correlated with postoperative NVG in patients with DR[13].According to our results,the risk of postoperative NVG in posterior capsular defects during cataract surgery was 11.868 times that of posterior capsular integrity defects.The potential mechanism of NVG induced by posterior capsule defects during cataract surgery involves the release of several cytokines (such as VEGF) because of DR retinal ischemia and hypoxia.Simultaneously,the vitreous fluid fills up,and VEGF spreads faster,resulting in a posterior capsule defect.VEGF enters the posterior chamber through the damaged barrier and circulates to the iris and horn of the atrium along with the aqueous solution to form new blood vessels,resulting in NVG[14].Palfi Salavatetal[15] have shown that anti-VEGF drugs can effectively prevent the formation of posterior capsule blood vessels and that the postoperative use of anti-VEGF drugs can prevent the occurrence of NVG.In addition,Simhaetal[16] showed that anti-VEGF drugs effectively reduced intraocular pressure in patients with NVG.Our study also found that VEGF is a risk factor for NVG formation and a sensitive factor for predicting postoperative NVG in patients with DR,which is consistent with the results of previous studies by other medical researchers.Excessive secretion of VEGF can promote the generation of new blood vessels at the iris surface and anterior chamber angle,which further suggests that VEGF may be involved in the mechanism of NVG secondary to DR.Haseetal[17] found high VEGF-C expression in the trabecular meshwork tissues of patients with glaucoma.In the case of severe retinal ischemia,VEGF expression promotes the formation of NVG[18].HbA1c is the product of the combination of hemoglobin in red blood cells and serum sugars (mainly glucose) through a non-enzymatic reaction,which mainly reflects the changes in blood sugar in the previous 2 months.It can change the affinity of red blood cells to oxygen such that tissues and cells are deprived of oxygen.HbA1c is one of the biomarkers of DR,and the gradual accumulation of HbA1c concentration is closely related to the occurrence and progression of the disease[19].Sakamotoetal[20] believe the HbA1c difference to be a risk factor for NVG occurrence.Tissue hypoxia caused by increased HbA1c content may lead to retinal hemorrhage,exudation,edema,ischemia,and eventually neovascularization.
Extensive and in-depth studies on NVG have found that its pathogenesis is not only related to angiogenesis caused by ischemia and hypoxia-induced increase in VEGF expression but also to inflammation[21].Several studies[22,23] have found that inflammatory factor levels in serum and aqueous fluid are increased in patients with NVG and are positively correlated with VEGF levels,suggesting that inflammatory factors have angiogenesis-promoting activities.Our study also showed that high levels of IL-6 and IL-10 in the aqueous humor increased the risk of postoperative NVG in patients with DR.IL-6 is a major cytokine secreted by immune T cells and is involved in immune response and inflammation.Ocular IL-6 is produced by the retinal pigment epithelium,corneal epithelium,and other cells[24,25].An abnormal increase in IL-6 expression in the aqueous humor indicates an active ocular inflammatory response.Polidoroetal[26] reported that IL-6 increased VEGF expression.IL-10 may promote ocular neovascularization by stimulating VEGF expression[27].It also promotes ocular neovascularization by regulating macrophages during retinal hypoxia[27].In this study,there was no significant correlation between the levels of inflammatory factors in the serum and aqueous humor.A possible reason is that although the ocular inflammatory response was active and the blood-eye barrier was damaged to some extent during the test period,its function was not destroyed,and the entry of some inflammatory factors into the blood was blocked;therefore,there was no consistency between the serum and aqueous humor cytokine levels.
Based on the individual risk indicators,this study established a nomogram model for predicting individual risk factors.The model expressed the contribution rate of each risk index based on the length of the line segment,which is intuitive,concise,readable,and practical in clinical practice.We used internal and external data to verify the accuracy of the model,finding the model to have a high degree of differentiation and the actual prediction curve matching the ideal.Clinically,the risk of postoperative NVG in patients with DR can be predicted according to the scores of each risk factor to strengthen the intervention of controllable factors.Clinical staff should attach great importance to patients with posterior capsular defects during combined cataract surgery or elevated concentrations of preoperative VEGF,HbAlc,aqueous humor IL-6 and aqueous humor IL-10,and actively adopt preventive measures.However,because the sample size of this study was limited to a single center,there is a potential selectivity bias.It was also difficult to obtain more clinical data,which may have resulted in missing potential risk factors.Therefore,the results of this study need to be verified by multicenter and large sample size research and mass data mining.
CONCLUSlON
In summary,the primary influencing factors for NVG in patients with DR after surgery include posterior capsular defect,preoperative VEGF,HbAlc,aqueous fluid IL-6,and aqueous fluid IL-10.Furthermore,constructing a demographic model based on risk factors yields high prediction accuracy.This study can provide a reference for clinical personnel to screen high-risk groups and formulate relevant preventive and treatment measures.
ARTlCLE HlGHLlGHTS
Research background
Pars plana vitrectomy (PPV) can effectively treat diabetic retinopathy (DR);however,some patients are prone to neovascular glaucoma (NVG) after surgery,affecting treatment efficacy.An in-depth understanding of the risk factors for NVG formation and the construction of an effective prediction model are important for clinical intervention to reduce the occurrence of NVG.
Research motivation
Previous studies on NVG risk factors did not include inflammatory factors in their analysis,and there is a lack of a quick and effective clinical risk prediction model.A thorough understanding of the risk factors for NVG and the construction of an effective risk assessment model can promote the clinical identification of high-risk patients and guide interventions.
Research objectives
To analyze the risk factors (including inflammatory factors) for NVG after PPV in patients with DR,build a nomogram model based on this,and evaluate the effectiveness of the model.
Research methods
Binary logistic regression was performed to analyze the risk factors for NVG in patients with DR after PPV.The R language (R 4.0.3) software was used to construct the nomogram,and its accuracy and effectiveness were evaluated using receiver operating characteristic (ROC) and calibration curves.
Research results
Risk factors for NVG after PPV in DR include posterior capsule defect during combined cataract surgery,preoperative VEGF,HbAlc,aqueous fluid IL-6,and aqueous fluid IL-10,and the column nomogram model constructed based on this had good differentiation [AUC: 0.962 (95% confidence interval,95%CI): 0.932-0.991),sensitivity: 91.5%,and specificity: 82.3%].The external validation of the model was also good [AUC: 0.878 (95%CI: 0.746-0.982),sensitivity: 66.7%,specificity: 95.7%].
Research conclusions
NVG influencing factors in patients with DR after surgery are related to many factors,including posterior capsular defects,preoperative VEGF,HbAlc,aqueous fluid IL-6,and aqueous fluid IL-10.The nomogram built based on risk factors had a high prediction accuracy and clinical applicability and is expected to expand the scope of application and reduce the occurrence of NVG.
Research perspectives
This study confirmed that the constructed column diagram is suitable for DR after PPV at our hospital.Future research should aim to expand the sample size to multiple centers to enhance the reliability of the results and facilitate the popularization and application of the model.
FOOTNOTES
Co-first authors:Yi Shi and Yan-Xin Zhang.
Co-corresponding authors:Ai-Hua Liu and Xiao-Rong Li.
Author contributions:Shi Y and Zhang YX designed and performed the research and wrote the paper;Liu AH and Li XR designed the research and supervised the report;Jiao MF,Ren XJ,and Hu BJ contributed to the analysis;all authors were involved in the critical review of the results and have contributed to,read,and approved the final manuscript.Shi Y and Zhang YX contributed equally to this work and are co-first authors;Liu AH and Li XR contributed equally to this work and are co-corresponding authors.The reasons for designating Liu AH and Li XR as co-corresponding authors are threefold.First,the research was performed as a collaborative effort,and the designation of co-corresponding authorship accurately reflects the distribution of responsibilities and burdens associated with the time and effort required to complete the study and the resultant paper.This also ensures effective communication and management of post-submission matters,ultimately enhancing the paper's quality and reliability.Second,the overall research team encompassed authors with a variety of expertise and skills from different fields,and the designation of co-corresponding authors best reflects this diversity.This also promotes the most comprehensive and in-depth examination of the research topic,ultimately enriching readers' understanding by offering various expert perspectives.Third,Liu AH and Li XR contributed efforts of equal substance throughout the research process.The choice of these researchers as co-corresponding authors acknowledges and respects this equal contribution,while recognizing the spirit of teamwork and collaboration of this study.
Supported bythe Tianjin Key Medical Discipline (Specialty) Construction Project,No.TJYXZDXK-037A.
lnstitutional review board statement:The study was reviewed and approved by the Tianjin Medical University Eye Hospital [Approval No.2021KL(L)-54].
lnformed consent statement:The requirement of informed consent was exempted.
Conflict-of-interest statement:The authors declare no conflict of interest.
Data sharing statement:Statistical data used in this study can be obtained from the corresponding author.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Yi Shi 0000-0001-6435-5263;Yan-Xin Zhang 0009-0001-8693-911X;Ming-Fei Jiao 0000-0001-7207-1199;Xin-Jun Ren 0000-0002-8113-2069;Bo-Jie Hu 0000-0001-7840-8290;Ai-Hua Liu 0000-0001-7761-4498;Xiao-Rong Li 0000-0003-0641-2797.
S-Editor:Chen YL
L-Editor:A
P-Editor:Guo X
杂志排行
World Journal of Diabetes的其它文章
- Nε-carboxymethyl-lysine and inflammatory cytokines,markers and mediators of coronary artery disease progression in diabetes
- Non-pharmacological interventions for diabetic peripheral neuropathy: Are we winning the battle?
- Application and management of continuous glucose monitoring in diabetic kidney disease
- Role of renin-angiotensin system/angiotensin converting enzyme-2 mechanism and enhanced COVlD-19 susceptibility in type 2 diabetes mellitus
- Are treatment options used for adult-onset type 2 diabetes mellitus (equally) available and effective for children and adolescents?
- Prevalence and risk factors of wound complications after transtibial amputation in patients with diabetic foot
