Role of renin-angiotensin system/angiotensin converting enzyme-2 mechanism and enhanced COVlD-19 susceptibility in type 2 diabetes mellitus
2024-04-22AshwinKumarShuklaKomalAwasthiKauserUsmanMonishaBanerjee
Ashwin Kumar Shukla,Komal Awasthi,Kauser Usman,Monisha Banerjee
Abstract Coronavirus disease 2019 (COVID-19) is a disease that caused a global pandemic and is caused by infection of severe acute respiratory syndrome coronavirus 2 virus.It has affected over 768 million people worldwide,resulting in approximately 6900000 deaths.High-risk groups,identified by the Centers for Disease Control and Prevention,include individuals with conditions like type 2 diabetes mellitus (T2DM),obesity,chronic lung disease,serious heart conditions,and chronic kidney disease.Research indicates that those with T2DM face a heightened susceptibility to COVID-19 and increased mortality compared to nondiabetic individuals.Examining the renin-angiotensin system (RAS),a vital regulator of blood pressure and pulmonary stability,reveals the significance of the angiotensin-converting enzyme (ACE) and ACE2 enzymes.ACE converts angiotensin-I to the vasoconstrictor angiotensin-II,while ACE2 counters this by converting angiotensin-II to angiotensin 1-7,a vasodilator.Reduced ACE2 expression,common in diabetes,intensifies RAS activity,contributing to conditions like inflammation and fibrosis.Although ACE inhibitors and angiotensin receptor blockers can be therapeutically beneficial by increasing ACE2 levels,concerns arise regarding the potential elevation of ACE2 receptors on cell membranes,potentially facilitating COVID-19 entry.This review explored the role of the RAS/ACE2 mechanism in amplifying severe acute respiratory syndrome coronavirus 2 infection and associated complications in T2DM.Potential treatment strategies,including recombinant human ACE2 therapy,broad-spectrum antiviral drugs,and epigenetic signature detection,are discussed as promising avenues in the battle against this pandemic.
Key Words: Angiotensin-converting enzyme 2;Angiotensin-converting enzyme inhibitors;Angiotensin-II receptor blockers;Complex diseases;COVID-19;Type 2 diabetes
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is caused by a novel virus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),which globally expanded from Wuhan,China at the end of 2019.The World Health Organization declared COVID-19 a global pandemic on March 11,2020 (World Health Organization Situation Reports 51).According to weekly epidemiological updates globally,over 4500 deaths and more than 836000 new COVID-19 cases had been recorded from June 19 to July 16,2023.More than 6.9 million deaths and 768 million confirmed cases were recorded worldwide[1].Most severe COVID-19 cases have been observed in elderly people or comorbid individuals,including type 2 diabetes mellitus (T2DM),hypertension,cardiovascular diseases (CVDs),chronic renal and lung disorders,and cancer[2-5].According to a meta-analysis including 1527 patients in China,patients with diabetes or hypertension had a two-fold increase in risk of severe COVID-19 or requiring intensive care unit (ICU) admission,while it increased to three-fold in patients with cerebrovascular disease[6].Several studies have shown that SARS-CoV-2 along with pneumonia,may be detrimental to the other body organs including the heart,liver,and kidneys[7,8].In addition to this,a high prevalence of comorbidities was also reported in COVID-19 patients[4,9-12].Therefore,the treatment of comorbidities should be given more att-ention,especially in elderly patients with severe underlying conditions.
To effectively manage patients with comorbid disorders,it is crucial to comprehend the relationship between COVID-19 and chronic diseases.Additionally,it enables medical professionals to lessen the side effects linked to this disease.Hence,understanding these mechanisms will help develop a potential treatment for COVID-19.Herein,this review offered proof of the severe COVID-19 manifestation in T2DM and other chronic illnesses,including heart disease,hypertension,and myocardial infarction.More importantly,it analyzed the specific management techniques for patients with the aforementioned comorbid disorders to obtain the greatest therapeutic outcome,described in detail the molecular pathways through which COVID-19 causes these diseases,and highlighted the importance of these strategies.
OVERVlEW OF THE RENlN-ANGlOTENSlN-ALDOSTERONE SYSTEM AND INTRODUCTION TO ANGlOTENSlN CONVERTlNG ENZYME 2
Angiotensin converting enzyme 2 (ACE2) is a key enzyme in the renin-angiotensin-aldosterone system (RAAS),which plays a critical role in the regulation of several physiological processes in the human body.ACE2,a component of RAAS,oversees vasoconstriction to manage blood pressure,electrolyte and fluid balance,and renal salt reabsorption[13].In response to diminished renal blood flow,juxtaglomerular cells convert prorenin to renin,which is then released into the circulation spontaneously.Plasma renin converts hepatic angiotensinogen to angiotensin I (Ang-I),and Ang-I is further transformed into the vasoconstrictor Ang-II by ACE (Figure 1).By converting Ang-II into Ang-(1-7),a vasodilator,ACE2 is essential for maintaining system balance[13].Thus,ACE2 controls several pathological states,including oxidative stress,fibrosis,vasoconstriction,and inflammation (Figure 1).To mitigate RAAS hyperactivity,specific medications disrupt this system through various approaches.For instance,to reduce the synthesis of robust Ang-II,ACE enzyme inhibitors (ACEi) are commonly employed.Another method involves the use of angiotensin-II receptor blockers (ARBs) to inhibit angiotensin-II type 1 receptor (AT1R),thereby controlling an overactive RAAS[14].
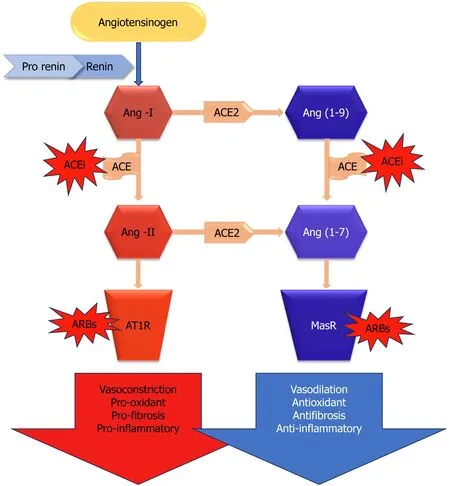
Figure 1 Renin angiotensin aldosterone system: lts components and workflow. ACE: Angiotensin-converting enzyme;ACE2: Angiotensin-converting enzyme 2;ACEi: Angiotensin-converting enzyme inhibitor;Ang(1-7): Angiotensin 1-7;Ang(1-9): Angiotensin 1-9;Ang-I: Angiotensin I;Ang-II: Angiotensin II;ARBs: Angiotensin-II receptor blockers;AT1R: Angiotensin-II type 1 receptor;MasR: MasR receptor.
Role of renin-angiotensin system,ACE,and ACE2 in diabetes susceptibility
Additionally seen in the glomerulus,ACE2 colocalizes with ACE on the apical surface of the proximal tubules[15].Similar to ACE in distribution,ACE2 was shown to be confined to pancreatic acini and islets[16].It has been shown that in diabetic animal models,ACE2 pharmacological suppression[15,17] and genetic ablation[18] both exacerbate albuminuria and the associated glomerular lesions.Additionally,diabetic nephropathy patients’ kidney biopsies[19] and diabetic rodent models[15] have demonstrated decreased glomerular expression of ACE2.Thus,in order to address renal impairment linked to diabetes,increasing the activity of this enzyme by therapeutic targeting of ACE2 has been suggested[15,20].
Changes in glucose tolerance and decreased first-phase insulin production have been seen inACE2knockout mice,indicating that diabetes development may be influenced by ACE2[21].According to reports,pancreatic β cells contain collectrin,another ACE homolog,which may play a role in insulin production and β cell proliferation despite its unknown function[22].Concentrating on comprehending the involvement of ACE2 in diabetes,emphasizing ACE2 within the kidney could emerge as a crucial focal point for addressing and preventing diabetic kidney disease.This progressive condition is marked by thickening and expansion of the mesangial matrix around the glomerular basement membrane,the presence of elevated protein in urine,and associated features linked to kidney disease.In recent times,several research studies have also suggested that the loss of podocytes[23-27] is connected to the deterioration of glomerulopathy[28].The kidney exhibits a completely operational local renin-angiotensin system (RAS),with the ability to produce Ang-II in hyperglycemic circumstances[29,30] as observed by its activation[31].
The presence of ACE is reported in the kidney,pancreas,adipose tissue,and liver.Its expression level is elevated in the endocrine system involving the pancreas in diabetes and in the early stages of diabetic nephropathy.The proposition suggests that ACE2 plays an equal role in compensating for both diabetic nephropathy and diabetes.Furthermore,it appears that mice lacking ACE2 also had higher blood glucose levels following fasting,as evidenced by their elevated fasting blood glucose levels.A novel method for examining the role of ACE2 in diabetes research may be found in mice[32].On the apical surface of the proximal tubules and in the glomerulus,ACE2 colocalizes with ACE[15].Similar to ACE,ACE2 was shown to be distributed in the pancreas and localized to acini and islets[16].
More than 20 years ago,Chappelletal[33] discovered that the pancreas,like the kidney,expresses significant components of a local angiotensin peptide-producing machinery.With decreased levels of Ang-(1-7) and undetectable quantities of Ang-I,the investigators found that Ang-II was the most prevalent angiotensin peptide in the pancreas.As it has been speculated to do in the kidneys and other organs,Ang-(1-7) may mitigate the effects of Ang-II in the pancreas[34].In comparison to the plasma,both peptides were found in much larger concentrations in the pancreas[33].Ang-II has the ability to inhibit blood flow in a dose-dependent manner and delay the generation of insulin in the Langerhans islets,as revealed by Carlssonetal[34].The effects of AngII are consistent with increasing blood flow by RAS blockage with ACE inhibitors or AngII receptor antagonists[34].Moreover,it has been demonstrated that these medications lessen pancreatic fibrosis and inflammation[35].In a noteworthy study,Tikellisetal[16] found that in the Zucker diabetic fatty rat,RAS inhibition enhanced structural parameters in relation to increased first-phase insulin secretion and reduced islet fibrosis.Given clinical evidence that RAAS suppression may be associated with a decreased risk of T2D with a new start,these findings are particularly noteworthy[36].Though more recent studies have cast doubt on these results[37],ongoing current investigations have demonstrated that RAAS blockage aids in the prevention of T2DM.
Based on animal research,it was hypothesized that ACE/ARB therapy increases ACE2 expression.After the recognition of ACE2 as the receptor for SARS-CoV-2[5,38],there is apprehension that an excessive expression of ACE2,resulting in a faster uptake of the virus,might worsen lung damage and elevate the risk of a fatal outcome in individuals with COVID-19.Current clinical analyses[39] and assessments[40] regarding the COVID-19 pandemic raised these concerns without thoroughly examining data from studies involving both animals and humans,resulting in prompt conclusions and even instilling fear among physicians and patients utilizing ACEis or ARBs.There are current investigations aimed especially at demonstrating that RAS blockage aids in the prevention of T2DM.
ACE2 shedding and RAAS overactivity: Implications for glycemic homeostasis
Over time,expression of ACE2 and functioning in several tissues decrease in complications such as T2DM and hypertension[19,41-43].The mechanisms behind the reported decrease in ACE2 in these illnesses are unclear.One explanation for declining levels of ACE2 might be its depletion from tissues.Cell-membrane-bound ACE2 has been discovered to be cleaved by a sheddase,disintegrin,and metalloproteinase (ADAM17) or tumor necrosis factor-α (TNF-α) converting enzyme[44],releasing some of the tissue-resident protein into the bloodstream.In fact,ADAM17 may be the cause of ACE2 shedding in T2DM patients due to its elevation in human carotid atherosclerotic plaques[45].Interestingly,the pancreas,brain,adipose tissues,and vascular smooth muscle cells all express more ADAM17 when exposed to Ang-II[46,47].The phenomenon of ACE2 downregulation in response to RAS overactivity in the islets of mice can be attributed to ACE2 shedding mediated by ADAM17[42].The surge in ADAM17 expression is lowered by ACE2 treatment,suggesting that levels of Ang-II can affect levels of ADAM17[42].In summary,preliminary data points to a possible link between RAS hyperactivity and decreased glycemia;restoring lost ACE2 in the islets seems to control activity of RAS and hence raise glycemia.Restoring lost ACE2 seems to lower RAS activity,which in turn lowers blood sugar levels.To corroborate these early findings,more research into the connection between ACE2 shedding and glycemia is needed.
Role of ACE and ACE2 in COVID-19 susceptibility
SARS-CoV-2 belongs to the Beta-coronavirus genus,which encompasses other notably contagious viruses including the Middle East respiratory syndrome coronavirus and the SARS coronavirus (SARS-CoV)[48].SARS-CoV and SARS-CoV2 have about 80% sequence similarity[49].Both viruses enter cells by binding to the ACE2 with their spike proteins (Sprotein)[49].An extraordinarily high rate of human transmission makes SARS-CoV-2 different[50].Indeed,SARS-CoV-2 exhibits a heightened attraction to ACE2,contributing to its increased transmissibility.Through its S-protein,SARS-CoV-2 binds to human cells.ACE2[51],which is widely expressed on the surfaces of pulmonary alveolar epithelial cells,venous and arterial endothelial cells,arterial smooth muscle cells,and small intestine enterocytes,is one of the main SARS-CoV-2 receptors[38,52-55].
The S-protein is split into S1 and S2 subunits after viral infection[54].While the S2 subunit aids in membrane fusion,the S1 subunit directly interacts with the host cell’s ACE2 receptor through its receptor binding domain[54].SARS-CoV-2 and SARS-CoV have very similar receptor binding regions.In contrast,the SARS-CoV-2 binding site exhibits enhanced stability and compactness,displaying a greater affinity for ACE2 and its ability to infect the host cell is increased by the presence of a Furin at the cleavage site[56,57].After the binding is complete,ACE2 activity is downregulated[54].ACE2 downregulation is due to the activation of ADAM-17/TNF-α converting enzyme by the SARS S-protein,which cleaves and releases ACE2[44,58].Increased circulating levels of ACE2 were found interconnected with the severity of lung injury and the viral load[56].The downregulation of ACE2 after SARS-CoV-2 infection increases Ang-II concentration in the lungs causing severe lung injury.Another study reported ACE2 downregulation in the heart and severe heart injury[59].
There are a number of potential causes for the increased severity of COVID-19 in individuals with concomitant illnesses.The increased expression of ACE2 in certain organs,including the kidneys,small intestine,lungs,and heart[52,56,60],might elucidate the severe manifestation of COVID-19 in specific patient groups.This is because ACE2 serves as the functional receptor through which SARS-CoV-2 enters cells[61].In addition,the overactive immune response to the virus that contributes significantly to the disease severity is known as the “cytokine storm”[62,63].It is intriguing to note that COVID-19 was shown to trigger the abrupt beginning of long-term organ damage in people who did not already have chronic illnesses.One of these consequences is cardiac injury[64],along with kidney injury[65],renal injury[65],liver damage[67],gastrointestinal disorders[66],and acute or chronic diabetes[68].This makes COVID-19 more challenging and increases the likelihood that many infected people may develop chronic morbidities that are incapacitating.Interestingly,there is a scarcity of clinical data that supports these pathophysiological processes.
Mechanism of SARS-CoV2 entry into cells
Typically,coronaviruses require two cleavage events of the S-protein for their entry process (Figure 2).Two cleaves occur: One at the S1-S2 subunit junction and the other at the S2’ location inside the S2 subunit[69].Regarding SARS-CoV-2,the polybasic sequence cleaves at the S1-S2 border as the virus matures in an infected cell.Nevertheless,once ACE2 binds,the target cell cleaves at the S2’ location.The S2’ cleavage site in the S2 subunit is made visible by conformational changes in the S1 subunit caused by the interaction between the virus and ACE2.The particular proteases that cause the cleavage at the S2’ site differ according to the SARS-CoV-2 entry pathway that is selected.

Figure 2 Mechanisms of how the virus enters cells via the angiotensin-converting enzyme 2 receptor. ACE2: Angiotensin converting enzyme 2;FP: Fusion peptide;SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2;TMPRSS2: Transmembrane protease serine 2.
When transmembrane protease,serine 2 (TMPRSS2) is insufficient in the target cell or when a virus-ACE2 complex is not in contact with TMPRSS2,the virus-ACE2 complex is internalizedviaclathrin-mediated endocytosis[70].Cathepsins within endolysosomes cleave the S2’ site as a result of this internalization,and they require an acidic environment to function.Conversely,in the presence of TMPRSS2,S2’ cleaves at the cell surface.The fusion peptide is exposed in both routes by the cleavage of the S2’ site,and the S2 subunit undergoes major conformational changes as a result of S2 splitting from S1,especially in heptad repeat 1[69].This starts the process of membrane fusion by pushing the fusion peptide into the target membrane.Viral RNA is released into the host cell cytoplasm for uncoating and replication through a fusion hole created when the viral and cellular membranes fuse[69].
Impact of RAAS expression on T2DM patients suffering from COVID-19
Increased blood glucose and glycosylation product synthesis in patients with diabetes could enhance the working of RAAS,notably Ang-II.When exposed to excessive amounts of Ang-II,a variety of tissues can stimulate dihydro nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity and increase oxidative stress,which can lead to increased insulin resistance,complications from diabetes,and a worse prognosis for COVID-19[71-73].Moreover,Ang-II worsens diabetes by promoting fibrosis,cell death,and a reduction in the islet blood supply and early stages of insulin synthesis[73].Ang-I is converted to Ang-(1-9) by ACE2,whereas Ang-II is converted to Ang-(1-7).These two physiologically active downstream peptides encourage vasodilation,which has been linked to anti-inflammatory,antifibrotic,and antiproliferative properties[74].In addition to partially protecting islet cells and preventing diabetes,ACE2 suppresses conventional RAAS activation[75].These receptors are downregulated as a result of the interaction of SARS-CoV-2 and ACE2,which is followed by membrane fusion and viral entry into the cell.This exacerbates cellular damage,hyperinflammation,and respiratory failure[76,77].Increased ACE2 insufficiency may exacerbate the dysregulation between the “protective” ACE2 Ang-(1-7) Mas receptor axis and the “adverse” ACE Ang-II AT1R axis following viral invasion[77].Insulin secretion may be hampered by ACE2 downregulation upon viral entry if Ang-II is not inhibited.These factors may play a role in the rapid degradation of pancreatic cell function and the development of diabetic ketoacidosis[78],suggesting that the prognosis for patients with diabetes after SARS-CoV-2 infection may be bad.Nevertheless,it is uncertain if ACE2 has a mechanistic role in the development of dysglycemia or elevated complications in diabetics[79].Evidence suggests that RAS inhibitors,which include ACEi and ARBs,increase the expression of ACE2,which may have a protective effect against diabetes and hypertension but also make it easier for viruses to enter host cells,which may increase the likelihood or severity of infection[80].However,recent studies have not demonstrated that using ACEi or ARBs raises the likelihood of SARS-CoV-2 infection or the risk of serious consequences in COVID-19 individuals[74,81-83].
LlNK BETWEEN DlABETES,COVlD-19,AND ACE2: A CLOSER LOOK
Diabetes is considered to be one of the leading causes of death worldwide.Elderly people with T2DM are more severely prone to infections such as pneumonia and influenza[84,85].The International Diabetes Federation estimated that 537 million adult individuals were diagnosed with diabetes in 2021[86],with this figure anticipated to climb to 643 million by 2030 and 783 million by 2045[87].
Several studies could not find a clear association between COVID-19 severity and diabetes[12,88].However,studies in Italy[89] and China[2] showed the perilous threat of mortality and severe COVID-19 in older patients with some chronic diseases,including diabetes.Diabetes has been identified as an elevated risk for severe COVID-19 since the pandemic began.It is indeed one of the most frequently documented comorbidities among COVID-19 patients who were brought to the ICU and later passed away[3,11].In a study that included 52 COVID-19 patients who were brought to the ICU,32 (61%) of the patients had passed away 28 d after discharge;the most prevalent comorbidities in these patients were diabetes (22%) and cerebrovascular illnesses (22%)[4].
A different study involving 44672 individuals with SARS-COV-2 found that the COVID-19 case fatality rate was 7.3% in diabetic individuals and 2.3% in non-diabetic individuals[3].Numerous variables may be responsible for the severe presentation,even if the cause of the poor prognosis in patients with diabetes is unknown[90].First of all,poorly managed diabetes compromises the ability of the immune system to fight viral infections[91].The capacity to eliminate viruses is particularly hampered by poor T cell function,which weakens the natural defense system[92].Second,plasminogen levels are increased in diabetic individuals[93].This particular protein cleaves the S-protein of SARS-CoV-2,which enhances the viral cellular entry;further,it leads to an increase in the viral contagion and infestation of the virus[93].Alongside,inflammatory biomarkers like interleukin-6 (IL-6),C-reactive protein,and D-dimer were found to have increased levels in diabetic SARS-COV-2 individuals in comparison to those without diabetes,which indicates that the risk of worsening COVID-19 outcomes might be increased due to T2DM[64].
Due to their renal protective properties,ACEi is frequently prescribed to diabetic patients.This class of drugs raises the expression of ACE2,due to which viral entrance could be facilitated into the cells of the host[94].FURINandTMPRSSare two additional genes linked with cellular entry of SARS-CoV-2 that remain upregulated in T2DM,according to microarray-based transcriptome profiling for the disease[95].Finally,patients with diabetes having additional ailments such as coronary artery disease,chronic renal disease,and hypertension have a substantially poorer COVID-19 outcome[90].COVID-19 puts patients with diabetes at a higher risk of serious consequences.Diabetes,as previously noted,is regarded as one of the most frequent comorbidities in this pandemic.As a result,greater effort should be expended to comprehend the molecular mechanisms behind this danger.
Data are scarce regarding the advancement of diabetic complications and glucose metabolism in individuals having COVID-19.According to retrospective research,10% of patients with COVID-19 and T2DM had at least one episode of hypoglycemia (< 3.9 mmol/L)[5].However,SARS-CoV-2 infection in diabetic individuals may result in elevated stress levels and the release of more hyperglycemic hormones,such as glucocorticoids and catecholamines,which raises blood sugar levels[96].
Possible mechanisms for SARS-CoV-2 infection in patients with diabetes
Numerous vascular and metabolic problems associated with diabetes might impact our body’s ability to respond to infections[97].Hyperglycemia as well as insulin resistance enhance the synthesis of adhesion molecules,which promote inflammation of tissues,and increase the production of proinflammatory cytokines,advanced glycation end products,and oxidative stress[97,98].The undivulged phenomena that increases the risk of infestation in patients with diabetes may be this inflammatory response[97].
Some studies have reported an association of hyperglycemia with defects in immunity[99].Compliment activation dysfunction[100] and abnormal delayed-type hypersensitivity reaction[99] have been reported in diabetic individuals.Studies conductedinvitrorevealed that exposure to elevated concentrations of glucose enhanced influenza virus infection and replication in pulmonary epithelial cells,suggesting elevated viral replicationinvivoduring hyperglycemic circumstances[101].
COVID-19 epidemiology and its relationship to cardiometabolic diseases are poorly known.Current statistics reveal that people with COVID-19 and diabetes mellitus have a higher risk of death in comparison with individuals without diabetes mellitus.Resistance to insulin is caused by COVID-19 in individuals,resulting in persistent metabolic problems that did not exist before infection.Patients with diabetes are more vulnerable to SARS-CoV-2 infection than individuals who do not have diabetes.With infection,ACE2 expression declines,magnifying Ang-II activity and leading to insulin resistance,an increased immunological response,and severe SARS-COV-2 infection[102].Exocrine and endocrine pancreatic synthesis of ACE2 is likely to be associated with an increased presentation of diabetes in subgroups of critically unwell SARS-CoV-2 infected persons[79].Through binding to the ACE2 receptor as seen in SARS-Cov-1[103],SARS-CoV-2 promotes pancreatic islet destruction and the development of acute diabetes[4].
ACE2 is one of the main receptors of both SARS-CoV-2[51] and SARS-CoV[104].When CoVs get introduced to host cells,the CoVs attach to specific ACE2 receptors with the help of their S-proteins.The S-protein is then cleaved by host cell protease,allowing the virus to enter and multiply within the host cells[51].Patients with diabetes and hypertension frequently use medications called ACEi and ARBs[105].These drugs are known to increase the ACE2 concentration,and thus their use is controversial as it might negatively affect the outcomes of COVID-19 patients[55].On the other hand,some recommended ARBS and ACEi might be beneficial[106].
COVlD-19 VULNERABlLlTY: UNRAVELLlNG THE ROLE OF COMORBlDlTlES
SARS-CoV-2 infection has been potentiated by primary complications that are illustrated as T2DM and its associated metabolic comorbidities.Mentioned below are the other related comorbidities and their possible mechanisms involved in the pathophysiology of SARS-CoV-2 infection and different strategies involved in the treatment of COVID-19.
Hypertension and COVID-19
The initial study in China indicated a high occurrence (30%) of hypertension in severe SARS-COV-2 cases[107],which is not very surprising as 23.2% of the adult population of China are estimated to have hypertension[108].According to another report,out of 406 patients who died from COVID-19,39.7% of patients had hypertension[109],which is higher than the general population.Hypertension may also result in other cardiovascular risk factors like hypertensionmediated organ damage,diabetes,or cardiovascular complications[110],and their prevalence increases with age.
Italy was the first European country that was most severely affected by COVID-19[111,112].According to a report published on June 18,2020 byEpidemiologyofPublicHealth,the median age of patients in Italy who died with COVID-19 was 82 years,and 66.8% deceased COVID-19 patients had pre-existing hypertension (https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-analysis-of-deaths).These numbers were much higher than reported in China but were consistent with estimated high prevalence of hypertension of this age group in Italy[113].Furthermore,17% and 30% of deceased patients with COVID-19 were being treated with ARBs and ACEi,respectively.These data suggest that COVID-19 patients with hypertension exhibit an increased fatality rate as compared with overall infected patients,with elderly patients being at greatest risk.
Possible mechanisms for SARS-CoV-2 infection in hypertension patients
A recent study conducted to understand the role of immune dysregulation in hypertension[114] provided the possible mechanism linking immune dysregulation and COVID-19 severity.An increased level of systemic IL-7,IL-6,IL-2,C-X-C motif chemokine 10,chemokine ligand 2,granulocyte colony-stimulating factor,and TNF-α was observed in COVID-19 patients[7].Interestingly,these are the same cytokines that are associated with hypertension development in interventional[114],clinical observational[115],and experimental studies[116,117].
Another study reported dysregulation of CD8+and CD4+cells in hypertension[118],indicating elevated productivity of proinflammatory cytokines,which includes those related with COVID-19 (IL-6,IL-7,IL-17,TNF-α,and interferon-γ)[119].Hypertension was associated with a distinct immune senescent profile of CD8+cells[114,119,120],which were likely to overproduce cytokines but are weak in antiviral defense.These mechanisms significantly contribute to accelerated end-organ damage[121,122].All these studies somewhat demonstrate the association of hypertension with the severity of COVID-19.In order to test this hypothesis,observational studies are recommended on a large scale to analyze the relationship between hypertension and COVID-19.
ARBs are usually used for the treatment of hypertension and are considered the main therapy for hypertension and associated renal and cardiovascular comorbidities[110].Several reports showed upregulation of ACE2 upon treatment with ACEi and/or ARBs[123].Hence,some authors hypothesized that RAAS blockers may enhance the susceptibility and COVID-19 risk.They recommended avoiding ARBs and ACEi during the COVID-19 pandemic[40,124].Several studies conducted to investigate the effect of RAS blockade on ACE2 showed variable results.These variations may depend on different pathological conditions,like hypertensionvsnormotension,animal strains used,organs investigated,and most importantly type and dosage of the drug.Moreover,very little human research has been conducted to look at how RAS blockage affects the expression of ACE2.A study showed that olmesartan,an ARB,can significantly increase urinary ACE2 in hypertensive patients when treated for > 1 year[125].Similar results were seen in patients with diabetic nephropathy upon treatment with olmesartan for 24 wk[126].On the other hand,urinary ACE2 levels were not affected by ACEi or ARBs in diabetic patients[127].
There is no evidence that shows the usage of ACEi and/or ARBs that directly enhances the susceptible SARS-CoV-2 and its severity and infectivity.Therefore,it is recommended to continue the use of RAAS blockers in stable COVID-19 patients with hypertension and other related diseases,like T2DM,heart failure,and chronic kidney disease[110,128].
CVDs and COVID-19
Several studies of COVID-19 showed extreme variability ranging from asymptomatic to some mild symptoms like dry cough,fatigue,and fever.According to some early reports,a high prevalence and association of comorbidities was seen with severe COVID-19 and mortality[2,4,5,7,10,12,82].Among different comorbidities,the involvement of heart-related ailments appeared more significant.In a report from the Chinese Control for Disease Control and Prevention of 72314 cases,1023 deaths were reported among 44672 confirmed cases (i.e.2.3% mortality rate).However,this percentage went up to 10.5% in instances of diabetes,7.3% in cases of cardiovascular illness,6.3% in situations of chronic respiratory conditions,and 6.0% in cases of hypertension[3].
Myocardial damage during COVID-19 might be asymptomatic,similar to other acute diseases.It can only be identified by detecting troponin levels beyond the 99th percentile upper reference range.Patients with CVDs were shown to be particularly affected by COVID-19[2],and many of them had myocardial stress[9],active myocardial damage[5,9,129],and cardiomyopathy[9] while they were unwell.There was an increase in the cardiac troponin levels in 8%-12% of unselected COVID-19 patients[5,7,64,129].Other studies also reported higher plasma levels of N-terminal pro-brain natriuretic peptide in cases with myocardial injury,although these increased levels were not independently associated with the outcomes[64,129].
Some of the cardiac complications,like cardiomegaly,hypotension,and heart failure were previously seen in SARSCoV infections[130].Acute myocarditis or myocardial damage may lead to severe cardiac dysfunction or heart failure in COVID-19 cases.This is often difficult to diagnose and controversial.Although very few COVID-19-associated acute myocarditis cases were reported[131-134],they might be severe with low cardiac output and hypotension that needed end-stage heart failure inotropic therapy.The endomyocardial biopsy showed limited or no myocardial necrosis and different levels of myocardial inflammation[131-134].
Only 1 of the 2 patients who underwent endomyocardial biopsy met the criteria for acute myocarditis[133].SARS-CoV-2 was also detected in macrophages but not in cardiomyocytes in a different instance.The biopsy results from these cells revealed only modest interstitial cardiac inflammation and some non-specific alterations in heart myocytes,such as lipid droplets and myofibrillar lysis[134].These data indicate that the virus does not have any direct pathogenetic role,even though it can reside within the heart[5,135].Hence,it can be assumed that apart from viral infection,other mechanisms responsible for myocardial injury may exist[5,135,136].
Possible mechanisms for SARS-CoV-2 infection in patients with CVDs
Cardiomyocytes,coronary endothelial cells,and cardiac fibroblasts all have ACE2 on their surface.It has been discovered that individuals with diabetes,cardiovascular conditions,and those using ARBs and/or ACEi had elevated levels of ACE2[136-140].This has been reported in myocardium tissue samples of patients with end-stage heart failure[139,140],experimental models[137,138],and circulating plasma levels of ACE2[140].ACE2knockout mice develop heart failure with reduced ejection fraction and systolic dysfunction in left ventricle[59].Several studies conducted on experimental models showed thatACE2gene overexpression reduced fibrosis,oxidative stress,and myocardial hypertrophy,which improved the diastolic function of the left ventricle[141,142].Notably,ACE2 also has immunomodulatory properties,both indirectly by decreasing Ang-II concentration,which favors inflammation and interacts with macrophages[56,143].
For the first time,Samaetal[140] assessed the levels of ACE2 in circulation in a group of patients with heart failure that included 1485 males and 537 females from Europe.The findings were confirmed in a separate survey.The ACE2 plasma levels were found to be increased in males in both the cohorts explaining high susceptibility and fatality rate in males[140],and this elevated ACE2 concentration was consistent with a higher prevalence and severity of COVID-19[2,3,140].Consequently,elevated ACE2 expression may heighten the sensitivity to COVID-19 and may intensify the infection by augmenting the viral load within the cell.Because the administration of ARBs/ACEi upregulates the expression of ACE2,there are concerns regarding the usage of these drugs,which have not yet been verified[40,144-146].
Secondly,SAR-CoV-2 infection downregulates ACE2 and thus increases the Ang-II levels,which stimulates AT1R.Acute respiratory distress syndrome (ARDS),heart and/or lung damage,and other COVID-19 problems may be primarily brought on by elevated Ang-II activity[145,147,148].This demonstrates the protective function of ACE2,suggesting that the use of ARBs may mitigate the organ damage caused by COVID-19.When the ACE2 concentration in two heart failure cohorts (validation cohort: 1123 males and 575 females;index cohort: 1485 males and 537 females) was assessed,there was no correlation seen between RAAS inhibitor medication and increased plasma ACE2 concentration[140].This study suggested that ARB/ACEi treatment may not increase COVID-19 vulnerability through elevated plasma ACE2 levels[140].
At present several things remain uncertain regarding cardiac injury before COVID-19.The clinical implication of detecting myocardial injury remains uncertain as cardiac injury can only be diagnosed using biomarker measurements.At present there is no specific treatment.Regarding the use of ARBs and/or ACEi,no evidence is found to support a higher risk of COVID-19 or severity in individuals who are on these medications.Therefore,their use should be continued until any study proves its harmful effects.
Possible mechanisms involved in myocardial injury during COVID-19
There are several mechanisms through which COVID-19 may promote myocardial damage.COVID-19 has harmful effects caused by sympathetic activation,fever,and tachycardia with elevated energy expenditure and myocardial oxygen consumption[149].Prolonged bed rest is another consequence of acute COVID-19,and this infection can cause thrombosis,a serious complication[150].Another feature of COVID-19 is hypoxemia,which is associated with elevated oxidative stress brought on by the production of reactive oxygen species,damage to the mitochondria,intracellular acidosis,and even cell apoptosis[11,88,149,151].
Several more pathways are connected to the aberrant inflammatory reactions brought on by COVID-19.After 7-10 d from the start of COVID-19,a hyperinflammatory reaction with cytokine release (cytokine storm) may happen.These reactions may result in cardiac failure,thromboembolic events,shock,renal failure,and possibly multiorgan failure.They may increase the risk of pneumonia and ARDS[5,7,64,129].The oxidative stress and activation of the inflammatory response may lead to severe clinical conditions in these patients,and this might elevate the possibility of mortality in these individuals with heart failure[152,153].
An association was seen among different inflammatory markers (like ferritin,C-reactive protein,IL-6,and d-dimer) and major complications and high mortality[5,7,129].A constant increase in inflammatory markers was also associated with myocardial damage in line with hyperinflammation,which leads to cardiac dysfunction[64,129].Currently,the beneficial roles of anti-inflammatory therapies in COVID-19 are being studied[154,155].On the other hand,drugs working on endothelial functions,like ACEi or ARBs and statins may also be beneficial[156].
DlFFERENT STRATEGlES FOR THE TREATMENT OF COVlD-19
COVID-19 treatment strategies of T2DM patients
Treatment plans for patients with diabetes were determined based on their blood glucose and/or glycated hemoglobin levels,as well as their urine ketone levels,at the time of admission.At the time of admission,several factors were considered,including age,nutritional status,food intake,the existence of different organ dysfunctions,and conditions related to the heart and brain.This took into account both the potential risks associated with fluctuations in blood glucose levels and the severity of the patient’s condition[157].
As per the guidelines provided by the United Kingdom National Diabetes Inpatient COVID Response Group[158,159],it was recommended that all patients hospitalized with COVID-19 should diligently monitor their blood glucose levels.This would enable prompt identification of any changes in glucose levels.Individuals who had a history of diabetes or who showed up with blood glucose levels more than 12 mmol/L were regularly checked (2-4 times per hour) and treated for their diabetes.The aim for blood glucose management might be changed to 7-12 mmol/L if insulin therapy is implemented and blood glucose levels continue to surpass the 12 mmol/L threshold or if oral medication is not practical.When treatment for COVID-19-related symptoms did not provide the desired outcomes and real blood glucose readings did not match expectations,more frequent blood glucose monitoring (4-6 times per hour) was recommended.When blood glucose levels exceeded 15 mmol/L,subcutaneous administration of short-acting insulin was performed as required.
Dexamethasone emerged as a prominent treatment option for severe COVID-19 cases due to its demonstrated ability to mitigate fatalities among individuals requiring ventilator and oxygen therapies.However,given its classification as a glucocorticoid,special caution was urged for COVID-19 patients also managing diabetes when utilizing dexamethasone,to avert the onset of ketoacidosis.In scenarios involving dexamethasone treatment,patients and healthcare practitioners were advised to increase the frequency of blood glucose level assessments to ensure optimal glycemic control.Alternatively,consideration for alternate therapeutic approaches was recommended for patients grappling with uncontrolled hyperglycemia[160].
COVID-19 treatment strategies involving ACE2/RAAS system: Recombinant human ACE2 treatment therapy
Adenoviral ACE2,also known as recombinant human ACE2 (rhACE2),has been used as a therapeutic intervention in animal models of sickness and a human trial including 44 patients with ARDS[161].rhACE2 is a crucial negative regulator of Ang-II and inhibits harmful cardiac remodeling[142].When rhACE2 significantly increases ACE2 activity in the circulation,Ang-II levels significantly decrease and Ang-(1-7) synthesis from Ang-II increases.A chimeric fusion of immunoglobulin fragment Fc region restored induced hypertension in mice and increased total Ang-II-conversion activities in blood by up to 100 times.
For COVID-19 patients,Vermaetal[162] suggested a combination treatment that included rhACE2 and GapmeR technology.While rhACE2 stops the virus from infecting host cells,GapmeR is an antisense single-stranded DNA molecule that binds to the SARS-CoV-2 RNA.The resulting DNA-RNA hybrid is then destroyed by intracellular RNAase H[162].For the treatment of this sickness,cyclodextrin soluble ACE2 (sACE2) insertion has been suggested as an alternative method[163].Many aerosolized sACE2 compounds that may be inhaled directly into the lungs,intravenous sACE2 infusions,and ocular and nasal drops made from cyclodextrin sACE2 inclusion compounds have all been proposed as treatments for COVID-19[164].
Use of RAAS inhibitors
Recent trials have shown that RAAS inhibitors and increased ACE2 levels are beneficial for COVID-19 individuals.As mentioned earlier,ACE2 is downregulated when it binds to the SARS-CoV-2 S-protein,which raises Ang-II levels.Breathing problems and lung congestion result from this[148,165].Therefore,some drugs that increase ACE2 concentration may help lessen the severity of COVID-19[106].Patients treated with RAS inhibitor had a lower mortality rate than patients taking other hypertensive drugs,according to many retrospective studies on people with COVID-19 and hypertension[166,167].
Lametal[168] showed that hypertensive COVID-19 patients who continued to use ACEi or ARBs had better clinical results and were advised to stick with their current course of treatment.Mengetal[169] separated their study subjects into two separate groups: Those taking RAS inhibitors (including 17 participants);and those not taking RAS inhibitors (including 25 participants).As opposed to those receiving on-RAS inhibitors,they found that those receiving RAS inhibitors had lower viral loads,lowered serum IL-6,increased blood CD8+and CD3+T cells,and decreased sickness severity.Thus,medications such as RAS inhibitors have been associated with better clinical results in COVID-19 hypertension patients[14].
COVID-19 treatment strategies of T2DM patients involving the ACE2/RAAS system
Several clinical trials have been performed for the efficacy and safety of potential alternatives,including arbidol,chloroquine phosphate,tocilizumab,ribavirin,and remdesivir,among others.Chloroquine and its hydroxy analog hydroxychloroquine are a promising pharmacological option for patients with diabetes as it is a broad-spectrum antiviral drug and is commonly used to treat malaria and autoimmune diseases.Chloroquine increases endosomal pH and inhibits ACE2 glycosylation,thereby blocking the SARS-CoV entry in the host cell[170].It has been shown that chloroquine increases the C-peptide response,which indicates enhanced function of the pancreatic β cell[171].Several studies showed improved glycemic control by hydroxychloroquine in decompensated diabetic patients,which were refractory to other antidiabetic drugs[171,172].
The severity of the condition,age,the existence of comorbidities,and problems associated with diabetes,among other things,should all be taken into consideration while developing therapeutic methods and ideal glucose levels.A greater number of COVID-19 tests in outpatient diabetes clinics may exhibit a positive impact on their outcome.
Identifying the epigenetic signatures linked to COVID-19 and their changes during viral entry and infection (such as going from asymptomatic to mildly symptomatic,severe infection,and long-lasting symptoms) could be helpful in facilitating prompt diagnosis and the advancement of treatments that could lessen the severity of the virus and its associated mortality.Major metabolic issues such as T2DM,hypertension,and CVD are factors that lead to COVID-19 patient death.Finding epigenetic markers linked to these comorbidities and their impact on COVID-19 severity may also be helpful in guiding treatment to prevent the development of sequelae that increase the catastrophic death rate associated with COVID-19[173].
CONCLUSlON
Early diagnosis,early isolation,and early management might prove to be important in controlling COVID-19.ACE2 is the major receptor for the SARS virus and the interaction between the S-protein and ACE2 is proposed to be the potential factor for infectivity[174,175].There are concerns regarding the use of RAAS blockers as they may alter ACE2 and whether the altered ACE2 expression is responsible for COVID-19 virulence[39,40,124,146].There are uncertainties regarding the association of elevated ACE2 expression and infectivity of SARS-CoV-2.The available evidence suggests that RAAS inhibitors should be continued until it is demonstrated that there is an association of ARB/ACEi use with increased severity of COVID-19.
ACKNOWLEDGEMENTS
AKS acknowledges the Research and Development Scheme,Government of Uttar Pradesh,Lucknow,India for providing a Junior Research Fellowship.The authors also acknowledge ICMR,Department of Biotechnology (DBT),Department of Science and Technology (DST),New Delhi,India,and Centre of Excellence,Higher Education,Government of Uttar Pradesh,Lucknow,India.
FOOTNOTES
Co-first authors:Ashwin K Shukla and Komal Awasthi.
Author contributions:Shukla AK and Awasthi K contributed equally to the study;Shukla AK contributed to the conceptualizing and writing;Awasthi K contributed to the conceptual writing of the manuscript;Usman K provided expert clinical input and interpretation of the reviewed literature;Banerjee M contributed to critical reading and reviewing the article and making critical revisions related to the intellectual content of the manuscript.
Conflict-of-interest statement:The authors declare having no conflicts of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:India
ORClD number:Ashwin Kumar Shukla 0000-0003-1554-090X;Komal Awasthi 0009-0007-3069-4356;Monisha Banerjee 0000-0002-5371-8791.
S-Editor:Chen YL
L-Editor:Filipodia
P-Editor:Zhang YL
杂志排行
World Journal of Diabetes的其它文章
- Nε-carboxymethyl-lysine and inflammatory cytokines,markers and mediators of coronary artery disease progression in diabetes
- Non-pharmacological interventions for diabetic peripheral neuropathy: Are we winning the battle?
- Application and management of continuous glucose monitoring in diabetic kidney disease
- Are treatment options used for adult-onset type 2 diabetes mellitus (equally) available and effective for children and adolescents?
- Prevalence and risk factors of wound complications after transtibial amputation in patients with diabetic foot
- Prevalence and risk factors of diabetes mellitus among elderly patients in the Lugu community
