高灵敏、多响应的镧系金属有机骨架的设计、合成和荧光传感性能
2024-04-17闫睿魁陈小莉崔华莉王记江
闫睿魁 陈小莉 蔡 苗 任 静 崔华莉 杨 华 王记江
(延安大学化学与化工学院,陕西省反应工程重点实验室,新能源新功能材料实验室,延安 716000)
0 Introduction
Metal - organic frameworks (MOFs) are mainly composed of metal/metal clusters and organic linkers[1-3]. Due to its high specific surface area, large pore size, high thermal and chemical stability, and novel and diverse structure, MOFs are widely used in gas separation, catalysis, fluorescence detection, molecular magnetism,ion recognition,and other fields[4-10].
With the development of modern industry and agriculture, serious environmental pollution has become an insurmountable obstacle to the sustainable economic development of all countries in the world.Environmental problems such as metal, organic molecules, and antibiotic pollution have been widely discussed by people, and it is urgent to detect and control environmental pollutants efficiently. Therefore, the highly selective and sensitive detection of these pollutants is not only an important guarantee for ecological development and life safety but also a major challenge faced by contemporary scientists[11-15]. So far, many MOFs have been successfully used for the sensing of small organic molecules and metal ions[16-20]. In particular, lanthanide metal-organic frameworks (Ln-MOFs)exhibit stronger fluorescence lifetime and luminescence characteristics than transition metals due to their unique electronic configuration[21-26], so they can often be used for environmental monitoring, such as the detection of pesticide residues, nitro explosives(NACs)[27-31]. However, most fluorescence sensors usually respond to only one or a few signals[32-34]. If fluorescence sensors can respond to multiple analytes, then they can perform well in complex objects, multidimensional storage materials, and multiple sensors, so designing multi-response fluorescence sensors becomes an urgent challenge.
Aniline(ANI)is a widely used chemical raw material that has been identified as a carcinogen. Nitrobenzene (NB) is widely used in industries such as fireworks, pharmaceuticals, and dyes, but it causes serious harm to the environment due to its explosive nature[35-37].Pyrimethanil (PTH) as a pesticide will remain in plants and animals, and this undoubtedly poses a serious threat to human health and the ecological environment.Therefore,rapid detection of pesticides is essential[38-42].Antibiotics are currently used worldwide in human therapeutics and the agricultural industry, and their derivatives are considered to be one of the most obvious environmental pollutants[43-48]. Therefore, various techniques are widely used in the detection of antibiotics, but most methods rely on complex instruments,have poor repeatability, and are time-consuming and expensive,which greatly limits their application in routine analysis[49-50].
5-(3,4-Dicarboxyphenoxy)isophenic acid(H4dppa)has four carboxyl groups and eight potential donor atoms, which can construct structures with different topological and dimensional from different directions.In addition, the four carboxyl groups can be fully or partially deprotonated, providing hydrogen bond donors and acceptors, making H4dppa an ideal candidate for constructing multi - dimensional MOFs and supramolecular networks. In this work, we designed and synthesized two Ln-MOFs:{(dima)[Dy(dppa)(H2O)2]·2.5H2O}n(Dy-MOF) and {(dima)[Eu(dppa)(H2O)2]·1.5H2O}n(Eu-MOF) (dima=dimethylamine cation), and explored their fluorescence characteristics. It was found that they have excellent luminescence performance, and Dy-MOF can efficiently, selectively, and sensitively detect various pollutants: ANI, NB, tetracycline (TC), PTH, and tryptophan (Trp) in water, which has potential application prospects in multifunctional fluorescence sensing.
1 Experimental
1.1 Reagents and physical measurements
All chemical reagents were purchased from the reagent platform and were not specially treated.Elemental analyses (H, C, N) were performed with an Elementar Vario EL Ⅲelemental analyzer. Thermogravimetric analysis (TGA) was performed with a NETZSCHSTA 449C thermogravimetric analyzer at a heating rate of 10 ℃·min-1and in a nitrogen atmosphere. IR spectra were recorded as KBr pellets on a Bruker EQUINOX55 spectrophotometer in the 4 000-400 cm-1region. The powder X-ray diffraction (PXRD)pattern was recorded by Rigaku D/Max Ⅲdiffractometer under the conditions of CuKαradiation(λ=0.154 18 nm), scanning rate of 2 (°)·min-1, 2θrange of 5°-50°,operating voltage of 40 kV and current of 30 mA.
1.2 Synthesis of Dy-MOF and Eu-MOF
A solid mixture of H4dppa (0.05 mmol, 0.017 3 g)with Dy(NO3)3·6H2O (0.1 mmol, 0.0435 g) was dissolved in 2 mL DMF and 2 mL H2O by sonication for 15 min. The pH of the solution was adjusted to 2-3,and then it was sealed in a 10 mL glass bottle, heated in an oven at 120 ℃for 5 d, and finally cooled to room temperature. The mixture was filtered, and the precipitate was washed well with H2O and DMF, and dried to obtain colorless bulk crystals of Dy-MOF with a yield of 58% (based on Dy). Elemental analysis Calcd. for C36H46N2O27Dy2(%): C 34.22, N 2.22, H 3.67; Found(%):C 34.26,N 2.25,H 3.63.IR(KBr,cm-1):3 368(s),3 165 (s), 1 561 (s), 1 374 (s), 1 250 (m), 1 089 (w),971(w),934(w),822(m),773(m),716(m),617(w).
Eu-MOF was synthesized in much the same way as Dy-MOF, except that Dy(NO3)3·6H2O was replaced with Eu(NO3)3·6H2O (0.1 mmol, 0.044 6 g). Finally,colorless massive crystals were obtained with a yield of 56% (based on Eu). Elemental analysis Calcd. for C36H42N2O25Eu2(%): C 35.83,N 2.32,H 3.51; Found(%):C 35.86,N 2.29,H 3.47.IR(KBr,cm-1):3 364(s),3 156 (s), 1 551 (s), 1 377 (s), 1 242 (m), 1 093 (w),968(w),928(w),825(m),771(m),714(m),612(w).
1.3 Luminescent sensing experiments
Organic solvent sensing experiments.Dy-MOF(3 mg) was dissolved in an aqueous solution (10 mmol·L-1) containing organic solvents, including cyclohexane(CYH), polyethylene glycol (PEG), 1,2-dichloroethane(DCE), acetonitrile (CH3CN),n-propanol (NPA),Nmethyl-2-pyrrolidone (NMP), ethylene glycol (EG),formaldehyde (HCHO),n-pentane(NP),1,2-propanediol (PG), DMF, and ANI (20 μL, 1 mmol·L-1). After ultrasonic treatment for 30 min and aging for 3 d,a stable emulsion was formed and a fluorescence test was carried out.
NACs sensing experiments.In the same way, as the organic solvents described above, some of the NACs were selected to detect the fluorescence sensing ability of Dy-MOF, including 4-NPH (p-nitrophenylhydrazine),PNBA(p-nitrobenzoic acid),DNP(2,4-dinitrophenylhydrazine), TRI (2,4,6-trinitrophenylhydrazine),4-NP(p-nitrophenol),O-NT(o-nitroaniline),TNP(2,4,6-trinitrophenol),2-NP(o-nitrophenol),3-NT(m-nitroaniline), and NB (20 μL, 1 mmol·L-1). After ultrasonic treatment for 30 min and aging for 3 d, a stable emulsion was formed and a fluorescence test was carried out.
Antibiotic sensing experiments.In the same way, as the organic solvents described above, some of the antibiotic sensing were selected to detect the fluorescence sensing ability of Dy-MOF, including PEN(penicillin sodium), ORN (ornidazole), ROX (roxithromycin), MET (metronidazole), LIN (lincomycin hydrochloride), GEN (gentamicin sulfate), CAP (chloramphenicol), AZM (azithromycin), CEF (cefixime), TC (20 μL, 1 mmol·L-1). After ultrasonic treatment for 30 min and aging for 3 d, a stable emulsion was formed and a fluorescence test was carried out.
Pesticides sensing experiments.Dy-MOF (3 mg)was dissolved in an aqueous solution containing pesticides (0.1 mL, 1 mmol·L-1), including emamectin benzoate(EMB),triadimefon(TRI),prochloraz(PRO),pyrimethanil (PTH),24-epibrassinolide (24-EPI),pyraclostronbin (PST), fluazinam (FLU), zhongshengmycin(MYC), and imazalil (IMA) (0.1 mL). After ultrasonic treatment for 30 min and aging for 3 d, a stable emulsion was formed and a fluorescence test was carried out.
Amino acid sensing experiments.In the same way, as the organic solvents described above, some of the amino acids were selected to detect the fluorescence sensing ability of Dy-MOF. The amino acids including cysteine (Cys), phenylalanine (Phe), histidine(His), tyrosine (Tyr), proline (Pro), glutamic acid (Glu),serine (Ser), arginine (Arg), leucine (Leu), isoleucine(Ile), methionine (Met), alanine (Ala), glycine (Gly),threonine (Thr), valine (Val), tryptophan (Trp) (20 μL,1 mmol·L-1). After ultrasonic treatment for 30 min and aging for 3 d, a stable emulsion was formed and a fluorescence test was carried out.
1.4 Application in real samples
To verify the feasibility of Dy-MOF as a fluorescence sensor to detect antibiotics, we added TC with different concentrations into actual water samples(water samples were taken from the local Yanhe River).Yanhe River water was retrieved after standing for some time, after centrifugation, and filtration treatment with buffer solution diluted 100 times after use.
1.5 X-ray crystallography
Intensity data was collected on a Bruker Smart APEX Ⅱ CCD diffractometer with graphitemonochromated MoKαradiation (λ=0.071 073 nm) at room temperature. Empirical absorption corrections were applied using the SADABS program. The structure was solved by direct methods and refined by the full-matrix least-squares based onF2using the SHELXTL-2018 program. All non-hydrogen atoms were refined anisotropically and hydrogen atoms of organic ligands were generated geometrically. Crystal data and structural refinement parameters for the Ln-MOFs are summarized in Table 1, and selected bond distances and bond angles are listed in Table S1(Supporting information).

Table 1 Crystal data and structural refinement parameters for Dy-MOF and Eu-MOF
CCDC:2284537,Dy-MOF;2067689,Eu-MOF.
2 Results and discussion
2.1 Structure description of Dy-MOF and Eu-MOF
Single crystal X-ray diffraction analysis showed that Eu-MOF and Dy-MOF are hetero-isomorphic, so Dy-MOF is described as an example. Dy-MOF shows a 2D network structure. Each Dy1 ion is associated with seven carboxy oxygen atoms (O1, O3A, O4A, O6B,O7B, O8C, and O9C) of four dppa4-ions and two oxygen atoms (O10, O11) of the coordination H2O molecules to form a nine-coordinated mode (Fig.1a).The distance of Dy1—O is in a range of 0.022 7(18)-0.025 0(19) nm. Interestingly, the Dy1 center exhibits a slightly distorted single-cap inverse quadrangular prism coordination geometry (Fig.1b). Four atoms O4,O8,O10,and O11 form the upper bottom surface of the quadrangular prism, and the other four atoms O1, O3,O6, and O7 form the lower bottom surface of the quadrangular prism, and the O9 atom is at the cap position of the quadrangular prism[51].
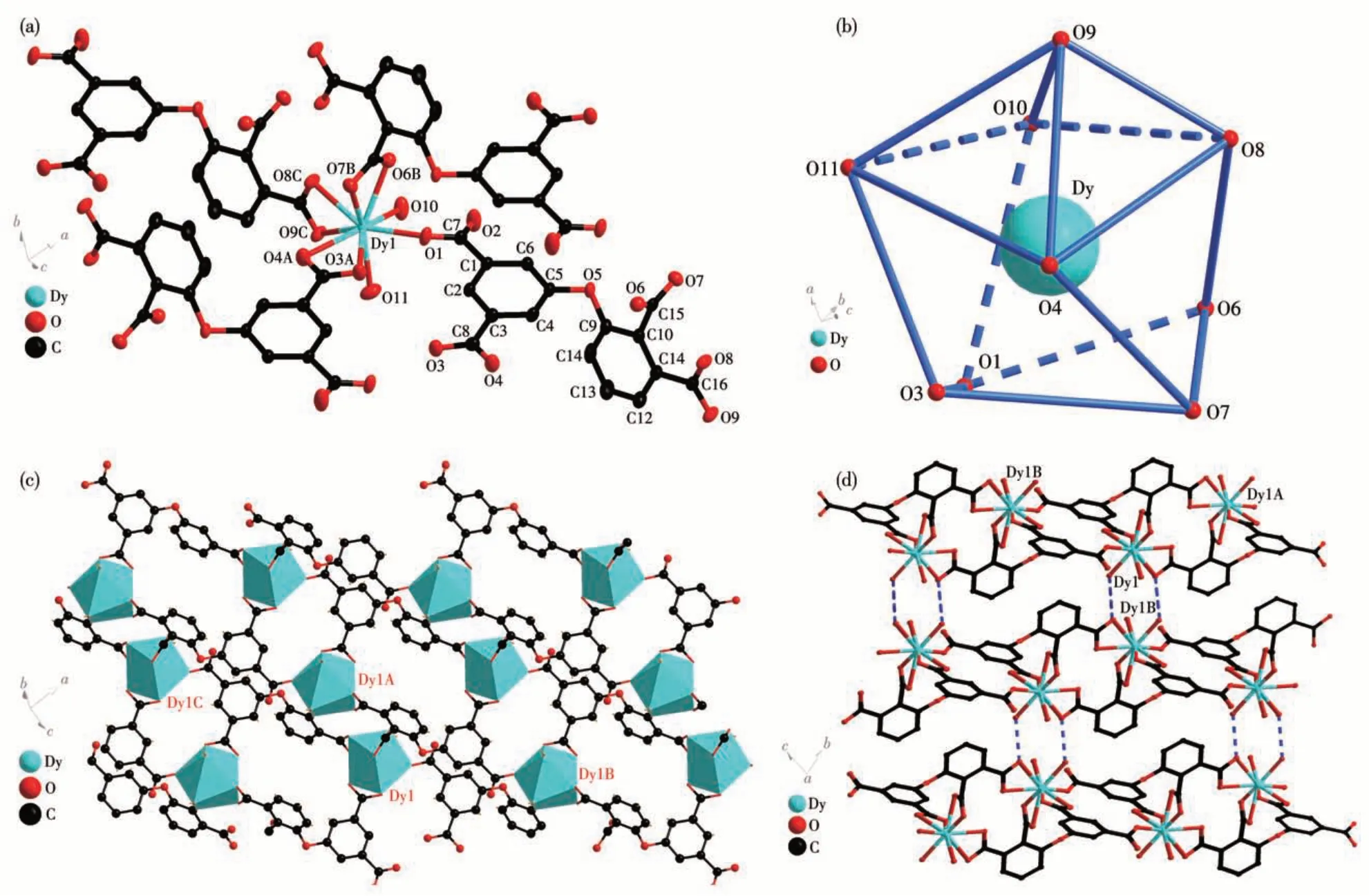
Fig.1 (a)Coordination environment of the Dy(Ⅲ)ion in Dy-MOF;(b)Capped inverse four-prism geometry of Dy ion;(c)2D mesh structure of Dy-MOF;(d)3D supramolecular structure of Dy-MOF
The dppa4-ligand in Dy-MOF is fully deprotonated and showing aμ4∶η1,η2,η2,η2coordination mode.Two Dy (Ⅲ)ions are linked to four carboxyl groups of two dppa4-ions to form dimeric units, where the distance of Dy1…Dy1 is 0.067 nm. The adjacent Dy dimer unit is linked to two dppa4-ions to form a 1D chain. The intrachain distance of Dy1…Dy1 is 1.130 3 nm. Then the adjacent ring chain is connected by dppa4-ions to form a 2D wave network structure and the interchain Dy1…Dy1 distance is 0.823 1 nm(Fig. 1c). Adjacent 2D networks are connected by hydrogen bonding (O10—H10…O9: O10…O9 0.280 1(3) nm)and further assembled in the form of[ABAB]to form a 3D supramolecular network(Fig.1d).
2.2 PXRD and thermal stability of Dy-MOF and Eu-MOF
To obtain the purity of Dy-MOF and Eu-MOF,PXRD experiments were performed.The PXRD diffraction peak positions of Dy-MOF and Eu-MOF powders were consistent with the simulated peak positions,respectively, indicating that Dy-MOF and Eu-MOF are single-phase and have high purity(Fig.S1).
The thermal stability of Dy-MOF and Eu-MOF was investigated by TGA. As shown in Fig.S2, because Dy-MOF and Eu-MOF are isomorphic, the weight loss process was roughly the same with the increase in temperature. So we take Dy-MOF as an example. Dy-MOF first lost its coordination water and guest molecules,and the weight loss rate below 220 ℃was 20.1%,which is consistent with the calculated value (20.6%).The structure was relatively stable in the range of 220-405 ℃. Skeleton collapse began at 405 ℃and continued until 610 ℃, corresponding to the loss of the dppa4-ligand, the loss rate is 54.9% (Calcd. 54.2%).The last remaining is Dy2O3with a residual value of 30.1% and a calculated value of 29.5%.
2.3 Luminescent properties of Dy-MOF and Eu-MOF
It is well known that the 4felectron transition produces unique luminescence properties, and the luminous intensity of rare earth ions in Ln-MOFs is greatly enhanced through the “antenna effect” of the ligand.Therefore, the luminescence of Dy-MOF with Eu-MOF and the ligands was studied at room temperature using the F-7100 fluorescence spectrometer.As shown in Fig.S3, the H4dppa ligand showed a maximum emission peak at 450 nm (λex=350 nm)[52]. The typical narrow emission band of Dy3+could be4F9/2→6H15/2and4F9/2→6H13/2orbital energy level transition, corresponding to wavelengths of 488 and 578 nm, where the maximum fluorescence emission peak was displayed at 578 nm(λex=372 nm).The typical narrow emission band of Eu3+occurred with5D0→7F1,5D0→7F2,5D0→7F3, and5D0→7F4orbital transitions, corresponding to wavelengths of 559, 595, 620, and 699 nm, where the maximum fluorescence emission peak was shown at 620 nm (λex=398 nm)[50]. The emission peak of the ligand was not shown in the fluorescence pattern of emission from the complex, indicating sufficient energy transfer from the ligand to the rare earth metal center.
2.4 Organic solvent sensing
The excellent fluorescence properties of Dy-MOF led us to explore its ability to detect common solvent molecules. When different organic solvents were added to 1 mL solution of Dy-MOF,it was found that Dy-MOF exhibited significant fluorescence quenching behavior when adding 1 mmol·L-1of ANI (20 μL), but with the addition of other organic solvents (20 μL, 1 mmol·L-1),there was no significant change in fluorescence intensity of Dy-MOF (Fig.2a).The quenching efficiency (Q)of Dy-MOF on ANI was calculated to be 99% by the equation:Q=(I0-I)/I0×100%. Therefore, Dy-MOF can be used as a sensor to detect ANI.

Fig.2 (a)Fluorescence responses of Dy-MOF to different solvents;(b)Emission spectra of Dy-MOF in ANI solutions with different concentrations;(c)Linear relationship of I0/I-1 vs cANI for Dy-MOF detecting ANI;(d)Anti-interference of Dy-MOF on detecting ANI after adding different solvents
The fluorescence intensity of Dy-MOF was studied upon the gradual addition of ANI solution, and the fluorescence peak height of Dy-MOF gradually dropped (Fig.2b). To further explore the relationship between ANI concentration and fluorescence intensity,the fluorescence quenching efficiency was quantitatively analyzed by the Stern-Volmer (SV) equation:I0/I-1=KSVcq,whereI0andIare the luminescence intensities of the sensor in the absence and presence of quenching agents,KSVis the quenching constant (L·mol-1), and cqis the molar concentration of the quenching agent. It was not difficult to see from Fig.2c thatI0/Ihad a good linear relationship withcANI(R2=0.993 6)in the concentration range of 4-20 μmol·L-1, and the calculatedKSVwas 1.449×106L·mol-1(Fig.2c). The detection limit(3σlevel) of Dy-MOF for ANI was 2.710 nmol·L-1(3σ/KSV).
Finally, the anti-interference test was performed.20 mL ANI solution (1 mmol·L-1) was dropped into Dy - MOF solution (20 μL) with CYH, PEG, DCE,CH3CN, NPA, NMP, EG, HCHO, NP, PG, and DMF,respectively, and the fluorescence intensity of Dy-MOF was detected (Fig.2d). It was found that the luminescence intensity of Dy-MOF did not decrease significantly when other organic solvents were added, but its luminescence intensity was sharply quenched when the same amount of ANI was injected. So, ANI can be selectively detected by Dy-MOF in the presence of other organic solvents.
In addition, the luminescence response rate of Dy-MOF to ANI(20 μL,1 mmol·L-1)in water at different times was measured (Fig.S4a). After the addition of ANI, the luminous intensity corresponding to Dy-MOF changed immediately, and reached a stable value after 25 s, and maintained for a while. The recyclability of Dy-MOF in solution was further tested. The Dy-MOF powder can be recovered by centrifugal filtration drying. After Dy-MOF detected the ANI, it could be reused four times (Fig. S4b). The results show that Dy-MOF can be used as the sensing material for ANI detection,and has good reliability and recyclability.
2.5 NAC sensing
In the same way as the organic solvents described above, some of the NACs were selected to detect the fluorescence sensing ability of Dy-MOF. As shown in Fig.3a, only the addition of 1 mmol·L-1NB was found to produce a significant fluorescence quenching effect.The fluorescence quenching efficiency was calculated to be 98.5%. While other 1 mmol·L-1NACs have less influence on the luminescence intensity of Dy-MOF aqueous solution than NB.
The fluorescence intensity of Dy-MOF was measured by adding different concentrations of NB. It is not difficult to see that the amount of quenching was related to the concentration of NB (Fig.3b). The SV equation was used to further analyze the fluorescence quenching.I0/Iof Dy-MOF was approximately linear withcNBin the range of 0-70 μmol·L-1at low concentrations (Fig. 3c), and theR2was 0.992 2. TheKSVobtained by linear regression equation was 5.323×105L·mol-1,and the detection limit was 7.780 nmol·L-1.
As shown in Fig.3d, in the absence of NB, the addition of other NAC solutions in Dy-MOF resulted in a slight change in its fluorescence intensity, and the fluorescence intensity was rapidly quenched after the addition of NB, which shows that Dy-MOF can achieve high sensitivity detection of NB.
The stability experiment of Dy-MOF on 20 μL 1 mmol·L-1NB was further explored. After adding NB to the Dy-MOF solution, the fluorescence intensity decreased rapidly after 25 s, and maintained for some time, indicating that Dy-MOF has a good response rate and stability to NB (Fig.S5a). Dy-MOF recyclability experiments show that Dy-MOF that detects NB multiple times can be recovered by centrifugal filtration drying, and the reused crystals can be restored to their original luminous intensity at least four times(Fig.S5b).
2.6 Antibiotic sensing
The procedure was similar to that of NACs, taking 1 mmol·L-1antibiotics to detect the fluorescence sensing ability of Dy-MOF. The fluorescence intensity quenching efficiency of Dy-MOF after the addition of TC was 94.7%, which means a good fluorescence quenching effect (Fig.4a). However, when adding other antibiotics,the fluorescence intensity of Dy-MOF did not change much.So,Dy-MOF has good selectivity for TC.

Fig.4 (a)Fluorescence responses of Dy-MOF to different antibiotics;(b)Emission spectra of Dy-MOF in TC solutions with different concentrations;(c)Linear relationship of I0/I-1 vs cTC for Dy-MOF detecting TC;(d)Anti-interference of Dy-MOF on detecting TC after adding different antibiotics
The fluorescence intensity of Dy-MOF was studied in different volumes of TC solution due to the gradual addition of TC solution. The fluorescence peak height of Dy-MOF gradually dropped (Fig.4b). When we further explore the relationship between TC concentration and fluorescence intensity, we could see from Fig.4c that the concentration range of 0-70 μmol·L-1has a good linear relationship (R2=0.993 7). TheKSVobtained by the linear regression equation was 4.279×105L·mol-1,and the detection limit was 7.933 nmol·L-1.
As shown in Fig.4d, in the absence of TC, the addition of other antibiotic solutions in Dy-MOF resulted in a slight change in its fluorescence intensity, and the fluorescence intensity was rapidly quenched after the addition of TC. The results show that Dy-MOF can achieve high-sensitivity detection of TC.
The luminescence response speed of Dy-MOF to detect TC in water was also tested (Fig.S6a).After adding TC (20 μL, 1 mmol·L-1), the fluorescence intensity was significantly reduced, the minimum value could be reached after 25 s, and the fluorescence intensity remained unchanged,which was conducive to the rapid detection of TC in practical applications.
In addition, Dy-MOF could be recovered by centrifugal washing and could be recovered back to their original luminous intensity at least four times (Fig.S6b). The test data show that Dy-MOF can be used as the sensing material for TC detection, and has good reliability and recyclability.
2.7 Pesticide sensing
The excellent fluorescence performance of Dy-MOF led us to explore its detection in the field of pesticides. In this experiment, 9 pesticides were selected for fluorescence sensing experiments. The photoluminescence spectra of Dy-MOF with different pesticides(20 μL,1 mmol·L-1)are shown in Fig.5a.PTH exhibited obvious luminescence quenching, and the quenching efficiency was 95.6%.
The fluorescence quenching intensity of Dy-MOF was measured by adding different concentrations of PTH. It is not difficult to find that the amount of quenching was related to the concentration of PTH(Fig.5b). The SV equation was used to further analyze fluorescence quenching.A good linear relationship(R2=0.993 4)was shown in the low concentration range of 2-20 μmol·L-1, theKSVwas 1.636×106L·mol-1, and the detection limit was 1.820 nmol·L-1(Fig. 5c), which show that Dy-MOF has high sensitivity to PTH.
The selectivity of PTH solution in the presence of other pesticide solutions in Dy-MOF was studied by an anti-interference experiment. In the absence of PTH,other anti-interference solutions were added to Dy-MOF so that its fluorescence intensity did not change much. However, if PTH was added to different pesticides, the fluorescence intensity decreased sharply(Fig.5d). This excellent immunity to interference opens up the possibility of detecting PTH in complex systems.
In addition,the luminescence response rate of Dy-MOF to PTH (20 μL, 1 mmol·L-1) in water at different times was measured (Fig.S7a). After the addition of PTH, the luminous intensity corresponding to Dy-MOF changed immediately and reached a stable value after 25 s, and maintained for some time. At the same time,Dy-MOF can be recovered by centrifugal washing and can be recovered back to their original luminous intensity at least four times(Fig.S7b).It can be seen that Dy-MOF can be used as a potential fluorescence sensing material for PTH.
2.8 Amino acid sensing
When different amino acids were added to 1 mL Dy-MOF,it was found that Dy-MOF showed significant fluorescence quenching behavior when adding 20 μL 1 mmol·L-1Trp, but after adding other amino acids,there was no significant change in fluorescence intensity of Dy-MOF(Fig.6a).

Fig.6 (a)Fluorescence responses of Dy-MOF to different amino acids;(b)Emission spectra of Dy-MOF in Trp solutions with different concentrations;(c)Linear relationship of I0/I-1 vs cTrp for Dy-MOF detecting Trp;(d)Anti-interference of Dy-MOF on detecting Trp after adding different amino acids
We added 10 mmol·L-1of different volumes of Trp solution each time, waited for 30 s, and recorded the fluorescence spectrum. It could be seen from the fluorescence spectrum that each time Trp was added,the fluorescence emission intensity would be quenched to varying degrees. the fluorescence intensity gradually decreased with the addition of Trp (Fig.6b). The fluorescence quenching efficiency was calculated to be 95.6%. For Dy-MOF, at low concentrations, the SV plot (Fig. 6c) had a good linear relationship (R2=0.992 4)and theKSVwas 1.794×105L·mol-1.The detection limit of Dy-MOF for Trp solutions was calculated to be 0.267 4 μmol·L-1.
Finally, the anti-interference test was performed.20 μL 1 mmol·L-1Trp solution was dropped into the Dy-MOF solution with 20 μL the other amino acids(Fig.6d). It was found that the luminescence intensity of Dy-MOF did not decrease significantly when other amino acids were added, but its luminescence intensity was sharply quenched when the same amount of Trp was injected. It can be seen that Trp can be selectively detected in the presence of other amino acids. In addition,the luminescence response rate of Dy-MOF to Trp(20 μL, 1 mmol·L-1) in water at different times was measured (Fig. S8). After adding Trp, the luminous intensity corresponding to Dy-MOF changed immediately, reached a stable value after 25 s, and was maintained for a while.
2.9 Possible mechanism
The mechanism of the analyte can be detected with high selectivity and sensitivity by testing the UVVis spectrum. For organic solvents, through the test of UV-Vis absorption spectra, it can be seen from Fig.7a that only the absorption peak of ANI was close to the excitation peak of Dy-MOF and had a large spectral overlap, while other organic solvents were relatively far away. This indicated that the excited light is partially absorbed by ANI, which inhibits the energy transfer transition of the ligand to the rare earth metal center,and finally leads to a certain degree of fluorescence quenching (Fig.7a). Therefore, the main mechanism of luminescence quenching is energy competitive absorption. Similarly, UV-Vis absorption spectra of NACs(Fig.7b), antibiotics (Fig.7c), and pesticides (Fig.7d)were respectively tested. It was found that the UV-Vis absorption peaks of NB in NACs,TC in antibiotics,and PTH in pesticides were close to the excitation peak of Dy - MOF. It is speculated that the luminescence quenching of the complex is caused by the energy competition absorption mechanism between the complex and the detected compound[53].
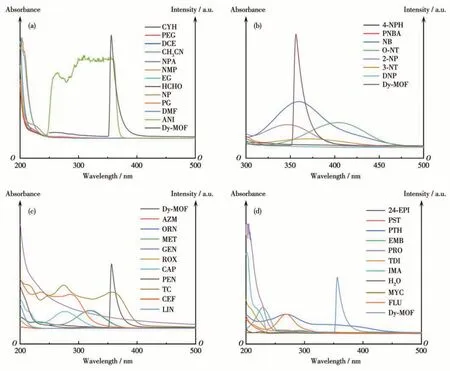
Fig.7 (a)Excitation spectra of Dy-MOF and UV-Vis absorption spectra of the organic solvents;(b)Excitation spectra of Dy-MOF and UV-Vis absorption spectra of the NACs;(c)Excitation spectra of Dy-MOF and UV-Vis absorption spectra of the antibiotics;(d)Excitation spectra of Dy-MOF and UV-Vis absorption spectra of the pesticides
In addition, fluorescence quenching has not only an energy resonance absorption mechanism but also other mechanisms. As shown in Fig.8a, since the fluorescence lifetime of Dy-MOF in the presence or absence of ANI remained unchanged, it is judged that the quenching process should be a static quenching mechanism rather than a dynamic quenching process.Since the fluorescence lifetime of Dy-MOF in the presence or absence of TC/NB/PTH remained unchanged,the quenching process should be judged to be a static quenching mechanism rather than a dynamic quenching process (Fig.8b-8d). Due to the change in the fluorescence lifetime of Dy-MOF in the presence or absence of Trp (Fig.9), it is judged that the quenching process should be a dynamic quenching mechanism,not a static quenching process.

Fig.8 Fluorescence lifetime attenuation curve of Dy-MOF before and after detection of (a)ANI,(b)TC,(c)NB,and(d)PTH

Fig.9 Fluorescence lifetime attenuation curve of Dy-MOF before and after Trp detection
2.10 Practical application in Yanhe River water
To prove the practicability of this method, TC was tested in Yanhe River water through the spiked recovery experiment. The spiked recoveries at different concentrations were obtained, ranging from 95% to 102%.The relative standard deviation (RSD) values were 2.2%-3% (Table 2), indicating the reliability and practicability of Dy-MOF to detect TC in real samples.
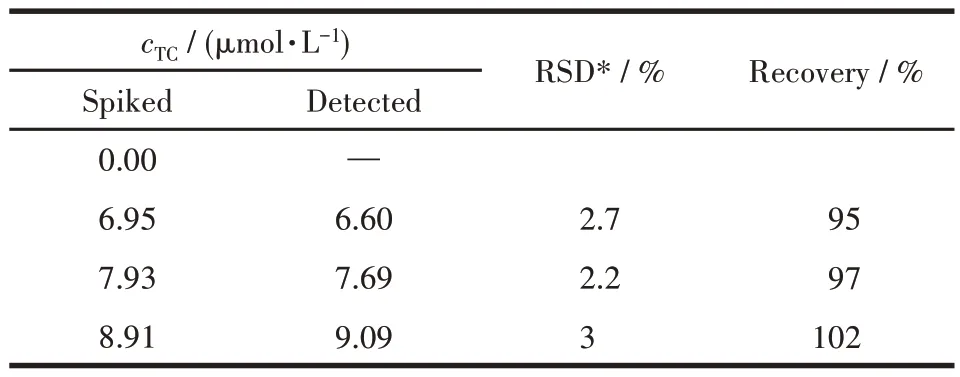
Table 2 TC detection in Yanhe River water by Dy-MOF
3 Conclusions
In summary, two Ln-MOFs (Ln=Dy, Eu) have been hydrothermally synthesized based on H4dppa ligands. The Ln-MOFs show a 2D network. Dy-MOF is a high sensitivity, good selectivity and multi-response fluorescence sensor to be used for the detection of ANI,NB, TC, PTH, and Trp. Among them, the detection limits of Dy-MOF for ANI,NB,TC,PTH,and Trp were 2.710, 7.780, 7.933, 1.820 nmol·L-1and 0.267 4 μmol·L-1, respectively. Dy-MOFs not only have good stability and recyclability in the detection process but also can be successfully used for the detection of TC in Yanhe River, indicating that it is a potential multifunctional fluorescence sensor for detecting contaminants in water. In addition, the mechanism of fluorescence quenching of ANI, NB, TC, PTH, and Trp to Dy-MOF was also discussed.
Supporting information is available at http://www.wjhxxb.cn
