Hematobiochemical and histopathological alterations in Nile Tilapia(Oreochromis niloticus) exposed to ethidium bromide: The protective role of Spirulina platensis
2024-04-16SreenAdullhMervtNguiAlElDinSlhElDinAlElDinSyed
Sreen Adullh ,Mervt Ngui ,Al El-Din Slh El-Din ,Al El-Din H.Syed ,*
a Zoology Department,Faculty of Science,Aswan University,Aswan,81528,Egypt
b Zoology Department,Faculty of Science,Assuit University,Assiut,71516,Egypt
Keywords: Ethidium bromide Spirulina platensis Oreochromis niloticus Liver Lipid peroxidation
ABSTRACT Ethidium bromide (EtBr) is one of the contaminants recorded in aquatic environments whose effects have been investigated;however,there is still limited knowledge about its remediation.This study examined the potential protective effects of Spirulina platensis (SP) against the effects of EtBr toxicity in the Nile tilapia (Oreochromis niloticus) fry.Fry were divided to five groups,viz.,a control and four treatment groups of low-dose EtBr (10 μg/L),low-dose EtBr with SP (10 μg/L EtBr +200 mg/L SP),high-dose EtBr (100 μg/L),and high-dose EtBr with SP(100 μg/L EtBr+200 mg/L SP);the exposure period was 2 weeks.Low and high doses of EtBr induced alterations in some hematological,biochemical,and histopathological parameters.Necrotic hepatocytes,degenerated area,vacuolated hepatocytes,pyknotic nuclei,constricted and dilated blood sinusoids,and infiltration of inflammatory cells were observed.Lipid peroxidation concentration was not significantly different in groups exposed to low doses of EtBr and EtBr with SP,but it was increased in groups exposed to high doses of EtBr and EtBr with SP,compared with the control group.After feeding with SP,most histological and histochemical parameters restored to normal values.Therefore,SP may possess the ability to preserve the structural integrity of the hepatic and renal membranes.
1.Introduction
Water pollution is considered as one of the major problems of this century,it may occur in several ways such as natural process and human anthropogenic activities in which the latter can alter the natural quality of water Elezaby et al.(2001).One of these contaminants is ethidium bromide (EtBr),an intercalating agent commonly used as a fluorescent tag which gets into the environment via water discharge making it one of the contaminants for the research industry.Ethidium bromide is a potent mutagen (may cause genetic damage) and moderately toxic after an acute exposure (Waring,1965).Although a highly sensitive stain,EtBr is notoriously unsafe (Waring,1965).It is not only a very strong mutagen but may also be carcinogenic or teratogenic.Its material safety data sheet documents state that it is harmful if swallowed and very toxic by inhalation,as well as irritating to the eyes,respiratory system,and skin (Hengen,1994).Furthermore,it has the risk of causing irreversible effects skin cancer,eye damage,and liver cancer (McCann et al.,1975).Therefore,EtBr can pose a major safety problem for researchers and can be an environmental hazard during disposal (Salah El-Din et al.,2021).Bioremediation can simply be defined as a biological process of the decontamination of a contaminated environment (Sayed et al.,2020).The environment may be either terrestrial,aqueous,or both.However,a more comprehensive definition of bioremediation is that it is a method of cleaning up contaminated environments by exploiting the diverse metabolic abilities of microorganisms to convert contaminants into harmless products by mineralization and generation of carbon (IV) oxide and water or by conversion into microbial biomass (Baggott,1993;Mentzer &Ebere,1996).Among the microorganisms reported to date,species such as cyanobacteria may provide a potential alternative to existing bioremediation methods (Balaji et al.,2015).
One of these cyanobacteria isSpirulina platensis(SP),a filamentous photoautotrophic cyanobacterium that inhabits various environments such as soils,lakes,and brackish and marine water,forming a green scum on the water surface (Borowitzka et al.,2003).It has a unique composition of nutritional and bioactive substances (e.g.,proteins,vitamins,minerals,pigments,and phenolic acids),with a wide range of medical applications (Ben Amor et al.,2017).SP has been reported to potentiate the immune system,leading to suppression of cancer development and viral infection (Hirahashi et al.,2002).
Hematological and biochemical parameters are an indication of health and physiological responses that may be determined by various stressors (Sayed,Farrag,Abdelaty,Toutou,&Muhammad,2018,Sayed&Authman,2018;Sayed et al.,2018,2020,2021).In general,increased susceptibility of fish to disease is associated with stress caused by unfavorable environmental conditions,inadequate management practices,or in response to dietary limitation of essential nutrients (Peres et al.,2015).Hematological indices are closely related to the response of an animal to its environment,an indication that the environment where fishes live could utilize some effect on the hematological characteristics(Gabriel et al.,2004).Fish are known to have a close relationship with the aqueous environment;hence,their blood would reveal the conditions within their body long before there is any visible reflection of disease (Musa &Omoregie,1999;Okechukwu et al.,2007).
Histopathological investigations have long been recognized as reliable biomarkers of stress in fish (Van der Oost et al.,2003).Histopathological changes have been widely used as biomarkers in the evaluation of health of fish exposed to contaminants,in both laboratory and field studies (Sayed et al.,2017).One of the major advantages of using histopathological biomarkers in environmental monitoring is that this category of biomarkers allows examining specific target organs,including the gills,kidney,and liver (Gernhöfer et al.,2001).Liver is considered as the principal organ of detoxification in vertebrates and particularly in fish.It is also the potential site for lipid deposition in these animals (Freeman et al.,1983).Meanwhile,fish liver is a indicator of aquatic environmental pollution,where one of the important functions of the liver is to clean off any poisons or pollutants from the blood coming from the intestine (Saleh,1982).
The Nile tilapia (Oreochromis niloticus) belongs to the cichlid family and has several distinctive features that support its suitability for use as a model species for the assessment of aquatic pollution.It is known for its ability to tolerate various environmental conditions (Fontainhas-Fernandes,1998).In fact,tilapia has previously been used as a sentinel organism for contaminants in various toxicological studies (Peixoto et al.,2006).Therefore,the study was done to provide insights into the hematological,biochemical and histopathological changes in response to EtBr and after feeding withSpirulina platensis.
2.Materials and methods
2.1.Chemicals
EtBr (purity >99%) was obtained from Sigma-Aldrich Chemical Co.(St.Louis,MO,USA).Biochemical kits of glucose,aspartate aminotransferase (AST),alanine aminotransferase (ALT),total protein,albumin,globulin,creatinine,urea,and uric acid were determined using kits,Stanbio LDH (UV-Rate) USA and RANDOX Laboratories Ltd.,PD410,United Kingdom,respectively.
2.2.Source of SP
SP 100% was purchased from Japan Algae Company (Tokyo,Japan).The nutritional composition of 100 g of SP was described in the product package (Lot number 3009).
2.3.Experimental animals
The fry of the Nile tilapia (O.niloticus) of both sexes were maintained in the Fish Biology Laboratory at the Zoology Department,Faculty of Science,Assiut University.Fish were fed with a commercial fish food(5% body weight) twice a day and maintained at approximately 28◦C with a 12 h:12 h light–dark cycle in tanks (100 L) for 2 weeks to acclimatize to laboratory conditions before experiments.During the acclimation period,approximately 50% of the water in each tank was replaced daily.The total body length of the fish ranged from was 2–3 cm and their weight was 9.5–13.5 g.The water used to culture the fish was dechlorinated and continuously aerated tap water (conductivity 0.4713 ms;pH 6.77;dissolved oxygen 18.68% saturation;temperature 18.8◦C;photoperiod 12:12 light:dark).
2.4.Experimental design
The acclimatized fish were randomly divided into five groups,control and four treatment groups.Each group contained 10 fish per 10 L of water,with three replicates.The first treatment group was exposed to low-dose EtBr (10 μg/L),the second group received low-dose EtBr with SP (10 μg/L EtBr +200 mg/L SP),the third group was exposed to highdose EtBr (100 μg/L),and the fourth group received high-dose EtBr with SP (100 μg/L EtBr+200 mg/L SP).The exposure period was 2 weeks,and the concentration of EtBr (10 and 100 μg/L) was selected based on the observed toxic value as reported previously (von Wurmb-Schwark et al.,2006;Ge et al.,2019).Experimental tanks were filled with dechlorinated water and treated with chemicals,followed by immediate addition of the fish to minimize the reduction of nominal dosing concentrations through the adhesion of particles or chemicals to the glass.Then,50% of tank water was changed daily and immediately redosed.
2.5.Blood collection and hematological parameters
At the end of exposure periods,six fish from each group were collected and anesthetized using ice (Hamed et al.,2019).Then,blood samples were collected from the caudal vein into heparinized tubes and placed immediately on ice for the estimation of hematological indices.Additional blood samples were used for blood smearing.
The total count of red blood cells (RBCs) and white blood cells(WBCs) was estimated using the hemocytometer method under a light microscope (Bancroft &Stevens,1982b).Hematocrit (Hct,%) was determined using the microhematocrit method (Hesser,1960).Hemoglobin (Hb) concentration (g/dL) was immediately evaluated colorimetrically as described previously (Lee,1998) by determining the formation of cyanomethemoglobin.Red cell indices,including mean corpuscular hemoglobin (MCH,pg),mean corpuscular volume (MCV,μm3),and mean corpuscular hemoglobin concentration (MCHC,%),were calculated from RBC count,Hb concentration,and hematocrit.All these red cell indices were calculated according to formulae described elsewhere (Lee,1998).
2.6.RBC alterations
Blood smears (four slides from each fish) were dried,fixed in absolute methanol for 30 s,and stained with hematoxylin and eosin.This was followed by dehydration in ascending grades of alcohol (70%,80%,90%,and 95%,absolute);finally,the slides were cleared in xylene and permanently mounted in Dibutylphthalate Polystyrene Xylene (DPX)(Pascoe &Gatehouse,1986).Several slides were selected based on the staining quality,then coded,randomized,and scored blindly.In each group,10,000 cells (minimum of 1000 cells per slide) were examined under a 40 × objective lens for micronucleated,morphological,and nuclear abnormalities in RBCs according to a previously described method (Al-Sabti &Metcalfe,1995).The established criteria for identifying micronuclei (Schmid,1976) were rigorously followed to ensure authentic scoring.
2.7.Biochemical analysis
A portion of the collected blood was allowed to clot in clean and dry centrifuge tubes at room temperature and centrifuged at 5000 rpm for 20 min at 4◦C,and then the serum was separated for the analysis of the following blood parameters: glucose,AST,ALT,total protein,albumin,globulin,creatinine,urea,and uric acid,which were measured spectrophotometrically using kits (SGMitalia Company,Egypt).
2.8.Lipid peroxidation and total protein measurements
The total protein content in muscles was determined according to the biuret method (Gornall et al.,1949) using bovine serum albumin (E.Merck-Darmstadt,Germany) as a standard.Lipid peroxidation (LPO)was determined using a previously described procedure (Utley et al.,1967).The absorbance of each aliquot was measured at 535 nm.The rate of LPO was expressed as nmol of thiobarbituric acid reactive substances (TBARS) formed per hour per milligram of protein using a molar extinction coefficient of 1.56 M-1cm-1(Buege &Aust,1978).
2.9.Histological and histopathological examination
At the end of the experiment,three fish from each group were removed and sacrificed.The liver was removed from each fish and immediately fixed with Davidson solution.The fixed livers were embedded in paraffin wax,sectioned at 5–7 μm in thickness,and then stained with hematoxylin and eosin (Bancroft &Stevens,1982a).Finally,the liver tissues were examined and imaged using an OMAX microscope equipped with a 14 MP USB Digital Camera (A35140U3;China).
Carbohydrates and protein represent one of the important histochemical parameters.For determining the polysaccharide status,the periodic acid–Schiff (PAS) technique (Mc Manus,1946;Mc Manus,1946) was used.In this method,carbohydrates are first oxidized with 0.1% periodic acid,and aldehyde groups (–HCO–HCO) are liberated from the glycol reagent,producing a compound of magenta coloration.For determining the protein status,the bromophenol blue method(Baker,1958) was applied.
2.10.Statistical analysis
The basic statistics,mean,standard error,and range were estimated.The pattern of variation was estimated by one-way analysis of variance using the SPSS package (IBM-SPSS,2012) at 0.05 and 0.00001 significance levels.Tukey’s test and Dunnett’s t-tests were used for multiple comparisons.
3.Results
3.1.Hematological parameters
The hematological parameters after exposure to EtBr and SP for 15 days are presented in Table 1.Significant differences atP<0.05 were observed among the groups in RBC count,Hb level,MCH,MCHC,Hct,platelet count,and neutrophil,lymphocyte,and WBC counts,whereas MCV and monocyte and eosinophil counts showed nonsignificant differences among all groups atP<0.05.Significantly lower RBC counts were observed in the group exposed to high-dose EtBr than in the control group (P<0.05).Furthermore,there was a significant decrease (P<0.05) in Hb concentration in groups exposed to high-dose EtBr and highdose EtBr +SP compared to that in the control group.Significantly (P<0.05) lower values were detected for MCH,MCHC,Hct,and platelet and neutrophils counts in the group exposed to high-dose EtBr alone than those in the control group.There was a significant increase (P<0.05) in WBC and lymphocyte counts in the group exposed to high-dose EtBr alone compared to that in the control group.However,there was no significant difference (P<0.05) in MCV and monocyte and eosinophil counts in all groups compared with the control group.
3.2.Morphological and nuclear alterations in erythrocytes
Fig.1A shows a blood smear from a healthy fish,demonstrating the normal structure ofO.niloticuserythrocytes,which are round or oval cells with a round or oval nucleus;the cytoplasm shows pink staining,whereas the nucleus shows blue staining.Exposure of the tilapia fry to low and high doses of EtBr resulted in the appearance of abnormal types of cells,such as sickle cells (elongated crescent-shaped RBCs),eccentric nuclei (nuclei deviating or departing from the center of the cell),acanthocytes (plasma membrane with abundant protrusions),crenated cells(plasma membrane with fewer protrusions),schistocytes (fragmented RBCs),elongated cells (elongated RBCs),swelling cells,and elliptocytes.
Table 2 shows the erythrocyte alterations and nuclear abnormalities after exposure to EtBr and SP for 15 days inO.niloticus.There was a significant increase (P<0.05) in morphological alterations in all groups compared with the control group.There was also a significant increase(P<0.05) in nuclear alterations in groups exposed to low-and high-dose EtBr alone compared with the control group.
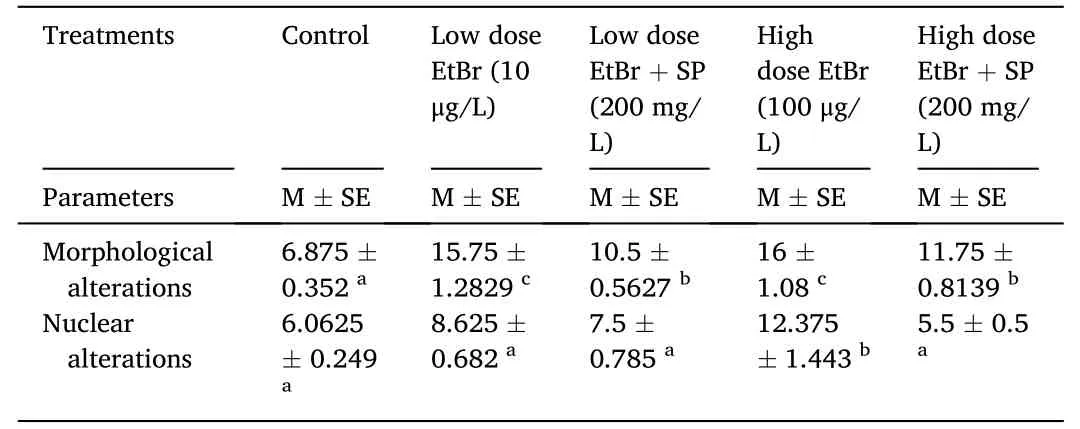
Table 2 Effect of ethidium bromide exposure and Spirulina platensis treatment on morphological and nuclear alterations of fry of Oryochromies niloticus for 15 days.
3.3.Biochemical analysis
The changes in biochemical parameters after exposure to EtBr and SP for 15 days are shown in Table 3.Significant (P<0.01) differences in the levels of ALT,AST,glucose,uric acid,creatinine,albumin,and albumin:globulin ratio were recorded among the groups exposed to EtBr compared with the control group.The levels of ALT,glucose,uric acid,creatinine,albumin,and albumin:globulin ratio were significantly (P<0.05) elevated in all groups compared with the control group,except in the group exposed to low-dose EtBr +SP,in which the levels were still similar.There was also a significant (P<0.05) increase in AST level in the group exposed to high-dose EtBr alone compared with the control group.However,there was no significant difference (P<0.05) in globulin levels in all groups compared with the control group.
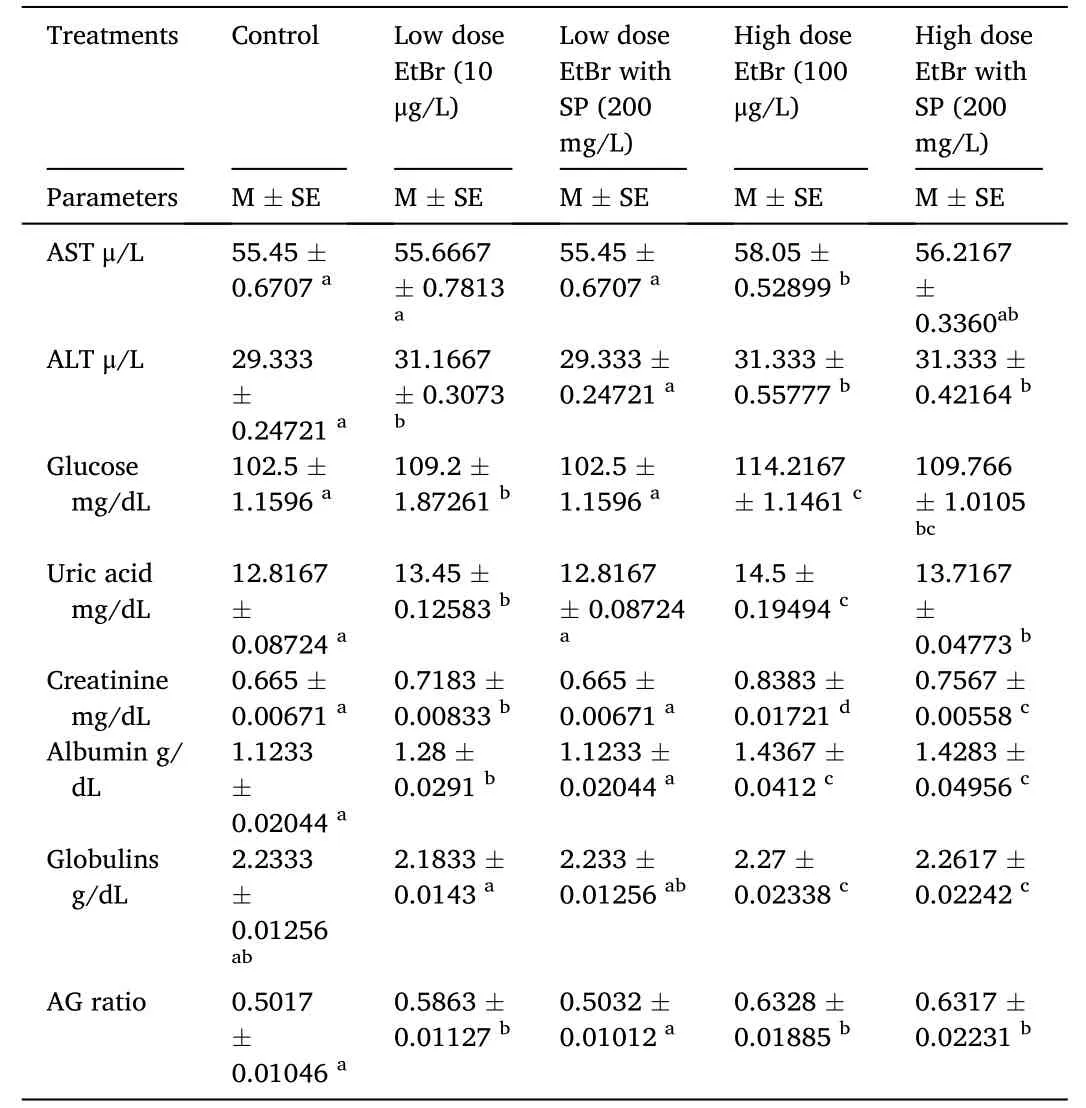
Table 3 Effect of ethidium bromide exposure and Spirulina platensis treatment on biochemical parameters of fry of Oryochromies niloticus for 15 days.
3.4.Total protein and LPO
The total protein and LPO levels after exposure to EtBr and SP for 15 days in the muscles ofO.niloticusare shown in Table 4.There was no significant difference (P<0.05) in the total protein content in all groups compared with the control group.However,there was a significant increase (P<0.05) in LPO in the group exposed to high-dose EtBr and the group exposed to high-dose EtBr +SP alone compared with the control group.

Table 4 Effect of ethidium bromide exposure and Spirulina platensis treatment on total protein and lipid peroxidation in muscles of fry of Oryochromies niloticus for 15 days.
3.5.Histopathological and histochemical examinations
3.5.1.Control liver
The liver tissue of the control fish exhibited normal architecture with polygonal hepatocytes containing vesicular nuclei located between blood sinusoids,and the majority of cells contained unstained cytoplasm around the central vein (Fig.2A).
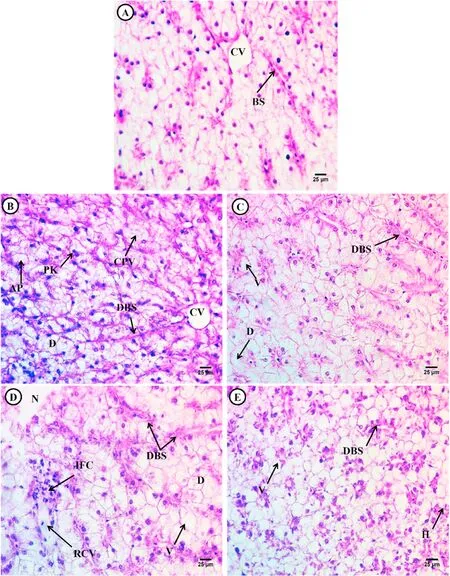
Fig.2.Photomicrograph of the liver of Tilapia fry(Orieochromies niloticus) after exposure to low and high dose EtBr and treatment with Spirulina platensis.(A) control,(B) low dose EtBr,(C) low dose EtBr +SP,(D) high dose EtBr,(E) high dose EtBr +SP.Control showing normal architecture of polygonal hepatocytes (H),blood sinusoids (BS),and central vein (CV).Treated groups showing alterations such as cytoplasmic vacuolation (CPV),pyknotic nuclei (PK),degeneration of hepatocytes(D),vacuolated hepatocytes (V),dilated blood sinusoids (DBS),necrotic hepatocytes (N),ruptured central vein (RCV) and inflammatory cells (IFC) and hemorrhage (H).(H&E × 400).
The PAS technique demonstrated distinct distribution of polysaccharide materials in the control fish hepatocytes.The positively stained materials were confirmed to be glycogen,as verified by PASstaining with and without pretreatment with diastase (Fig.3A).
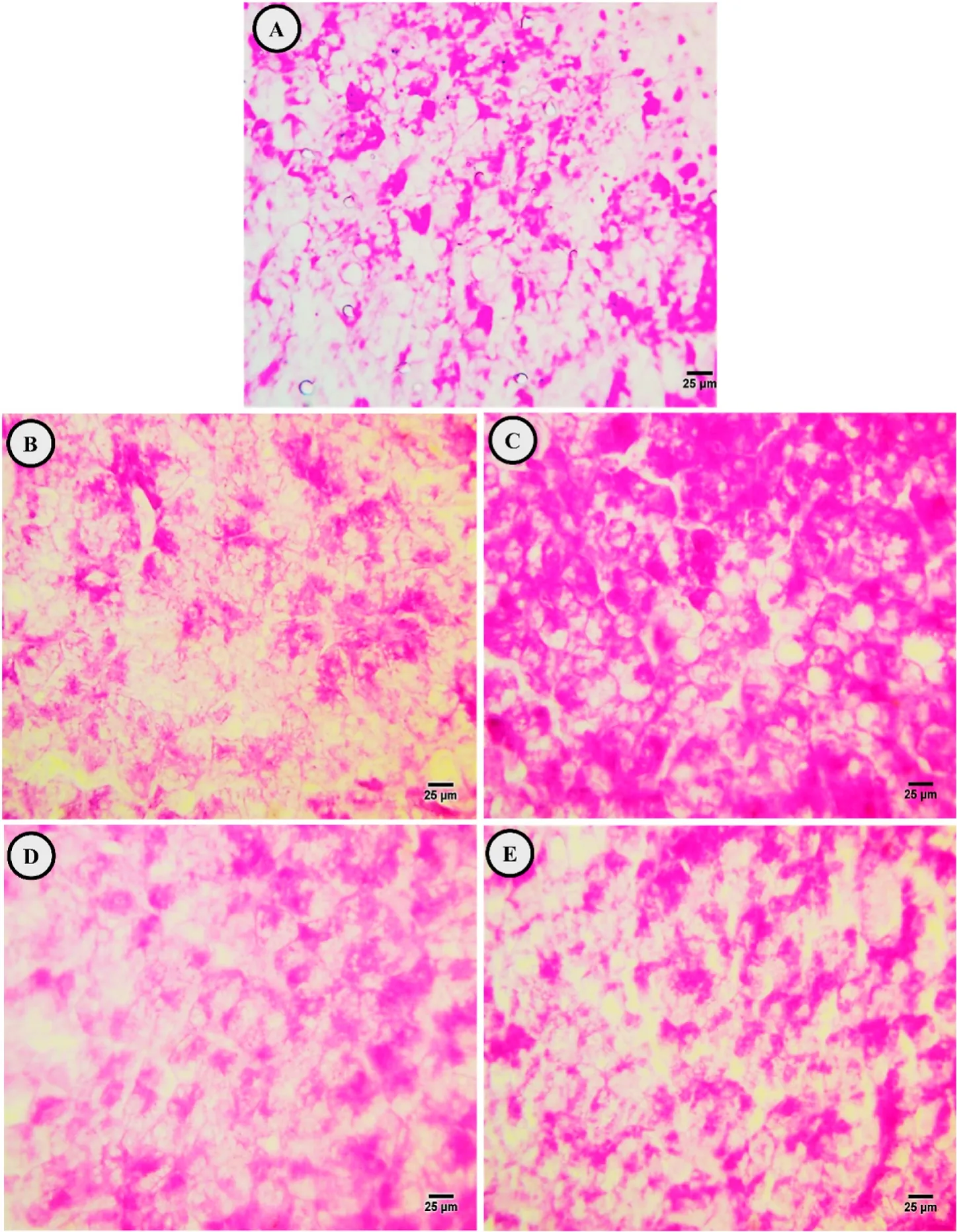
Fig.3.Photomicrographs of the control (A),exposure to low dose EtBr (B),treated with SP after low dose EtBr (C),exposure to a high dose EtBr (D),and treated with SP after high dose EtBr (E) in liver sections of fry of Oryeochromies niloticus.(PAS X 400).
In the liver section stained with mercury bromophenol blue,the total protein was positively stained in the hepatocyte outer membrane and nuclei,where the stored protein was concentrated or localized around the nucleus in the hepatocyte cytoplasm beside blood sinusoids(Fig.4A).
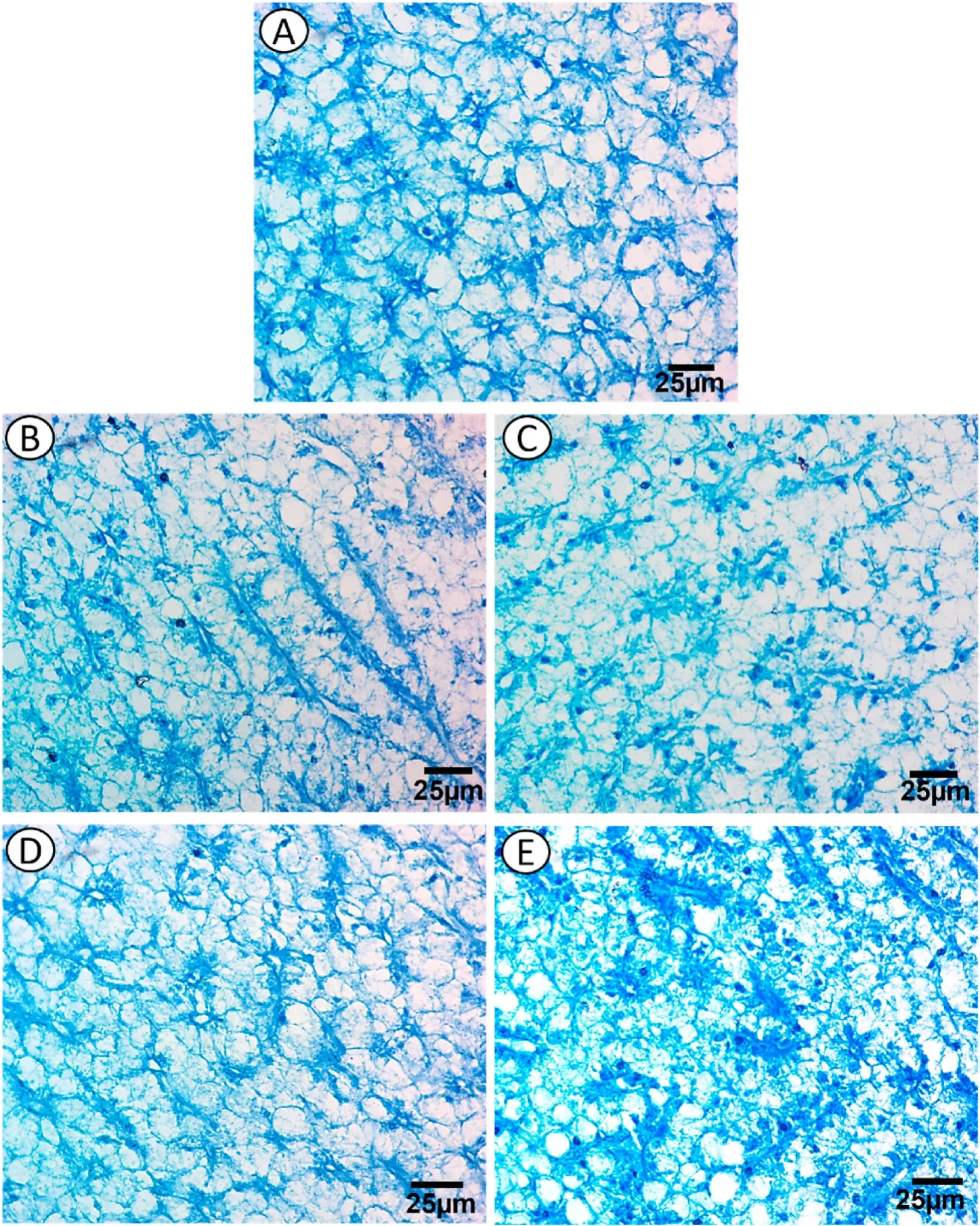
Fig.4.Photomicrographs of the control (A),exposure to low dose EtBr (B),treated with SP after low dose EtBr (C),exposure to a high dose EtBr (D),and treated with SP after high dose EtBr (E) in liver sections of fry of Oryeochromies niloticus.(Bromophenol blue × 400).
3.5.2.Exposure of O.niloticus to low or high dose of EtBr
Exposure to low and high EtBr doses for 15 days resulted in adverse effects on the liver,including the deformation of hepatic structures,patches of necrotic hepatocytes that lost their boundaries,degenerated areas,vacuolated hepatocytes containing scanty acidophilic cytoplasm with deeply stained nuclei (pyknotic),and constricted blood sinusoids(Fig.2 B &D).Moreover,dilated blood sinusoids and infiltration of inflammatory cells were observed in the group exposed to high-dose EtBr (Fig.2D).
The PAS technique revealed depletion in glycogen content in hepatocytes after 15 days of exposure to low and high doses of EtBr (Fig.3 B&D).The cytoplasm of the majority of hepatocytes exhibited a faint coloration compared to that in the control group.
Mercury bromophenol blue staining revealed depletion of total protein in hepatocytes after exposure to low and high doses of EtBr,which showed a faint coloration in the nuclei rim.Primary localization was observed around the nuclei near blood sinusoids,and this depletion increased in group with low dose of EtBr,whereas a slight shortage of total protein was noticed in group with high dose of EtBr (Fig.4 B &D).
3.5.3.Exposure of O.niloticus to low- or high-dose EtBr and SP
The livers of fish exposed to low-or high-dose EtBr +SP for 15 days showed restoration of the normal hepatic structure with a few degenerated hepatocytes and several vacuolated hepatocytes with increased acidophilic cytoplasm and a few pyknotic nuclei (Fig.2 C &E).In the other groups,there were some alterations such as hemorrhage and dilated blood sinusoids (Fig.2E).
The PAS technique revealed more accumulation of carbohydrate materials in the hepatocytes compared with that in EtBr-exposed fish(Fig.3 C &E).Mercury bromophenol blue staining revealed gradual increase in the total protein content from group with low-dose EtBr +SP to group with high-dose EtBr+SP,which aggregated near eccentric located nuclei beside blood sinusoids.Pale staining protein was observed in the rim of hepatocytes,and fine blue granules (stored protein) were observed in the cytoplasm (Fig.4 C &E).
4.Discussion
Environmental toxicant pollution has become one of the most critical problems throughout the world.Fish are particularly exposed to these pollutants because the pollution ends up in the aquatic environment irrespective of where it occurs (Fırat et al.,2011).One of these pollutants is EtBr (Singh &Singh,2018).Various hematological studies have described the variability in the effects of pollutants on hematological indices such as RBCs,Hb concentration,and Hct with an increase,a decrease,or no effect (Osman et al.,2010;Sayed,2016;Sayed &Authman,2018;Sayed et al.,2007).In the present study,there were no significant differences in the RBC count,MCH,MCHC,and Hct parameters compared with the control group,except in the group exposed to high-dose EtBr,in which these values were decreased,but no significant difference was observed in MCV in all groups compared with the control group.Furthermore,we observed a significant decrease in Hb content in the groups exposed to high-dose EtBr and high-dose EtBr +SP compared with the control group.This result is consistent with previously reported data (Osman et al.,2010;Sayed,2016;Sayed &Authman,2018)working on the catfish after UVA,nanoparticles,and SDS,respectively.Previous studies (Mekkawy et al.,2011c) onO.niloticushave also reported a significant decrease in RBC count,Hb concentration,and Hct after exposure to cadmium.Similar results have been described in the carpAphanius dispar,Cyprinus carpio,andCtenopharyngodon idellaafter exposure to mercuric chloride (HgCl2) (Beena &Viswaranjan,1987;Hilmy et al.,1980;Shakoori et al.,1991).The reduction in these parameters might be due to the destruction of mature RBCs and the inhibition of erythrocyte production due to a reduction of hemo-synthesis that affects hematopathology or causes acute hemolytic crisis,which in turn results in severe anemia in most vertebrates,including fish species,exposed to various environmental contaminants (Khadre,1988).It may also be attributed to one or more of the following factors: (i)heam-dilution of blood due to damage and subsequent bleeding in the gills (Heath,1995),(ii) destruction of mature RBCs and inhibition of erythrocyte production (Musa et al.,2013),and (iii) hemolytic crisis that results in severe anemia in fish exposed to heavy metals (Desai &Parikh,2012).
In addition,the present study revealed a significant decrease in platelet and neutrophil counts but a significant increase in WBC andlymphocyte counts in fishes exposed to high-dose EtBr alone compared with the control group.However,there was no significant difference in monocyte and eosinophil counts in all groups compared with the control fish.This result is in agreement with those reported inO.niloticusafter feeding with pomegranate peel (S¸ahan et al.,2016),Oncorhynchus mykissafter feeding with ginger (Haghighi &Rohani,2013),inO.niloticusafter feeding with lactocel (Yones et al.,2019).In addition,it was reported an increase in WBC count in fishes collected from polluted sites (Musa et al.,2013).
Changes in the differential leukocyte count are recognized as a sensitive indicator of environmental stress (Li et al.,2011).Lymphocytes are known to be responsible for the immune response (Singh &Tandon,2009) by producing antibodies and chemical substances,which serve as a defense against infection (Musa et al.,2013).An increase in lymphocyte count may be a compensatory response of lymphoid tissues to the destruction of circulating lymphocytes (Shah &Altindağ,2005).Changes in the number of leukocytes can also be caused due to the stress which make alteration in the blood physiology of the fish then weak the fish’s immune system,which makes it susceptible to disease attacks(Ariweriokuma et al.,2016).
Fish erythrocytes represent a good biomarker for monitoring water quality and pollution (Mekkawy et al.,2011c;Sayed et al.,2007,2013,2017).In the present study,there was a significant increase in morphological alterations in all groups compared with the control group and a significant increase in nuclear alterations in the groups exposed to low and high doses of EtBr compared with the control fish.These results are similar to those of previous studies,which indicated that the micronucleus frequency inClarias gariepinuswas increased on exposure to 4-nonylphenol (Mekkawy et al.,2011b),arsenic (Sayed et al.,2015),heavy metals (Sayed et al.,2015),methyltestosterone (Sayed et al.,2016),gamma radiation (Sayed et al.,2017),SDS (Sayed &Authman,2018),and UVA exposure (Osmon et al.,2019;Sayed et al.,2013).
Measurement of serum biochemical parameters can be especially useful in identifying the target organs of toxicity as well as the general health status of animals and has been advocated to provide early warning of potentially damaging changes in stressed organisms(Jacobson-Kram &Keller,2001).The present study indicated a significant increase in the levels of ALT,glucose,uric acid,creatinine,albumin,and albumin:globulin ratio in all groups compared with the control group,except in the group exposed to low-dose EtBr+SP,which still showed the same value.Moreover,there was no significant difference in globulin levels in all groups compared with the control group.In addition,the AST levels were significantly increased in the group exposed to high-dose EtBr alone compared with the control group.This result is similar to those reported by (Sayed,2016) inBufo regularisafter exposure to UVA,Shokr (2007) inTilapia zilliafter exposure to aluminum chloride,Kaoud et al.(2011) inO.niloticusafter exposure to cadmium,and Osmon et al.(2019) inO.niloticusandC.garepinuscollected from the contaminated area.The results are also consistent with those recorded inO.niloticusandC.garepinusafter exposure to heavy metals (Öner et al.,2008),pesticides (Adedeji et al.,2009),UV rays (Osmon et al.,2010),and nonylphenols (Mekkawy et al.,2011a).
As previously reported,increased levels of AST and ALT in blood plasma indicate impairment of the liver (Shalaby,2007).Moreover,an increase in creatinine level might be induced by glomerular insufficiency,increased muscle tissue catabolism,or impairment of carbohydrate metabolism (Hadi et al.,2009).Abnormal increases in the albumin:globulin ratio may occur as a result of disturbances in liver function or failure to excrete albumin if the animal is stressed due to xenobiotic treatment (Sharafeldin et al.,2015).Furthermore,an elevation in glucose levels is a manifestation of the increased needs of tissues to fuel the metabolic requirements and an important source of energy for maintaining homeostasis in fish during chronic stress (Braun et al.,2013),or it may be due to energy requirements to confront long-term exposure to free radicals (Sayed et al.,2017).
LPO is an important parameter indicative of oxidative stress and is generally reflected by increased levels of malondialdehyde and TBARS(Rajasekar &Anuradha,2007).In the present study,there was a significant increase in LPO in the groups exposed to high-dose EtBr and high-dose EtBr +SP compared with the control group.Moreover,there was no significant difference in the total protein content in all groups compared with the control group.These results are in agreement with previous studies conducted by (Bruneau et al.,2015;Choi et al.,2010;Govindasamy &Rahuman,2012;Wu &Zhou,2013).These authors observed a highly significant increase in LPO levels in the zebra fish,the Mozambique tilapia (O.mossambicus),the medaka (Oryzias latipes),and the rainbow trout,respectively,after exposure to silver nanoparticles.Furthermore (Azza,2014;Federici et al.,2007;Scown et al.,2010),observed increases in LPO inOncorhynchus mykiss,mice,andO.niloticusafter exposure to titanium dioxide nanoparticles,silver nanoparticles,and copper oxide nanoparticles,respectively.The increase in LPO levels in the liver indicates a modification in the physical characteristics of cell membranes (Ursini et al.,1991,pp.319–336),because LPO causes hydrolysis of phospholipids into hydroperoxy fatty acids (Salgo et al.,1993),thereby resulting in tissue damage and failure of antioxidant defense mechanisms to prevent the formation of excessive free radicals(Kim et al.,2010).
Histopathological biomarkers have been primarily used in fish to identify and evaluate the toxic effects of exposure to contaminants(Oliveira-Ribeiro et al.,2006).Extensive research has demonstrated a range of changes in the liver ofO.niloticusdue to exposure to a variety of toxic chemicals (Figueiredo-Fernandes,Fontaínhas-Fernandes,Monteiro,et al.,2006;Figueiredo-Fernandes,Fontaínhas-Fernandes,Peixoto,et al.,2006).In the present study,different histopathological alterations were recorded in the liver,including deformation of hepatic structures,patches of necrotic hepatocytes,degenerated areas,vacuolated hepatocytes containing pyknotic nuclei,dilated blood sinusoids,hemorrhage,and infiltration of inflammatory cells.These alterations are similar to those recorded inO.mossambicusby (Bhuyan et al.,2014),in gobies by (Cuevas et al.,2016),in Indian major carps by (Faheem et al.,2016),inL.rohitaby (Bantu et al.,2017;Sayed et al.,2018) inO.niloticusexposed to methyltestosterone by Sayed et al.(2018),and inO.niloticusexposed to heavy metals by (Mahboob et al.,2020).Similar alterations were also recorded by (Ayadi et al.,2015) inO.niloticusafter exposure to Red 195 dye (E Nofal et al.,2019),inO.niloticusobtained from different localities of the Delta Barrage.
In contrast,the groups exposed to low-and high-dose EtBr+SP showed restoration of the normal hepatic structure with a few degenerated hepatocytes and numerous vacuolated hepatocytes with increased acidophilic cytoplasm and a few pyknotic nuclei.This result is in accordance with those reported by (Abdalla et al.,2014) and (Dohaish et al.,2018) inO.niloticusand (Mokhbatly et al.,2020) inC.gariepinusafter exposure to chlorpyrifos.
In the present investigation,the histochemical results showed a decrease in glycogen amount in the fish liver exposed to low-and highdose EtBr.This finding is consistent with other studies,which showed a reduction in hepatic glycogen content following exposure to xenobiotic compounds (Biagianti-Risbourg,1997) and herbicides (Wassif et al.,2000).This result might be due to increased glycolytic activity to meet the energy demands imposed by enhanced metabolic activity and the hormone-mediated stress phenomenon (Gluth &Hanke,1985).Heath(1995) explained loss of glycogen as a result of depressed feeding and/or elevated levels of the stress hormones cortisol and adrenaline.Glycogen levels can also be depleted in response to some physiological processes such as sexual maturation (Yamamoto &Egami,1974) or nonchemical stresses such as temperature (Braunbeck et al.,1987) and hypoxia(Yuness,2005).
However,in the groups exposed to low-and high doses of EtBr +SP,the PAS technique revealed greater accumulation of carbohydrate materials in the hepatocytes than that in EtBr-exposed fish.This finding was similar to that reported by (Khalila et al.,2018) where there were improvements in liver and intestine histological observations in the group fed with a soybean meal-based diet compared with groups fed with diets based on corn gluten meal and distiller dried grains.Our data are also consistent with those reported by Abd-Elmoneim and Darwish (2016),where SP feeding successfully normalized the cytotoxicity effect of monosodium glutamate in the liver of treated mice,and also with those reported by (Torres-Durán et al.,1999) who corroborated the potential hepatoprotective role of SP.The improvement in liver histology may be attributed to its content of phytopigments such as phycobilins,phycocyanin,allophycocyanin,and xanthophylls,which may be related to its antioxidant activity (Bermejo et al.,2008).
The present study also showed that in groups exposed to low and high doses of EtBr,there was a depletion of total protein content in hepatocytes,which appeared with a faint coloration in the nuclei rim.Primary localization was observed around the nuclei near blood sinusoids,and this depletion was increased in the group exposed to low-dose EtBr,but there was a slight decrease in the total protein content in the group exposed to high-dose EtBr.This result is similar to those reported by (Gaber et al.,2015;Yadav et al.,2007).In contrast,in groups exposed to low-and high-dose EtBr +SP,there was a gradual increase in the total protein content from group with low-dose EtBr+SP to group with high-dose EtBr+SP,which aggregated near eccentric located nuclei beside the blood sinusoids.Pale staining protein was observed in the rim of hepatocytes,and fine blue granules (stored protein) were observed in the cytoplasm.This finding is similar to those reported by (Abdelmeguid et al.,2002) inTilapia zilliiafter exposure to water pollution and (Khan et al.,1987)) in rat after exposure to crude oil.
Our results showed that,after feeding with SP,most histological and histochemical parameters returned to normal values.This is in accordance with previous studies (Sayed et al.,2015,2017).Our findings could be attributed to the potential of SP due to its bioactive antioxidant and anti-inflammatory constituents,particularly C-phycocyanin,provitamin A (β-carotene),minerals,vitamins,proteins,carbohydrates,and lipids,to preserve the structural integrity of the hepatic and renal membranes,after the generation of scavenging free radicals (Salah El-Din et al.,2021).
5.Conclusion
EtBr is a potent toxicant that induces significant alterations in hematobiochemical parameters,morphonuclear characteristics of RBCs,LPO,and histopathological and histochemical characteristics ofO.niloticus.SP is recommended as a fish diet to reduce the harmful effects of EtBr.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Sabreen Abdullah:Methodology,Visualization,Investigation,Data curation,Writing– original draft,Final Draft,Writing– review &editing.Mervat Naguib:Final Draft,Writing– review &editing.Alaa El-Din Salah El-Din:Final Draft,Writing– review &editing.Alaa El-Din H.Sayed:Conceptualization,Methodology,Visualization,Investigation,Data curation,Writing– original draft,Final Draft,Writing–review &editing.
杂志排行
Aquaculture and Fisheries的其它文章
- Selected dietary plant-based proteins for growth and health response of Nile tilapia Oreochromis niloticus
- The roles of polysaccharides in tilapia farming: A review
- The effects of mixed prebiotics in aquaculture: A review
- Growth performance,fatty acid profile,gut,and muscle histo-morphology of Malaysian mahseer,Tor tambroides post larvae fed short-term host associated probiotics
- Evaluation of growth performance,feed efficiency and nutrient digestibility of red hybrid tilapia fed dietary inclusion of black soldier fly larvae(Hermetia illucens)
- Effects of dietary astaxanthin enrichment on enhancing the colour and growth of red tilapia,Oreochromis sp.
