Hydration Behavior and Cementitious Properties of Calcium Carbonate-aluminate Minerals Composite
2024-04-10WANGChongZHOUShuaiZOULuyaoLIUJiawenZHENGYalin
WANG Chong, ZHOU Shuai*, ZOU Luyao, LIU Jiawen, ZHENG Yalin
(1.College of Materials Science and Engineering, Chongqing University, Chongqing 400045, China; 2.China West Construction Group Co.,Ltd., Chengdu 610015, China)
Abstract: The purpose of this research is to investigate the hydration behavior and cementitious properties of the mixture of calcium carbonate and aluminate, and to explore whether it can be adopted as a new low-carbon cementitious material.The composite system of calcium carbonate and aluminate minerals is studied by measuring the component of hydration products, the hydration heat, setting time and compressive strength.The results prove that the composite system has certain cementitious properties and is feasible to prepare new low-carbon cement.
Key words: limestone; hydrated calcium carboaluminate; cementitious properties; mechanical properties
1 Introduction
In recent years, a large amount of carbon dioxide has caused serious greenhouse effect, which poses a great threat to the ecosystem.Cement production still has the characteristics of high emissions and high energy consumption.The cement industry is a major source of industrial greenhouse gas emissions,accounting for about 7% of global anthropogenic greenhouse gas emissions[1].The carbon dioxide mainly comes from limestone, which is one of the most important raw materials for Portland cement[2], and the carbon emission of cement is high[3].It is necessary to investigate new low-carbon special cement.
The main component of limestone is calcium carbonate, which reacts with the aluminum phase in Portland cement to produce hydrated calcium carboaluminate[4-12].Some scholars have produced hydrated calcium carboaluminate by chemical synthesis[13].At present, hydrated calcium carboaluminate mainly includes hemicarbonate(Hc) and monocarbonate (Mc).Their chemical formulas are C3A∙1/2CaCO3∙1/2Ca(OH)2∙11.5H2O and C3A∙CaCO3∙11H2O, respectively.In addition, a few scholars have found tricarbonate (Tc)[14-16].It is generally believed that calcium carbonate mainly acts as an inert filler in the Portland cement system.But it can participate in certain chemical reactions.The chemical reactions are listed as follows:
Studies have shown that Mc in the calcium carbonate-cement composite system is more stable than Hc.Hc will gradually convert into Mc after the early generation.Ipavecet al[17]investigated the composition change of the hydration products of Portland cement mixed with 20% calcium carbonate.They found that Hc was formed after 1 day of hydration and Hc began to transform into Mc after 3 days.By 100 days, Hc was not detected and the content of Mc reached the highest.Luzet al[18]researched the hydration products of calcium aluminate cement (W/B=0.3) mixed with 15% calcium carbonate at 37 ℃, and found that Mc would partially decompose within 30 days.The formation of Mc was a reversible reaction.There are researches[4,19-21]proving that when calcium carbonate reacts with Portland cement, and the hydration activity of calcium carbonate can be obviously manifested.But,when it is compounded with aluminate cement, calciumcarbonate presents higher hydration activity in the early period and more carboaluminate can be produced[22].
Based on the synergistic effect of calcium carbonate and aluminum-contained auxiliary cementitious materials, the development of new cementitious materials that can produce hydrated calcium carboaluminate is expected in the future.Recently, Scriveneret al[23-25]proposed limestone calcined clay cement (LC3), indicating that the cement clinker replaced by nearly 50% limestone and calcined clay has better mechanical properties and durability.However, the main raw material studied here is aluminate cement.The main active component of aluminate cement is aluminate minerals, while the essence of LC3is still a silicate composite system.
In this research, the purpose is to probe whether it can make full use of the reaction between aluminate minerals and calcium carbonate to greatly reduce carbon dioxide emission.Studying the hydration reaction characteristics and cementitious properties can explore its potential as a new low-carbon cementitious material.
2 Experimental
2.1 Raw materials
Aluminate minerals include C3A, C12A7, CA,etc.In general, C3A has the highest activity, followed by C12A7.C12A7is adopted here due to its availability.
The aluminate mineral (C12A7, TEP) used in this test was provided by Kerneos Aluminate Technology Co., Ltd.The density and specific surface area were 2.88 g/cm3and 591 m2/kg, respectively.The chemical composition is displayed in Table 1, and the XRD result is presented in Fig.1.The aluminate cement used in the experiment is a commercially available cement with C12A7as the main mineral, which also needs to be calcined.The main raw material of this cement also contains calcium carbonate, but its calcium oxide content is lower than that of Portland cement clinker.Hence, the direct carbon emission is lower.In addition,a large amount of calcium carbonate can be mixed into the cement.The overall carbon emission can be lower.The calcination temperature of aluminate cement is lower than that of Portland cement clinker, which can further reduce energy consumption.The calcium carbonate (precipitated calcium carbonate powder, LC)used in this experiment was produced by Shanghai Yuanjiang Chemical Co., Ltd.The specific surface area was 1 880 m2/kg.The chemical composition of LC is displayed in Table 2.
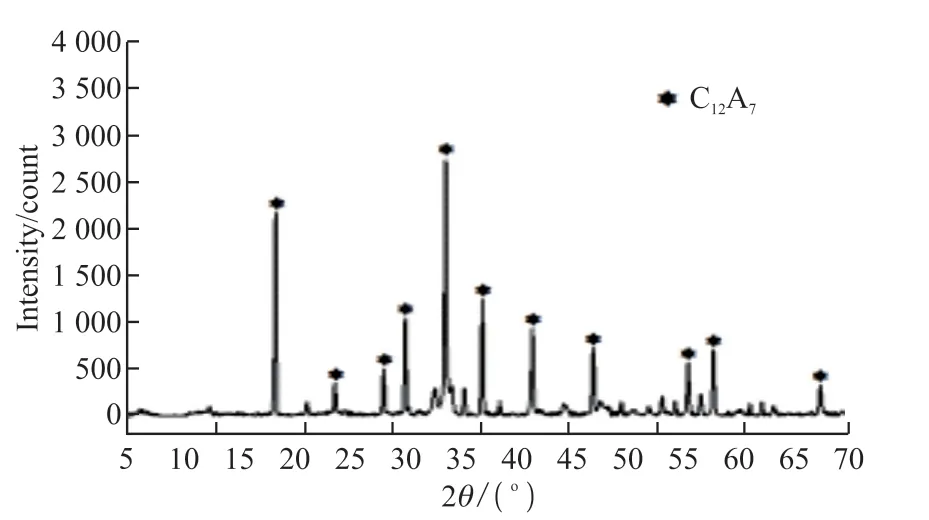
Fig.1 XRD pattern of aluminate minerals

Table 1 Chemical composition of TEP/wt%

Table 2 Chemical composition of LC/wt%
2.2 Test methods
2.2.1 Test of hydration heat
The hydration heat behavior of the CaCO3-C12A7composite system was tested by the TAM-Air multi-channel heat of hydration tester produced by TA Company.In the test, the water-binder ratio was fixed at 1.0.After the paste mixing was completed (as far as possible to ensure that the control group was carried out at the same time), around 15 g paste was poured into a special bottle.It was sealed and then put into the instrument.The test temperature was 20 ℃.After 72 hours, the data acquisition process was automatically completed by the computer.
2.2.2 Analysis of hydration products
The mix proportion of the cement paste sample is exhibited in Table 3.The sample was soaked in absolute ethanol for 48 h to stop hydration after curing to the specified age.It was dried in a vacuum drying oven at 40 ℃ for 48 h, ground and sieved (200 mesh)to prepare powdered samples.XRD was used to study the composition of the product.The test range of XRD was 5°-55°(2θ) with 5 min fast scan.N2atmosphere and a heating rate of 10 ℃/min were adopted.The VEGA 3 LMH type tungsten filament scanning electron microscope produced by TESCAN was used to observe the microscopic appearance of the sample.The working voltage of the electron microscope was 15 kV,the electron beam intensity was 8.0, and the working distance was controlled at 8-15 mm.
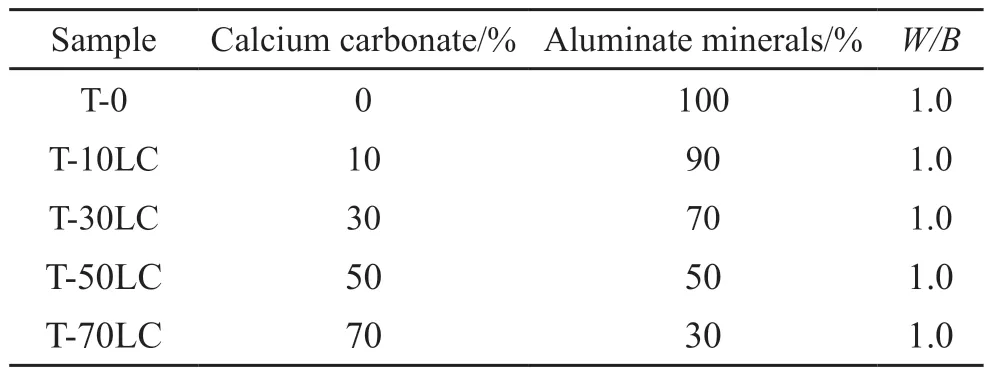
Table 3 Mix proportion for calcium carbonate-aluminate minerals composite
2.2.3 Measurement of the setting time
The water demand for normal consistency and setting time were measured according to the standard methods and steps in GB/T 1346-2011[26].
2.2.4 Tests of compressive strength
Based on GB/T 17671-1999[27], the specimen was 40 mm × 40 mm × 40 mm, with a water/cement ratio of 0.50.Although the standard flexural specimen is 40 mm × 40 mm × 160 mm, this research only focuses on the development of the compressive strength of the system, and does not pay attention to its flexural strength.Hence, the block of 40 mm × 40 mm × 40 mm can meet the standard requirements and the test requirements here.The manufacturing operation was carried out at 20 ℃.After demolding, they were placed in the standard curing room until test.
3 Results and discussion
3.1 Hydration heat
3.1.1 Hydration heat of calcium carbonate-aluminate composite
Here, the hydration of the composite is investigated.The related chemical equations are displayed as follows:
In Fig.2, the hydration heat of the composite is displayed.The hydration of C12A7was very rapid.There were multiple exothermic peaks.The initial exothermic peak of C12A7hydration was significantly higher than the subsequent hydration stage (Fig.2(a)).After adding calcium carbonate, the initial exothermic peak generally decreased with the increase of calcium carbonate content.Calcium carbonate did not significantly promote the early dissolution process of C12A7.But it advanced the second exothermic peak,indicating that calcium carbonate still had a certain promoting effect on the hydration of C12A7.Calcium carbonate reduced the number of hydration exothermic peaks of C12A7.The reason was that calcium carbonate and C12A7generated a large amount of carboaluminate,reducing the conversion of C2AH8to C3AH6.
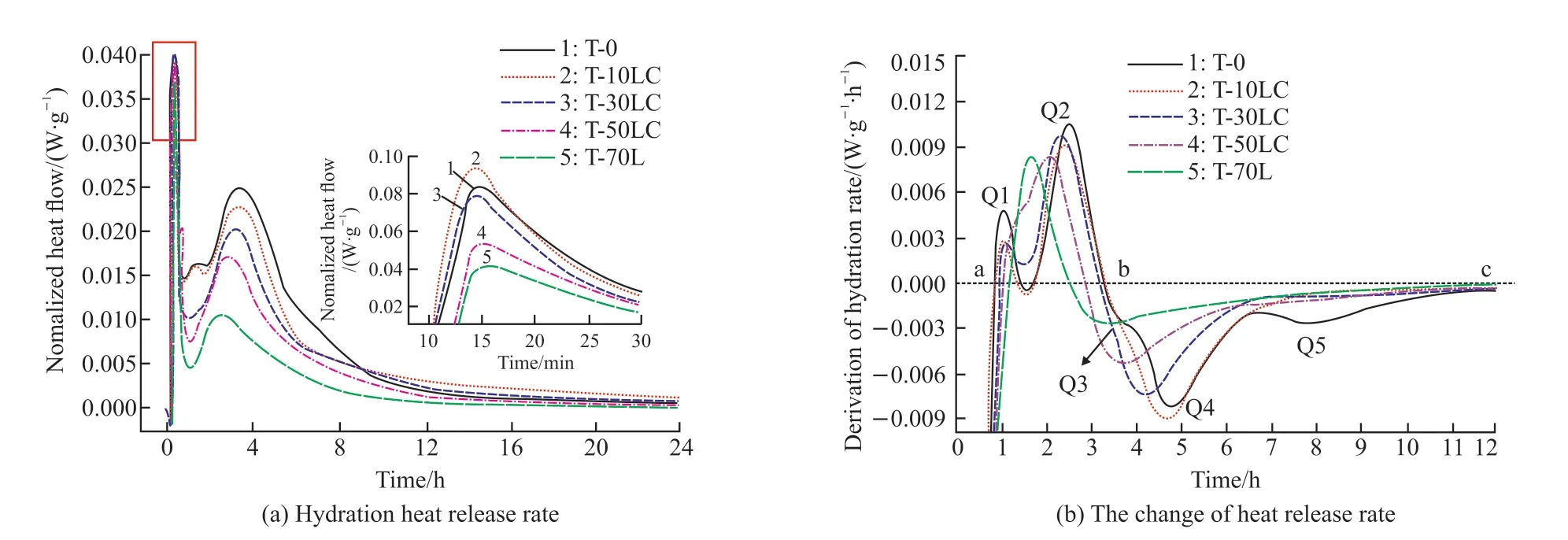
Fig.2 The hydration heat of CaCO3-C12A7 composite with different C/A ratios
In Fig.2(b), a and b were the start point and the endpoint of the acceleration period, respectively.c was the endpoint of the deceleration period.Q was the extreme point of change of heat release rate.Fig.2(b) illustrates that the hydration heat release rate of C12A7had multiple extreme points.There were two abrupt change points Q1 and Q2 in section a-b (i e,the acceleration period).The extreme value at Q2 was higher than Q1, indicating that the rate of the latter stage developed faster during the two acceleration periods of hydration of C12A7.It was because C12A7continuously released heat during hydration, thus intensifying the hydration process of C12A7.It could be seen from Q1 and Q2 that with the increasing content of calcium carbonate,the peak of Q1 decreased continuously, while the peak of Q2 decreased and shifted to the left.It indicates that the increase of the C/A ratio would mainly weaken the speed development in the early acceleration period.Moreover,the duration of the acceleration period was shortened.The speed of the deceleration period b-c was slowed down.The extreme point was reduced.Therefore, the incorporation of calcium carbonate made the reaction of C12A7more gentle.
The total amount of heat released by the composite within 72 hours was 610.6 J/g (Fig.3).Combined with the analysis of the change of hydration heat, the hydration promotion effect of calcium carbonate on the CaCO3-C12A7system was not obvious.In the early stage,the total amount of heat release showed a downward trend with the increase of the C/A ratio.However, the heat release of the 10% calcium carbonate system was basically the same as that of C12A7, and it was even slightly higher than that of C12A7in the later stage of hydration.Possibly, it was because the heat generated by the chemical reaction between CaCO3and C12A7could compensate for the heat loss due to the dilution effect caused by the addition of a small amount of CaCO3.However, excessive incorporation of calcium carbonate would make the dilution effect more significant.The heat generated by the chemical reaction of CaCO3and C12A7cannot compensate for the heat loss.Therefore,as the content of calcium carbonate increased, the heat release decreased on different levels.
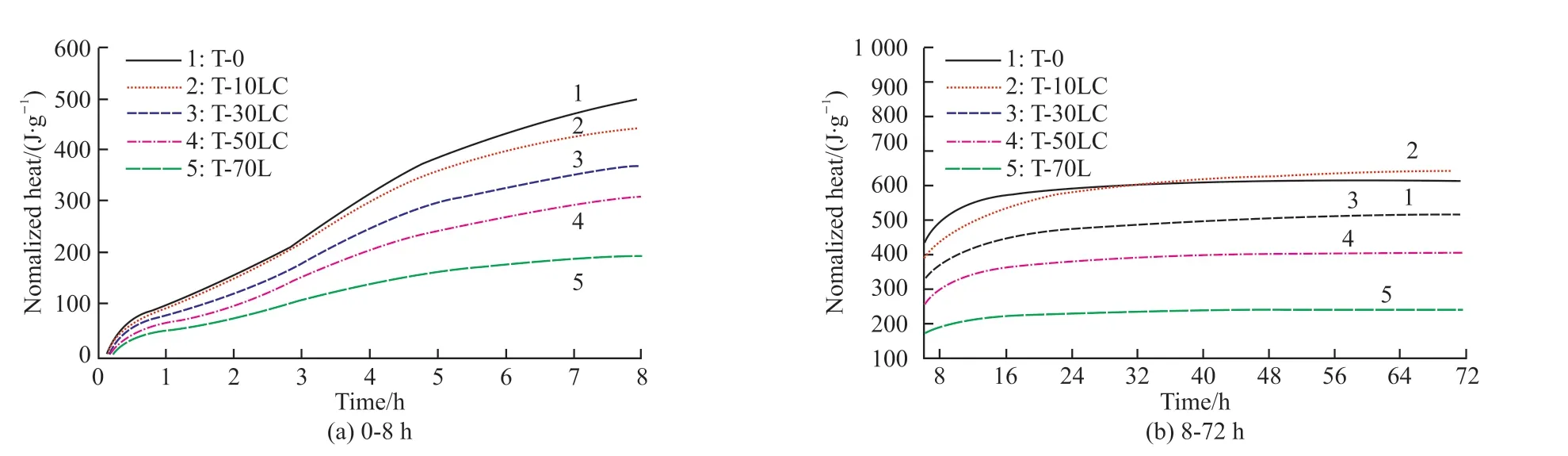
Fig.3 The curve of hydration heat of CaCO3-C12A7 systems with different C/A ratios
3.1.2 The heat of reaction between calcium carbonate and aluminate mineral
The hydration heat of calcium carbonatealuminate minerals was mainly composed of two parts, namely the hydration heat of the aluminate mineral and the chemical reaction between calcium carbonate and aluminate minerals.The hydration heat of the composite subtracted that of aluminate minerals according to the mass proportion, and the rest could be expressed as the heat released by the reaction of calcium carbonate.
It can be seen from Fig.4 that the hydration reaction between calcium carbonate and aluminate was fast.Hydration began immediately after adding water,and a great deal of hydration heat was released.The hydration heat release reached 60 to 100 J/g within 3 days.When the content of calcium carbonate did not exceed 50%, the hydration heat release rose with the increase of its content.However, when the content of calcium carbonate was too high, the heat release was reduced.
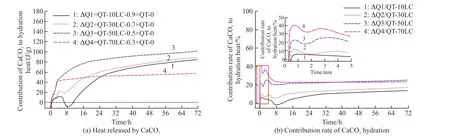
Fig.4 The behavior of hydration heat of CaCO3 in CaCO3-C12A7
Fig.4(a) displays an abnormal phenomenon.The system with 10% calcium carbonate began to have an endothermic peak after the hydration for 4 hours, while the whole system was still exothermic at this time.The heat release of other systems continued to increase.The reason may be that a less calcium carbonate content led to greater instability of hydration products and the crystal transformation.
As the C/A ratio increased, the amount of heat generated by the reaction of CaCO3also gradually increased.However, it increased significantly in 4 hours when the content of CaCO3increased to 70%.The heat release of the same system at the later stage(after 4 hours) was significantly lower than the other three blending ratios.The calcium carbonate may be excessive in the system.During the process of the reaction, the formation of calcium carboaluminate could not continuously supply enough alumina and the dilution effect of calcium carbonate had a greater impact on the reaction[4].Therefore, the calcium carbonate reacted quickly with hydrated aluminate in the early stage, then presenting an inert state.
The heat released by calcium carbonate in the reaction was divided by the total hydration heat of the calcium carbonate-aluminate mineral composite to obtain the contribution rate of the hydration heat of calcium carbonate.It can be seen from Fig.4(b) that in the early stage of hydration, the contribution rate of calcium carbonate hydration had a trend of increasing at first and then decreasing.It was speculated that calcium carbonate reacted with hydrated aluminate to continuously promote the hydration of aluminate minerals.At the same time, a large number of hydration products were formed and settled on the surface of the reaction particles, hindering the dissolution of aluminate minerals.
In the later period of hydration, the contribution rate of the hydration of CaCO3gradually increased with the rise of the C/A ratio.When the dosage of CaCO3was 50%, the contribution rate of heat release of CaCO3was close to 70%, even slightly higher.It showed that the increase of calcium carbonate content could not continuously promote the formation of calcium carboaluminate.
3.2 Composition of hydration products
Fig.5(a) and Fig.5(b) are the XRD patterns of the CaCO3-C12A7composite with different C/A ratios for 1 and 28 d.
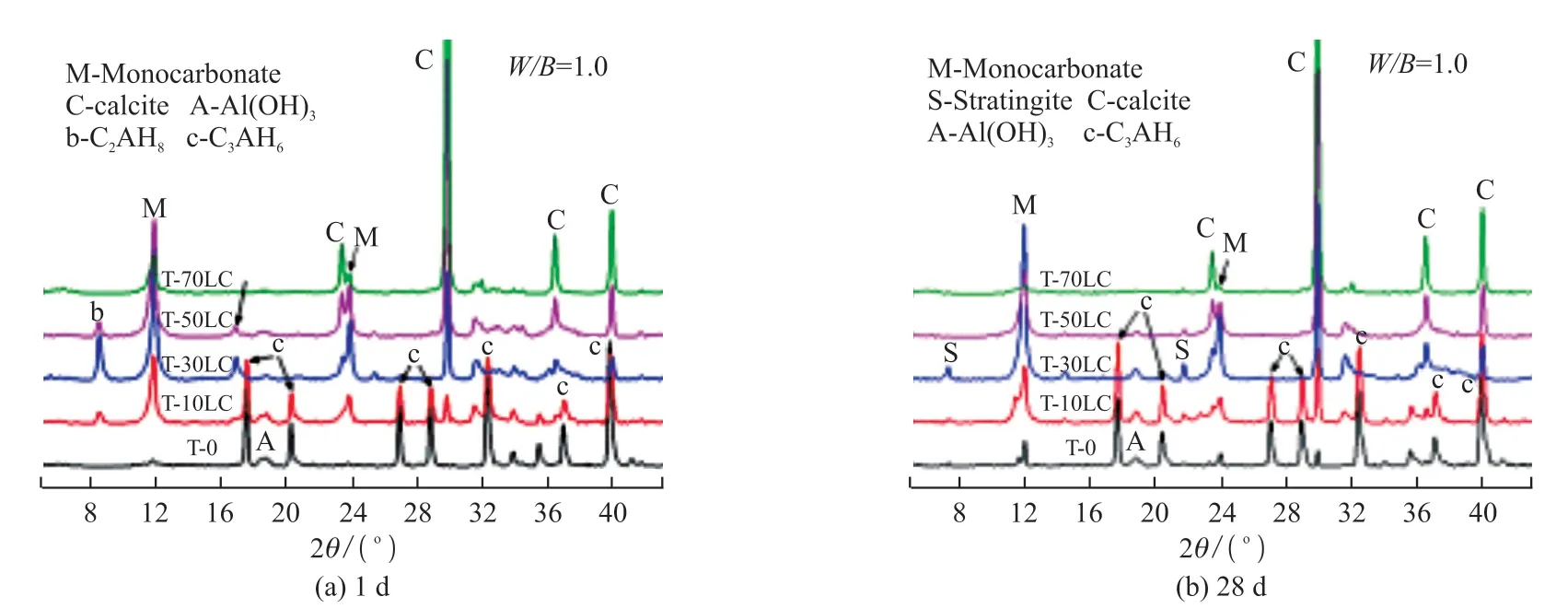
Fig.5 Hydration products of CaCO3-C12A7 with different C/A ratios
On the first day (Fig.5(a)), the diffraction peaks of C2AH8and calcium carboaluminate both reached the maximum when the C/A ratio was 3:7.The amount of C3AH6produced continued to decrease with the increase of calcium carbonate content since the incorporated calcium carbonate reacted with the alumina to generate calcium carboaluminate, inhibiting the crystal conversion of C2AH8to C3AH6.
On the 28th day (Fig.5(b)), the diffraction peaks of calcium carboaluminate appeared in the pure C12A7.The hydrated aluminate produced during the hydration process of C12A7was carbonized, generating calcium carbonate.Then calcium carbonate reacted with hydrated aluminate to generate a small amount of Mc.Carbon dioxide in the air was dissolved to produce carbonate in the later stage, then reacting with hydrated aluminate to form calcium carboaluminate.
After adding calcium carbonate (30%), a new product was formed, namely stratlingite(Ca2Al2SiO7·8H2O).Its formation was related to the Si phase[28], mainly from the relatively inert Gehlenite in aluminate cement.
Fig.6(a) and Fig.6(b) were the XRD patterns of the hydrated products of C12A7and the mixture of C12A7(70%) and calcium carbonate (30%) from 3 hours to 90 days, respectively.It can be seen from Fig.6(a) that a large amount of C2AH8was generatedby the hydration of C12A7for 3 hours, and then as the hydration was processed, the amount of C2AH8gradually decreased.At the same time, the diffraction peak of C3AH6continued to increase and there was an obvious crystal conversion phenomenon.Stratlingite was newly formed after C12A7was hydrated for 3 days, but it decomposed with time.After 28 days of hydration, the peak of monocarboaluminate was gradually significant and reached the highest at 90 days.The carbonization of hydrated aluminate would promote the formation of calcium carbonaluminate due to the increase of dissolved carbon dioxide.Then the carbonate in the solution reacted with hydrated aluminate to generate Mc.
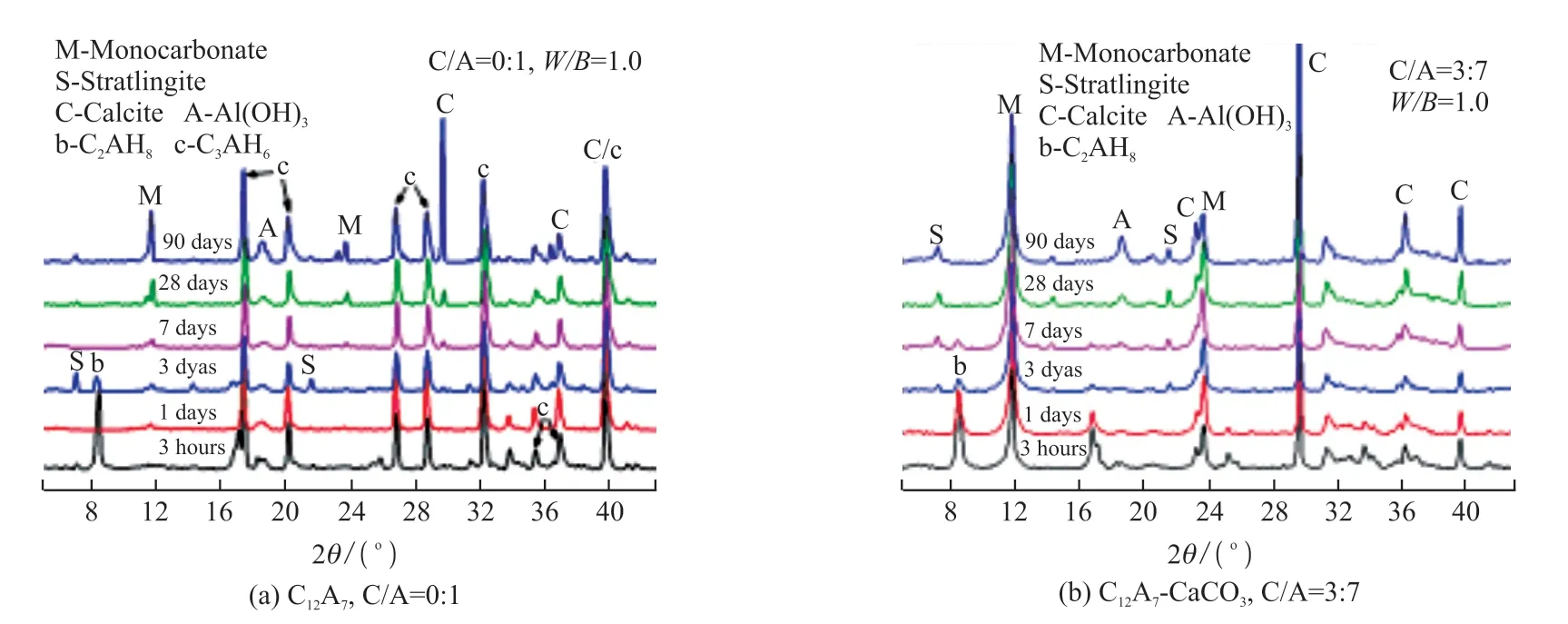
Fig.6 Hydration products during different curing ages
After 30% calcium carbonate was added in the C12A7system (Fig.6(b)), Mc and C2AH8were formed obviously after 3 hours.During the hydration process,C2AH8decreased until it disappeared, but the diffraction peak of C3AH6had not been detected.The formation of Mc inhibited the crystal conversion between calcium aluminate and also delayed the formation of stratlingite.However, calcium carboaluminate did not exist stably in the composite system and would decompose with time.
3.3 Cementitious properties
3.3.1 Setting time
Table 4 lists the results of the setting time of the CaCO3-C12A7composite with different C/A ratios.Fig.7 shows the effect of the C/A ratio on the setting time.
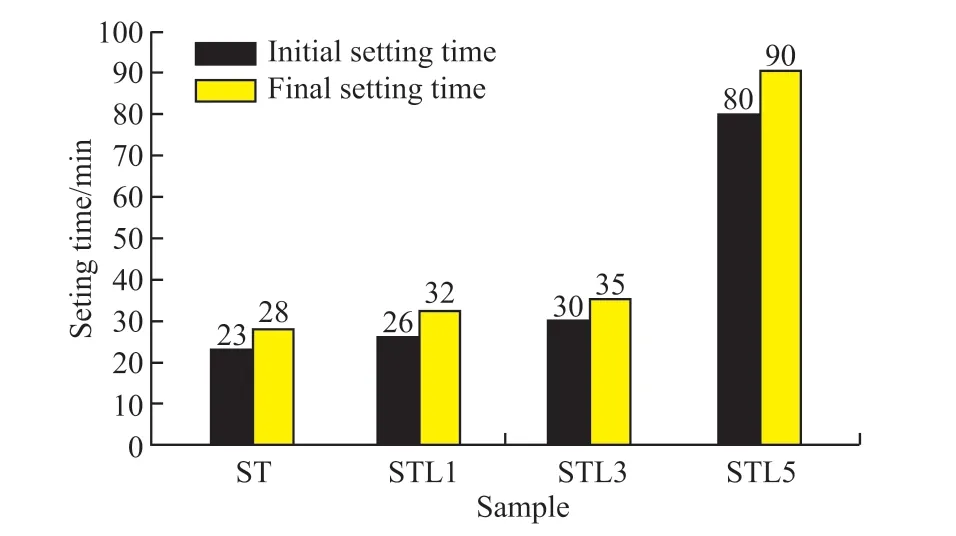
Fig.7 Effect of C/A ratio on the setting time of CaCO3-C12A7
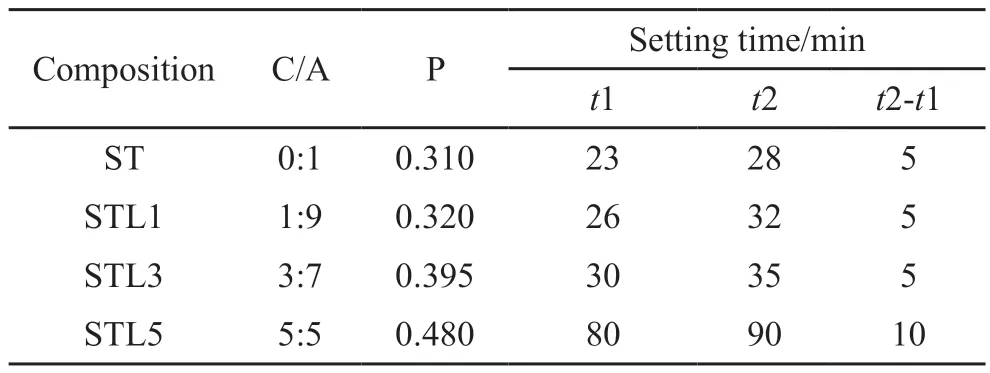
Table 4 Setting time of CaCO3-C12A7 composite with different C/A ratios
As shown in Table 4, the water consumption for the standard consistency of C12A7was 0.310.The initial setting was reached after 23 minutes.The interval between the initial setting time and the final setting time was only 5 minutes.After adding calcium carbonate, the water consumption for standard consistency gradually increased and the initial setting continued to be delayed as the C/A ratio increased.
When the calcium carbonate content was 50%, the initial setting time was 80 min.The interval between the initial setting and the final setting was extended to 10 min, indicating that the setting of C12A7was retarded by adding calcium carbonate.When the C/A ratio was higher, the degree of retardation was more significant.Combined with microscopic analysis, a large amount of C2AH8was produced at first during the hydration process of the pure C12A7, then quickly converted into C3AH6.During the hydration process, the crystal conversion occurred.Calcium carbonate reacted with aluminate to form a large amount of hydrated carboaluminate, reducing the content of hydrated aluminate and the occurrence of crystal transformation.
Fast setting is a feature of this system, which may be used as a repair material.The research is currently in its early stages.Later, suitable application research will be carried out for this feature.Meanwhile, the research on the retarder of this system will be carried out.
3.3.2 Compressive strength
Fig.8 displays the development of compressive strength of CaCO3-C12A7composite with different C/A ratios (W/B=0.5) and ages (3 d, 28 d).It can be seen that the compressive strength of C12A7at 3 and 28 d was only 14.9 and 12.3 MPa, respectively.Some literature pointed out that the reason for the low strength was the microstructure of C12A7had lots of holes[29].
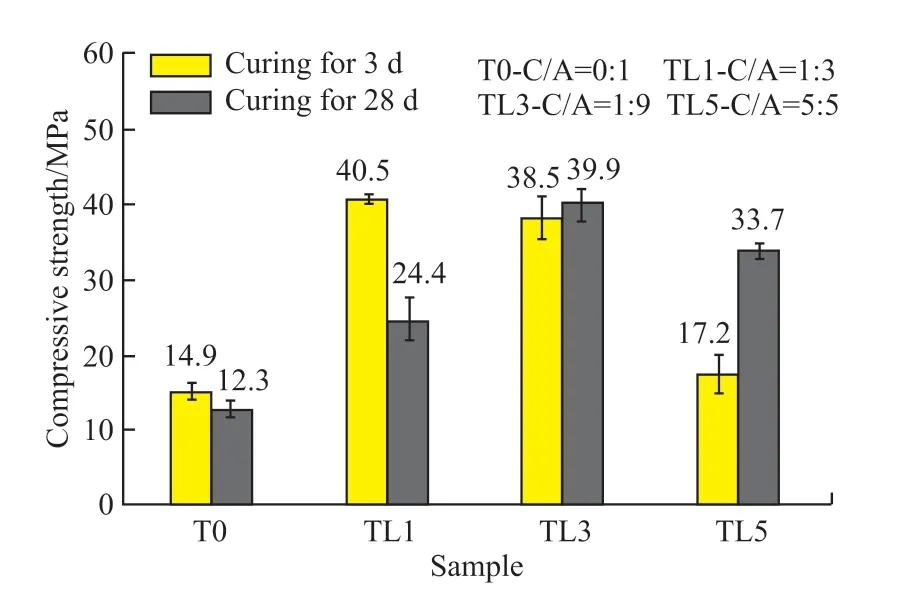
Fig.8 Effect of C/A ratio on the compressive strength of CaCO3-C12A7
After adding calcium carbonate, the mechanical properties of the composite were significantly improved.The compressive strength showed a trend of increasing at first and then decreasing with the increasing content of calcium carbonate after 3 days of hydration.When the content of CaCO3was 10%,the compressive strength reached the highest (40.5 MPa), which was 1.72 times higher than the strength of the C12A7at the same age.The changing trend of compressive strength for 28 days was the same as that of 3 days.When the dosage was 30%, it reached the highest (39.9 MPa).When the C/A ratio was low, the strength of the composite decreased significantly at later stages.
Regarding the compressive strength, some samples have obvious strength decreasing, which is a reasonable phenomenon.The strength of T0 and TL1 is reduced because of insufficient calcium carbonate,and there is a significant crystal transformation.When the calcium carbonate content is 0 or only 10%, the hydrated aluminate does not completely react with calcium carbonate, and cannot completely inhibit the crystal transformation phenomena of aluminate cement.When crystal transformation occurs, a large number of voids are created in the slurry, thus causing strength reduction.The dosage of TL3 is sufficient, and its strength shrinks slightly in the later stage because the slurry is not uniform during the preparation and stirring process, resulting in local crystal transformations.The calcium carbonate in TL5 is sufficient.Hence, there is no strength reduction.
In order to verify the experimental rules, the development curves of the compressive strength of the CaCO3-C12A7system from 3 to 90 d (W/B= 0.5)were explored, as exhibited in Fig.9.The compressive strength of C12A7presented a trend of V-type with curing time.It was inferred to be related to the obvious crystal conversion phenomenon of the C12A7hydration process.
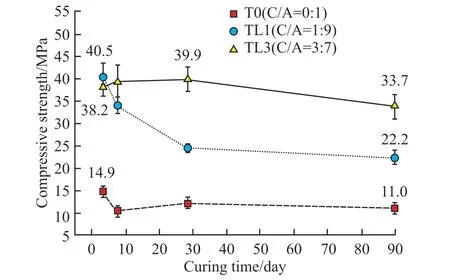
Fig.9 Development of compressive strength in CaCO3-C12A7
The compressive strength of C12A7at all ages increased significantly after adding calcium carbonate.However, the strength decreased in the later period of curing.In particular, the specimens mixed with 10%calcium carbonate began to show a significant strength decline after 7 days.The 90-day compressive strength was 22.2 MPa, 45.2% lower than the strength in the early 3 d (38.2 MPa).However, the strength of the mixture with 30% calcium carbonate increased within 28 days, then decreasing at later stages.Compared with the strength of 3 d (40.5 MPa), the rate of decrease was 15.5%.Therefore, the CaCO3-C12A7mixture was more prone to a decrease in strength with a lower C/A ratio at later stages of hydration.
It can be seen from the formation law of hydrated calcium carboaluminate that the composite would produce a large amount of monocarboaluminate only at the early stage of hydration (3 h).The reaction degree was more obvious when the C/A ratio was 3:7.Therefore, calcium monocarboaluminate would continue to be generated in the mixture when the C/A ratio was high, although it was unstable and decomposed.Calcium monocarboaluminate would inhibit the crystal conversion between hydrated calcium aluminate and increased the solid phase volume of the composite.Thus, the reduction in strength occurring with a high C/A ratio was smaller.
According to the setting time and mechanical properties of the composite, the addition of calcium carbonate would retard the setting of C12A7.The higher the C/A ratio, the greater the retardation degree.However, the mechanical properties of the composite containing calcium carbonate had been significantly improved.
4 Conclusions
a) The hydration reaction between calcium carbonate and aluminate minerals is obvious from the beginning of adding water, releasing great hydration heat.After testing and calculation, the heat release per unit mass of calcium carbonate can reach 60-100 J/g within 72 hours.
b) Calcium carbonaluminate (mainly Mc) could be formed obviously in the early 3 h.When the C/A ratio was 3:7, the amount of calcium carbonaluminate reached the maximum and the reaction degree was the highest (43%).
c) After 28 days of hydration, the main hydration product was hydrated calcium carboaluminate.The hydrated calcium aluminate in the reaction product was not obvious.The compressive strength of the composite was greater than that of pure aluminate minerals.
d) The results illustrate that it is feasible to prepare new low-carbon cementitious material using calcium carbonate and aluminate.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Effect of VEGF/GREDVY Modified Surface on Vascular Cells Behavior
- Evolution of Biofilm and Its Effect on Microstructure of Mortar Surfaces in Simulated Seawater
- Synthesis of Organic-Inorganic Hybrid Aluminum Hypophosphite Microspheres Flame Retardant and Its Flame Retardant Research on Thermoplastic Polyurethane
- Surface Metallization of Glass Fiber (GF) /Polyetheretherketone (PEEK) Composite with Cu Coatings Deposited by Magnetron Sputtering and Electroplating
- Effect of Size Change on Mechanical Properties ofMonolayer Arsenene
- Effects of Sinusoidal Vibration of Crystallization Roller on Composite Microstructure of Ti/Al Laminated Composites by Twin-Roll Casting
