Alkali Tolerance of Concrete Internal Curing Agent Based on Sodium Carboxymethyl Starch
2024-04-10CHENMeihuaLIURongjinCHENPingJINGDaiyanWANDandanFUSiyuan
CHEN Meihua, LIU Rongjin,*, CHEN Ping, JING Daiyan, WAN Dandan, FU Siyuan
(1.College of Materials Science and Engineering, Guilin University of Technology, Guilin 541004, China; 2.Guangxi Engineering and Technology Center for Utilization of Industrial Waste Residue in Building Materials, Guilin 541004, China; 3.Guangxi Maibu New Material Limited Company, Guilin 541004, China)
Abstract: Internal curing agents (ICA) based on super absorbent polymer have poor alkali tolerance and reduce the early strength of concrete.An alkali tolerate internal curing agent (CAA-ICA) was designed and prepared by using sodium carboxymethyl starch (CMS) with high hydrophilicity, acrylic acid (AA) containing anionic carboxylic group and acrylamide (AM) containing non-ionic amide group as the main raw materials.The results show that the ratio of CAA-ICA alkali absorption solution is higher than that existing ICA, which solves the low water absorption ratio of the ICA in alkali environment.The water absorption ratio of CAA-ICA in saturated Ca(OH)2 solution is 95.8 g·g-1, and the alkali tolerance coefficient is 3.4.The application of CAAICA in cement-based materials can increase the internal relative humidity and miniaturize the pore structure.The compressive strength of mortar increases up to 12.95% at 28 d, which provids a solution to overcome the reduction of the early strength.
Key words: alkali tolerance; sodium carboxymethyl starch; internal curing agent; compressive strength
1 Introduction
High strength and high-performance concrete(HSC/HPC) with low water/binder (w/b) ratios are prone to early cracking due to autogenous deformation caused by self-desiccation[1].Self-desiccation is caused by unsaturated humidity formed after the loss of water in the capillary pores inside the concrete.Traditional curing techniques do not allow significant penetration of external water into the loww/bcement-based material due to it’s denser microstructure and low permeability[2].Therefore, water needs to be provided in the cement mixture during the curing process to avoid self- desiccation[3].The internal curing technique,in which water is incorporated into the capillaries by preloading the absorbent material, is considered to be one of the most promising anti-shrinkage and anticracking techniques[4].
Super absorbent polymer (SAP) can be used in the internal curing of concrete, and is the most commonly used internal curing agent (ICA) at present[5,6].An ICA refers to a three-dimensional network polymer that is a water-swellable, water-insoluble, organic, or inorganic material.ICA has excellent water absorption performance and good desorption capacity.An appropriate amount of ICA is added to concrete, to promote a good internal curing effect[7,8].
The alkaline environment in concrete with high pH, complex ions and multivalent states requires ICA to be capable of absorbing alkali solution at powder state and retaining its water at aqueous state, that is,alkali tolerance.Alkali tolerance coefficient is defined as the ratio of ICA’s absorption of tap water to saturate calcium hydroxide, and the smaller the ratio, the better the alkali tolerance.Li[9]and Li[10]synthesized the polyacrylic acid-acrylamide internal curing agent.Under the optimal synthesis conditions, the water absorption ratio was 366-606 g·g-1, and the absorption ratio of saturated calcium hydroxide was 31-62 g·g-1.The water absorption ratio of polymer ICA in alkaline environment is low, while the alkali tolerance coefficient can reach 9-12.A high alkali tolerance coefficient is harmful to the internal curing.
Many studies have found that polymer ICAs have adverse effects on the strength of concrete, especially on early mechanical properties[11,12].Beushausen[13]found that when the water-to-cement ratio was 0.55,the addition of ICA would reduce the early strength of mortar.Zhu[14]and Wang[15]found that when ICA was single-doped, the compressive strength of cement-based materials would be lost 5%-15%.In order to restrain the reducing of early strength, Baloch[16]and Lefever[17]added nano-SiO2to make the mortar structure more dense and compensate for the strength loss by taking advantage of the pozzolanic properties and physical filling effect of SiO2.Zhang[18]introduced lime bulking agent into ICA mortar to promote the formation of micro pores and increase the compressive strength of mortar.
Sodium carboxymethyl starch (CMS) is a kind of anionic starch ether obtained by the etherification of high amylose starch.The molecular chain contains a large number of hydroxyl, carboxyl and ether groups,which have high reactivity, hydrophilic and excellent water retention ability.Some people used different synthesis methods to synthesize CMS based super absorbent polymer, which had a water absorption ratio of more than 1 500 g·g-1and absorbed 0.9% NaCl solution of more than 100 g·g-1.It was used in the food industry, agriculture and wastewater treatment[19-21].Therefore, CMS can be selected as the raw material of ICA.
At present, ICAs have poor alkali tolerance and reduce early strength of cement-based materials.Saturated ICA gel’s alkali tolerance is poor, easy to lose unstable water in the concrete mixing process, and a large amount of water release may lead to concrete bleeding and reducing strength.More importantly,ICA releases water and leaves behind pores that affect strength.Therefore, the molecular structure can be designed to improve alkali tolerance of cementbased materials.The size of ICA is reduced under the premise of guaranteeing the curing effect, and reduces the influence of pores on strength.In this study, the composition and structure of alkali tolerate internal curing agent (CAA-ICA) was selected based on the internal curing environment, and an alkali tolerate internal curing agent with expected water absorptionwater retention-water release properties was prepared by the appropriate synthesis method.The water absorption, water retention and water release properties of CAA-ICA were studied and verified by mechanical properties, internal relative humidity and pore structure analysis of cement-based materials.
2 Experimental
2.1 Materials
The CMS was obtained from Shanghai Roen Chemical Technology Co., Ltd.AA was obtained from Shanghai Aladdin Biochemical Technology Co.,Ltd., while AM, ammonium persulfate (APS), N,N’-methylene bisacrylamide (NMBA), anhydrous ethanol,and sodium hydroxide were provided by Xilong Scientific Co., Ltd.
The cement used was Conch PO42.5 cement.The sand used was ISO standard sand from Xiamen ISO Standard Sand Co., Ltd.The powder polycarboxylate superplasticiser was purchased from Shanghai Qinhe Chemical Co., Ltd.and its basic performance is list in Table 1.A polymer ICA was purchased for the comparative test and the information is list in Table 2.

Table 1 Basic performance of powder polycarboxylate superplasticizer

Table 2 Information of internal curing agents
2.2 Mixing proportion and preparation of specimens
The mixing proportion of mortar used in this study is according to JC/T 2551-2019 (Superabsorbent polymer for internal curing of concrete).Therefore,cement to sand ratio was 1: 2, the ratio of water to cement was 0.35, and the content of the water-reducing agent was 0.07% of the cement mass.CAA-ICA is a kind of concrete admixture, and its dosage is usually 0.3%-0.6% of the binder mass[22].Considering CAAICA’s high viscosity and easy to produce errors when weighing, appropriate dilution was performed before use.The dosage is 2%-5% of the binder mass, and the test was carried out after 100% moisture was deducted.
Mortar specimens for testing mechanical properties were prepared according to GB/T 19671-1999 (Test Method for Strength of Cement Mortar (ISO Method)).The mixed mortar was formed in a mold of 40 mm×40 mm×160 mm, and 3 test blocks were formed in each proportion.After curing for 24 h at an ambient temperature of 20±2 ℃ and relative humidity of 95%, the mortar was released from the mold and sealed until the test age.
When testing the relative humidity inside, after mortar was molded with 100 mm×100 mm×100 mm test moulds, inserted standard bar and set test hole.Standard bar insertion depth was 50 mm to ensure that the bottom of the standard bar be in the mortar center.After 24 h, remove the mold, immediately wrap the test block with plastic wrap, and block the standard bar with white Vaseline to prevent moisture exchange between inside and outside.Then, it was cured in a constant temperature and humidity environment of 20±2 ℃ and 60%±5% relative humidity.
2.3 Measurements
Weigh the dried and purified CAA-ICA(m1) into a beaker, add 1 L tap water, and saturate calcium hydroxide or synthetic pore solution at room temperature for 12 h.After it was fully swelled, the gel mass after saturated water absorption wasm2.The calculation formula of water absorption ratio is shown as follows: Water absorption ratio (g·g-1) = (m2-m1)/m1.
The saturated absorbent gel with the mass ofm1was laid in a dish with the mass ofm2and the diameter of 10 cm.At the humidity was 75%, the mass of the gel and the dish at different temperatures was measured to bem3every 1 h.Calculate the water retention ratio:Water retention ratio = (m2/m1) ×100%.
The water release ratio of CAA-ICA was measured by applying negative pressure.The saturated gel with a mass ofm1was filtered by circulating the water vacuum pump and the remaining gel mass were measured asm2.The water release ratio is calculated as follows: Water release ratio = (m2-m1)/m1×100%.
The mechanical properties were tested by automatic constant stress flexural and compressive testing machine.The internal relative humidity was monitored using Hygropin hygrometer.The internal relative humidity was tested every 24 h with 1%accurate (Fig.1).The pore structure of hardened cement mixed with CAA-ICA was determined using a mercury intrusion porosimeter AutoPore IV 9510.
2.4 Synthesis of CAA-ICA
CAA-ICA was synthesized under the conditions ofm(AA):m(CMS) = 9.33:1,m(AA):m(AM) =2.7:1,m(NMBA) = 0.02957%,m(APS) = 0.37%,neutralization degree = 65%, and reaction temperature 70 ℃, wherem(NMBA) andm(APS) are calculated by the quality of monomer (AA+AM).
The preparation process of CAA-ICA was divided into four steps.Firstly, CMS and deionized water were added into the reactor and stirred at 70 ℃ for 40 min.Secondly, AM was dissolved in deionized water to obtain AM solution for later use.Thirdly, 30% sodium hydroxide was slowly added into the AA to prepare a certain degree of neutralization of sodium acrylate solution.After cooling, initiator and cross-linking agent were added into the prepared sodium acrylate solution,and the mixture was obtained by stirring.Finally, the AM solution was added to the reactor, and after 20 min,the mixture was added to the reactor.The reaction was then continued at 70 ℃ for 1.5 h to obtain the crude product.The crude product was washed with anhydrous ethanol for 2-3 times, dried to constant weight at 60 ℃,and the CAA-ICA powder was obtained by grinding.The schematic diagram of CAA-ICA synthesis is shown in Fig.2.
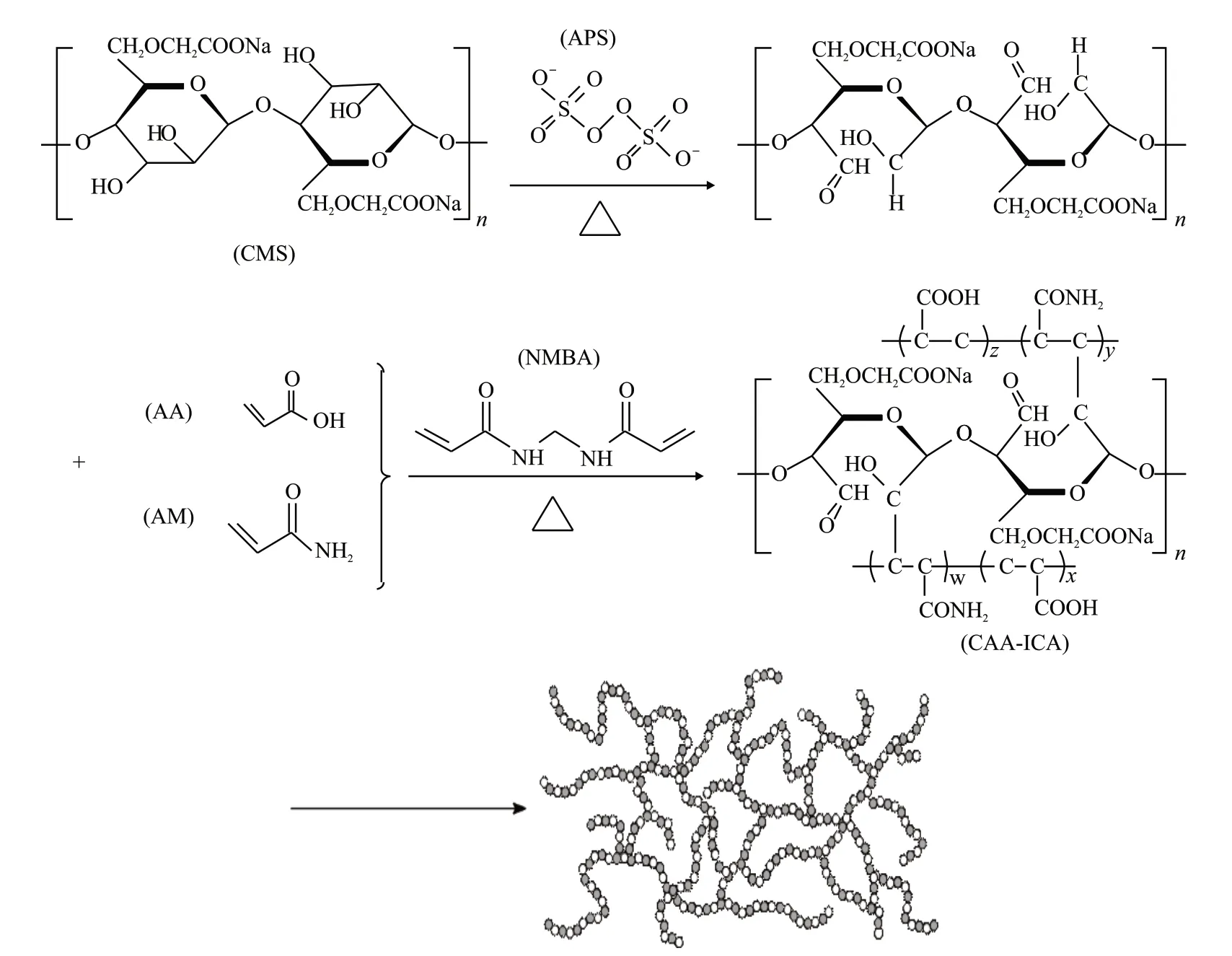
Fig.2 Preparation process of CAA-ICA
3 Results and discussion
3.1 Characteristic of CAA-ICA
3.1.1 Effect of different media on water absorption
Considering that the particle size of similar products is 40-60 meshes, CAA-ICA with the same particle size was used for testing.The water absorption ratio of CAA-ICA with size of 350 μm to tap water,saturated calcium hydroxide solution and synthetic pore solution was determined, as shown in Fig.3.In Fig.3, there was a positive relationship between water absorption ratio and absorption time.The water absorption ratio and rate of CAA-ICA for tap water,saturated calcium hydroxide solution and synthetic pore solution are shown in Table 3.
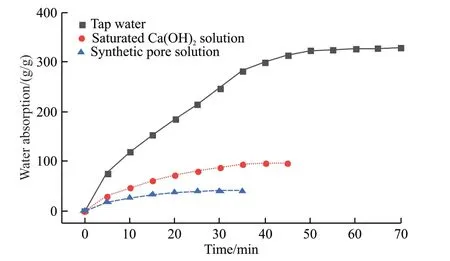
Fig.3 Relationship between water absorbency of CAA-ICA and time in different media
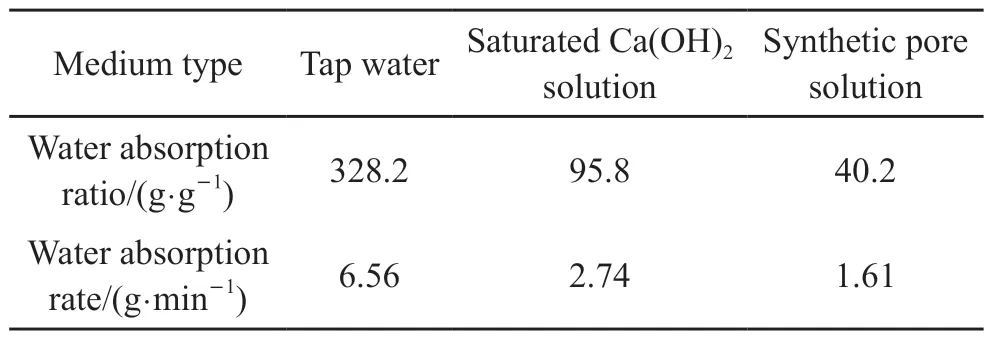
Table 3 Absorbency ratio and absorption rate of CAA-ICA in different media
According to Table 3, CAA-ICA’s absorption capacity and rate in tap water were higher than those of the other two solutions, and decreased with the complexity of ion species.CAA-ICA tap water absorption ratio was 328.2 g·g-1, saturated Ca(OH)2solution absorption ratio was 95.8 g·g-1, synthetic pore solution absorption ratio was 40.2 g·g-1, and alkali tolerance coefficient was 3.4.This is because the calcium ions in saturated calcium hydroxide andsynthetic pore solutions interact with carboxyl groups on CAA-ICA, which reduces the internal charge density and affects the CAA-ICA’s water absorption ratio.The higher the ionic concentration and valence of the external liquid, the lower the osmotic pressure of the CAA-ICA network.Simultaneously, the charge fixed in the CAA-ICA is shielded by external ions,which reduces electrostatic repulsion.Both factors lead to reducing water absorption ratio of CAA-ICA in saturated calcium hydroxide and synthetic pore solution, compared to tap water.
According to Flory’s hydrogel swelling theory,the non-ionic monomer can effectively improve the water absorption ratio of the polymer and improve the salt tolerance[23].The simultaneous introduction of ionic groups and non-ionic monomers can enhance the synergistic effect between different groups and effectively enhance the water absorption capacity.CAA-ICA is mainly synthesised by sodium carboxymethyl starch grafted anionic monomer acrylic acid and non-ionic monomer acrylamide.The anions and non-ions weaken the ionic effect between CAAICA and various ions in aqueous solution, and promote the movement of chain sections and diffusion ability in the network structure when CAA-ICA absorbs water.In terms of composition, after -COOH and -CONH2hydrophilic groups were grafted onto carboxymethyl starch sodium -C-C- backbone, water molecules can be adsorbed to CAA-ICA molecules through hydrogen bonding.In addition, -CONH2has good hydrolytic stability, alkaline resistance and thermal stability, and their compound superposition greatly reduces the sensitivity of the polymer network in alkaline solution.CAA-ICA has higher alkali tolerance compared with existing ICAs, indicating that it can better adapt to the complex alkali environment of concrete.
3.1.2 Water retention ratio at different temperatures
Water retention ratios of CAA-ICA with particle size of 350 μm were tested at different temperatures and 75% humidity.The results are shown in Fig.4.
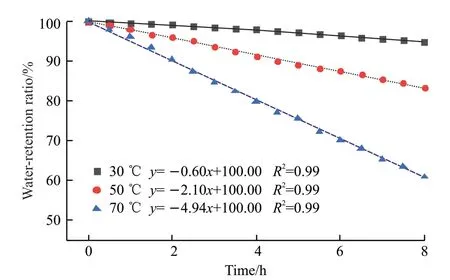
Fig.4 CAA-ICA water retention ratio changes with time
The water retention ratio of CAA-ICA decreased as temperature increased, and the higher the temperature, the lower the water retention ratio.The water retention ratio of CAA-ICA had a linear relationship with time at different temperatures.Through linear fitting, the CAA-ICA linear equation of water retention ratio and time can be obtained, and water retention ratio at different time or temperatures can be estimated as follows:
where,Wis the water retention ratio (%) andtis time.
CAA-ICA water retention ratios after 8 h were 94.80% and 60.88% at 30 and 70 ℃, respectively.These results showed that CAA-ICA possesses good water retention in a certain temperature range, because the CMS molecular chain contains a large number of hydroxyl, carboxyl and ether groups, all of which are polar hydrophilic groups, which impart high reactivity and hydrophilicity.Concurrently, these groups can bind to free water through hydrogen bonding, and the intermolecular forces make it difficult for water molecules to escape from the structure.In the graft copolymerization of CAA-ICA, crosslinking agent was added, and the spatial network structure was formed in CAA-ICA, and its water retention ability was enhanced.However, with the increase of temperature, the external energy increases and the moisture volatilization accelerates.When the temperature is too high, the chemical and hydrogen bonds in CAA-ICA begin to break, and the three-dimensional network structure is destroyed, resulting in the low water retention ratio of CAA-ICA at high temperature.
3.1.3 Water release capability
Release of water in the alkali environment is the key to reflect the internal curing function of CAA-ICA.Water release ratio is the proportion of water released by the gel under certain external effects.The greater the water release ratio, the better the internal curing effect.Negative pressure water release ratios of CAA-ICA with different particle sizes were investigated at 25 ℃,75% humidity and a vacuum filtration pressure of 0.02 MPa, and the results are shown in Fig.5.
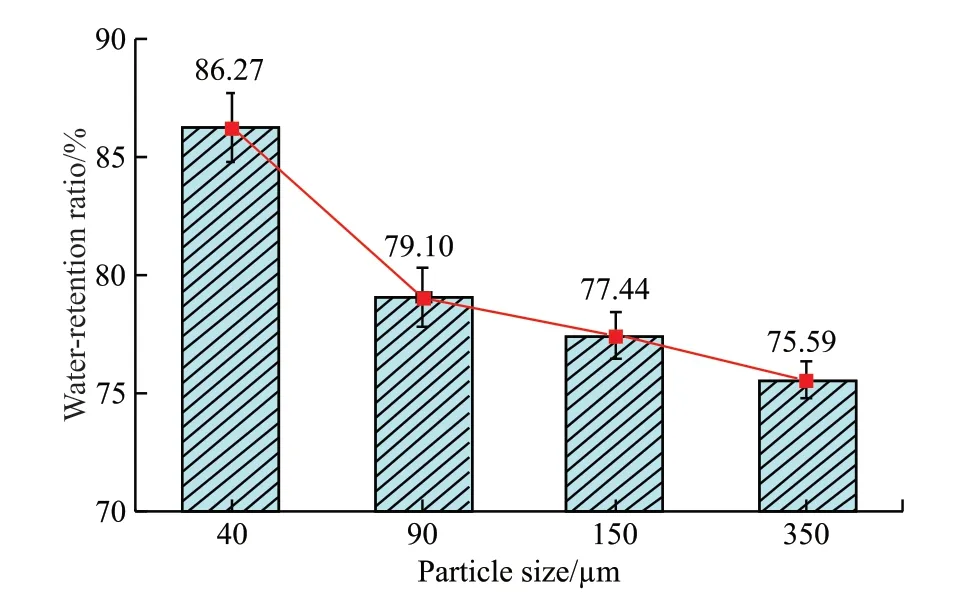
Fig.5 Relationship between CAA-ICA particle size and water release ratio
As can be seen from the Fig.5, as the particle size of CAA-ICA increased, its water release ratio decreased.With particle size of 40 μm, the water release ratio was 86.27%, and at particle size of 350 μm, the water release ratio was 75.59%, decreasing 10.68%.When subjected to external pressure,compared with the CAA-ICA with large particle size,the distance of water inside the CAA-ICA with small particle size to the outside becomes shorter and the binding force of water molecules is weakened by the three-dimensional network structure, so the water molecules are easier to release and the rate of water release increases.CAA-ICA water release ratio was greater than 75%, indicating that when the gel is dry inside and outside the concrete or in the state of capillary negative pressure, CAA-ICA gel can release most of the water absorbed by it for internal curing.3.1.4 Comparison of similar products
The water absorption, water retention and water release properties of CAA-ICA and similar products were compared respectively, and the results are shown in Table 4.CAA-ICA had higher absorption capacity of tap water, saturated calcium hydroxide solution and synthetic pore solution than XT, which was 2.52, 1.74,and 1.49 times, respectively.There was little difference in water retention ratio between them at 30 and 50℃, which were about 95% and 83%, respectively.However, the water retention ratio of CAA-ICA was only 60.9% after 8 h at 70 ℃, while XT was 70.4%.The water retention capacity of CAA-ICA was worse than XT at high temperature.The water release ratios of the two ICAs with the same particle size were 75.6%and 81.2% respectively, and XT was slightly higher than CAA-ICA.

Table 4 Performance comparison of similar products
CAA-ICA had better water absorption capacity,especially in alkali environment, while the water retention capacity and water release capacity ICAs were not different compared with XT.Therefore, CAAICA has an application prospect in the internal curing of concrete.
3.2 Influence of CAA-ICA dosage on mechanical properties of mortar
Fig.6 shows the change of mortar’s mechanical properties with the dosage of CAA-ICA at different curing ages.The addition of CAA-ICA reduced the flexural strength of mortar.The flexural strength of the reference sample at 3, 7 and 28 d was 7.5, 8.2 and 11.1 MPa, and CAA-ICA reduced the flexural strength by 9.3%, 8.5%, and 8.1%, respectively.The addition of CAA-ICA did not have a significant adversely influence on the compressive strength of mortar.When the additional dosage of CAA-ICA was less than 5%,the compressive strength of mortar was similar to the reference sample at each curing age.Compared with the existing ICAs, CAA-ICA had a less adverse effect on mortar strength, indicating that CAA-ICA exhibited advantages in retaining the strength of mortar.The decreasing amplitude in the compressive strength of CAA-ICA mortar decreased as time increased.For example, the sample with 5% CAA-ICA dosage exhibited a decrease in compressive strength of 5.9%, 4.7%, and 3.2% at 3, 7, and 28 d, respectively,compared with the reference sample.It showed that CAA-ICA can promote the development of 7-28 d strength of mortar.

Fig.6 Mechanical properties of mortar in relation to CAA-ICA dosage
This is due to the early absorption of the effective free water in the mortar by CAA-ICA, and the volume expansion forms holes in the mortar interior, which affects the compactness of the slurry and adversely affects the strength of the mortar.As the curing time extended, the cement hydration leads to insufficient internal relative humidity.When the external moisture is difficult to penetrate, the water absorbed by CAAICA is released to maintain the humidity inside the mortar, promote cement hydration, and reduce the strength reduction caused by shrinkage caused by negative pressure of capillary pores.Therefore, in the later stage, the pore structure of CAA-ICA mortar was refined, and the strength advantage compared with the reference sample was gradually reflected.
In general, the effect of the addition of CAA-ICA on the compressive strength of mortar was less than flexural strength and CAA-ICA had a positive effect on the compressive strength of mortar at 7-28 d.These results indicated that CAA-ICA improved the internal curing effect in the hydration process of cement, but the dosage of CAA-ICA should not be too large, and it was recommended to control the CAA-ICA dosage within the range of 3%-4%.
3.3 Influence of CAA-ICA on internal relative humidity of mortar
The 7 d internal relative humidity (IRH) of CAAICA mortar was determined by a hygrometer.Fig.7 shows the variation in IRH of the reference sample and the mortar mixed with CAA-ICA under sealed conditions.The IRH of the mortar decreased as the age increased and the IRH was positively correlated with the dosage of CAA-ICA.The small difference in IRH between the reference sample and the CAA-ICA mortar after 7 d indicated that CAA-ICA released most of its adsorbed water for compensation of the cement paste humidity loss.Compared to the reference sample,CAA-ICA inhibited the reduction of IRH.At 7 d, the IRH of the reference sample was 79.58%, a decrease of 20.42%; when 4% CAA-ICA was added, the IRH was 80.24%, a decrease of 19.76%.
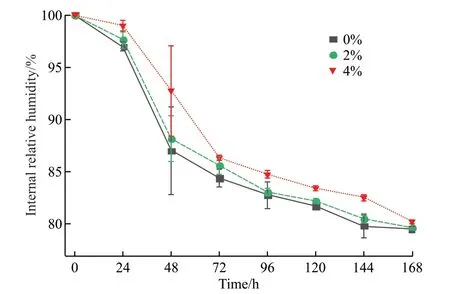
Fig.7 Effect of CAA-ICA on IRH of mortar
The addition of CAA-ICA makes the mortar more capable of maintaining the internal relative humidity.CAA-ICA has a high water release ratio and absorbs mixed water when it is mixed into the mortar.When cement hydration consumes water, most of the water inside CAA-ICA can be released, so the internal relative humidity is higher than that of a reference sample.
3.4 Influence of CAA-ICA on pore distribution of cement paste
The pore distribution results of the reference sample and the experimental sample with a dosage of 4% CAA-ICA are shown in Fig.8 and Table 5.The peak of the pore size distribution curve after CAA-ICA addition was lower than that of a reference sample,indicating that CAA-ICA can reduce the total pore volume of mortar.At 7 d, the maximum pore size of the experimental sample and the reference sample was about 60 nm.With the increase of curing age, at 28 d,the maximum pore size of the reference sample was about 32.4 nm, and the experimental sample was 21.1 nm, indicating that the CAA-ICA can refine pore size.
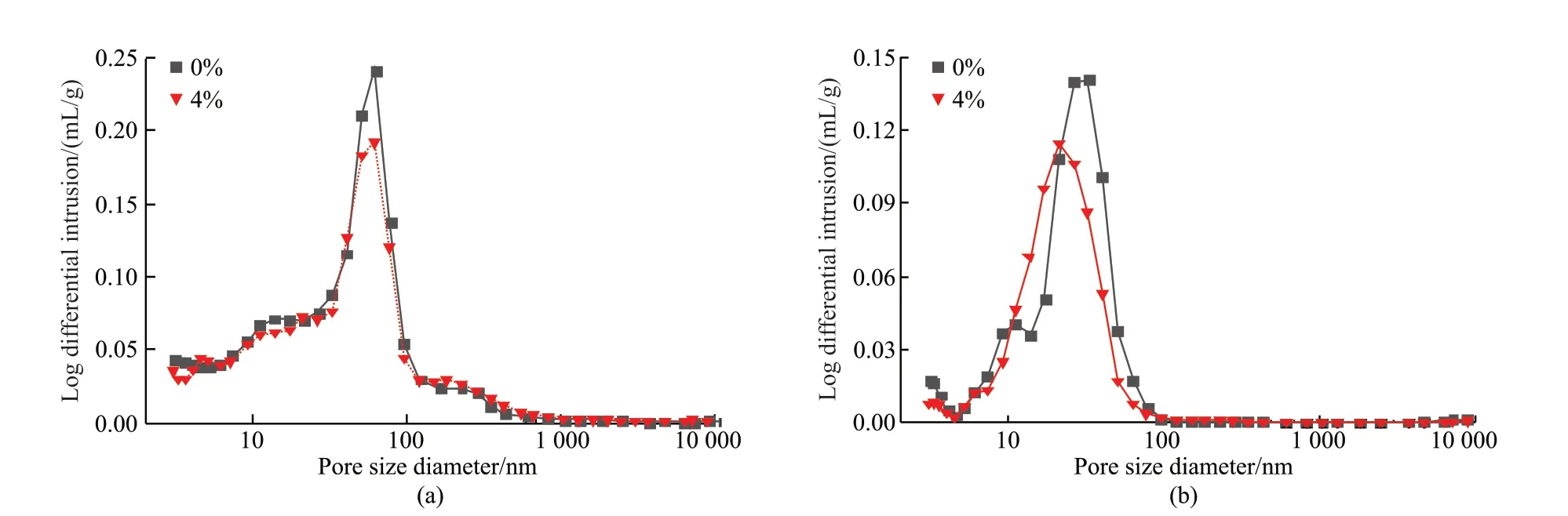
Fig.8 Pore size distribution of CAA-ICA cement paste

Table 5 Percentage of different pore sizes of hardened cement paste
At 7 d, the pore size distribution of the reference sample was 3-200 nm, and the pore size below 200 nm accounting for 95.30%; while experimental sample’s pore size below 200 nm reached 93.31%, lower than the reference sample.Therefore, the strength of mortar added with CAA-ICA decreased.At 28 d, with the hydration pores of cement were gradually filled by hydration products, the pore size distribution was 3-50 nm.The pore size of the reference sample below 50 nm was 91.33%, while the experimental sample was 94.51%, which is higher than the reference sample.The pore size distribution of harmful pore and more harmful pore of the experimental sample were both lower than the reference sample, which further proved that CAAICA had a promoting effect on the later strength of mortar.
Considering CAA-ICA has a certain volume,holes can be created in cement-based materials after water release.Therefore, the particle size of CAA-ICA in the liquid state was tested by Nano-ZS type Nano particle size and Zeta potential analyser produced by Malvern Company in the UK and the results are shown in Fig.9 and Table 6.The particle size distribution of liquid CAA-ICA was concentrated in 340-650 nm,accounting for 74.71%, which played a mainly role in the pore structure of cement paste.
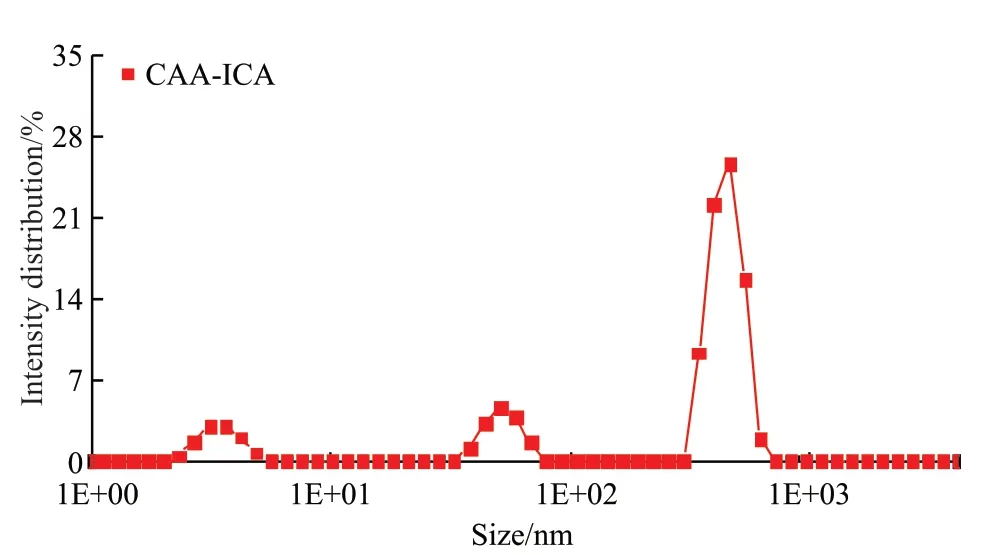
Fig.9 Particle size distribution of liquid CAA-ICA

Table 6 Particle size distribution for liquid CAA-ICA
CAA-ICA absorbed mixed water and reduced the water-cement ratio when specimen forming, then it released water and produced holes at the early age of hydration.The reduction of water-cement ratio and the promotion of cement hydration had a weaker effect on strength improvement than the reduction of strength by holes.Therefore, the strength of mortar at 7 d was slightly lower than the reference sample.At the late hydration age, CAA-ICA water release could fully maintain the mortar’s IRH, which on the one hand improved the degree of cement hydration and promoted the generation of ettringite.Hydration products filled the pores of hardened cement paste, reduced the porosity and increased the compactness.On the other hand, the degree of hydration around the holes was improved, which caused the decreasing effect of the holes on the strength.Therefore, CAA-ICA has little effect on the negative effect of mechanical properties of cement-based materials.
4 Conclusions
a) The water absorption ratio of CAA-ICA in different media solutions increased with time, and decreased with the increase of ions of type.CAA-ICA tap water absorption ratio was 328.2 g·g-1, saturated Ca(OH)2solution absorption ratio was 95.8 g·g-1,and alkali tolerance coefficient was 3.4.The water absorption ratio of CAA-ICA in alkali environment was higher than existing internal curing agent and could improve concrete internal curing efficiency.
b) When the CAA-ICA dosage was less than 5%, the compressive strength of the mortar was comparable to the reference sample.As the curing age increased, the decline in compressive strength was inhibited.With the increasing of CAA-ICA dosage,the flexural strength of mortar initially increased and then decreased.CAA-ICA can effectively promote the enhancement of mortar strength at 7-28 d and 28 d compressive strength of mortar can increase up to 12.95%.
c) CAA-ICA can improve the internal relative humidity of mortar.With the increase of CAA-ICA dosage, the higher the internal relative humidity, the better the curing effect.When the dosage was 4%, the internal relative humidity was 80.24% at 7 d.CAAICA miniaturized the pore diameter of hardened cement paste, and the maximum pore size decreased from 32.4 nm to 21.1 nm at 28 d.The improvement of internal relative humidity and miniaturization of pore diameter further proved the internal curing effect.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Effect of VEGF/GREDVY Modified Surface on Vascular Cells Behavior
- Evolution of Biofilm and Its Effect on Microstructure of Mortar Surfaces in Simulated Seawater
- Synthesis of Organic-Inorganic Hybrid Aluminum Hypophosphite Microspheres Flame Retardant and Its Flame Retardant Research on Thermoplastic Polyurethane
- Surface Metallization of Glass Fiber (GF) /Polyetheretherketone (PEEK) Composite with Cu Coatings Deposited by Magnetron Sputtering and Electroplating
- Effect of Size Change on Mechanical Properties ofMonolayer Arsenene
- Effects of Sinusoidal Vibration of Crystallization Roller on Composite Microstructure of Ti/Al Laminated Composites by Twin-Roll Casting
