Controllable Synthesis of Au NRs and Its Flexible SERS Optical Fiber Probe with High Sensitivity
2024-04-10XIONGWenhaoWANGWenboLONGYutingLIHong
XIONG Wenhao, WANG Wenbo, LONG Yuting, LI Hong*
(1.State Key Laboratory of Silicate Materials for Architectures, Wuhan University of Technology, Wuhan 430070, China; 2.National 111 Research Center Microelectronics and Integrated Circuits, School of Science, Hubei University of Technology, Wuhan 430068, China)
Abstract: The surface-enhanced Raman scattering (SERS) optical fiber probes were successfully prepared by self-assembling on polyelectrolyte multilayers.Gold nanorods (Au NRs) were used as SERS enhancement material to give excellent biological affinity and stability to the SERS optical fiber probes.Au NRs were synthesized by seed growth method.The synergistic effect between AgNO3 and surfactant was investigated, and the highest yield was found when AgNO3 was 500 uL.Meanwhile, different SERS optical fiber probes were obtained by selecting silane coupling agent, polyelectrolyte multilayer and graphene oxide(GO) to treat quartz fiber.It was found that the SERS optical fiber probes obtained by the self-assembled on polyelectrolyte multilayers method performed better than those by other methods.In addition, Mapping was combined with finite element simulation to analyze the electromagnetic field distribution at the fiber end face.The electromagnetic field distribution of Au NRs was investigated, the difference of electromagnetic field intensity around the Au NRs with different arrangements was compared, the strongest signal was obtained when the Au NRs were head-to-head.Finally, sensitivity of the optimized SERS optical fiber probes could reach 10-9 mol/L, with excellent stability and repeatability.
Key words: surface-enhanced Raman scattering (SERS); optical fiber probe; gold nanorods (Au NRs);polyelectrolyte multilayers; controllable synthesis
1 Introduction
Nanomaterials have made trace detection of pollutants possible due to their unique properties[1].Noble metal nanoparticles are widely used in sensing[2], energy harvesting[3], biomedicine[4]and catalysis[5]due to their surface plasmon resonance (SPR).
Surface-enhanced Raman scattering (SERS) is used as a common means of trace detection in which noble metal nanoparticles play an irreplaceable role.When noble metal nanomaterials are exposed to incident light radiation, the free electrons on the surface are excited because the wave of incident light is longer than the diameter of noble metal nanomaterials and interaction between photons and the free electrons on the surface of the material.As a result, free electrons will vibrate at a certain frequency on the surface of the nanoparticles, which is called locatized surface plasmon resonance (LSPR)[6,7].It is closely related to the shape, size, surface charge and incident electromagnetic field of nanomaterials.Because of LSPR, the localized electromagnetic field intensity on the surface of nanomaterials is amplified by two to five orders of magnitude[8].The molecules in close contact with noble metal nanomaterials are affected by laser, scattering Raman signals, which are further amplified under the influence of LSPR.This phenomenon is called electromagnetic enhancement, which is also an important part of SERS[9].As another important part of SERS,chemical enhancement had many influencing factors,which is mainly related to the charge transfer between precious metal nanoparticles and adsorbed molecules.Its contribution to SERS is far less than electromagnetic enhancement, and for different molecules to be tested, their interaction with the metal surface is also different[10].Therefore, signal amplification caused by chemical enhancement is not universal.
As a powerful optical detection method, SERS technology has been widely used in the detection of trace substances due to its high sensitivity[11].The traditional SERS substrate mostly used glass as the carrier,and the enhanced Raman signal was generated by direct contact between laser and SERS substrate.However, this simple and convenient testing method also has its shortcomings.The existence of the planar substrate limits its application and has more stringent requirements for testing conditions.In order to deal with more complex environment and the needs of various occasions, SERS optical fiber probes have gradually become a more appropriate choice[12].The introduction of optical fiber undoubtedly pushed SERS to a broader platform, and different kinds of optical fiber also enriched the selection of SERS optical fiber probes.Long[13]made a novel methimazole-functionalized SERS optical fiber probe for the first time for Cr (VI)determination with ultrahigh sensitivity.Wang[14]proposed a SERSin situdetection method for non-volatile complex liquid-phase systems by high-performance SERS optical fiber probes.The development of SERS optical fiber probes greatly promoted the development of SERS.It is precisely due to its advantages such as small size, flexible use, strong anti-electromagnetic interference and remote communication ability.SERS has gained new development in the field ofin situdetection, biomedicine and other fields[15].
Currently, challenges remain in the preparation of SERS fiber optic probes with optimal performance.The properties of noble metal nanomaterials and interface assembly have a significant impact on the performance of SERS fiber probes.Among the recently reported noble metal nanoparticles, gold nanoparticles have been favored by the majority of researchers[16,17].As the most stable metal nanoparticles, it has excellent biological affinity, which provides the possibility for the detection of molecules in organisms.Among numerous gold nanomaterials, Au nanorods (Au NRs) exhibit unique optical properties, such as high refractive index sensitivity and controllable longitudinal plasma resonance absorption peak, which can be achieved by adjusting their aspect ratio, thus making them display excellent characteristics as SERS sensing[18].
A large number of methods have been developed for the preparation of SERS optical fiber probes, which are mainly classified into physical and chemical methods.Physical methods refer to the use of advanced instrumentation to directly control the deposition of nanomaterials on the fiber end surfaces, such as thermal evaporation[19], oblique angle deposition methods[20]and magnetron sputtering[21].Although these methods can yield SERS fiber probes with good reproducibility, the equipment is expensive and severely limits their development and application.Chemical methods are used to treat optical fibers with silane coupling agents[22], polyelectrolytes[23]and self-assemble nanomaterials by electrostatic adsorption.This method is of very low cost but suffers from sensitivity, reproducibility problems.
In this paper, we prepared Au NRs by seed growth method, and obtained SERS optical fiber probes with high sensitivity, stability and repeatability by self-assembling on polyelectrolyte multilayers method.By changing the contents of AgNO3, AA, HCl and seed solution, the effects of each component on the morphology of Au NRs were analyzed.AgNO3and CTAB assisted to regulate the transverse and longitudinal ratio of Au NRs and to analyze the growth mechanism of Au NRs.Subsequently, the optical fiber end face was modified by silane coupling agent modification, graphene oxide (GO) and polyelectrolyte adsorption, and the best adsorption method was selected by combining with SEM images of SERS optical fiber probes and SERS signal intensity.The optimized SERS optical fiber probes for crystal violet (CV) detection showed a limit concentration of 10-9mol/L and had excellent stability and repeatability.Finally, combined with the Mapping analysis of SERS optical fiber probe, the SERS hot spots distribution was analyzed.The surface plasma distribution of Au NRs under electromagnetic field was simulated and the enhancement effects of Au NRs under different conditions were compared.
2 Experimental
2.1 Materials
Gold chloride trihydrate (HAuCl4⋅3H2O), silver nitrate (AgNO3), hexadecyl trimethyl ammonium bromide (CTAB), sodium borohydride (NaBH4), ascorbic acid (AA), poly dimethyl diallyl ammonium chloride (PDDA), poly sodium 4-styrenesulfonate (PSS),(3-mercaptopropyl) trimethoxy-silane (MPTMS),hydrochloric acid (HCl), and crystal violet (CV) were purchased from Shanghai Aladdin Chemical Co.LTD.Deionized water was used in all experiments.
2.2 Synthesis of Au NRs
Controlled synthesis of Au NRs by seed growth method[24].Firstly, 250 μL HAuCl4⋅3H2O (10 mmol/L)was added to 9.3 mL CTAB (0.1 mol/L) solution.Then 0.6 mL of NaBH4(10 mmol/L) aqueous solution (in ice water configuration) was added.The solution turned light brown in color, stirred for 2 minutes, and left for 1-2 hours in a water bath at 30 ℃.Subsequently, 2.5 mL of HAuCl4was added to 47 mL of CTAB water solution.Then added AgNO3, HCl and AA solution of different volumes, the solution became colorless immediately, stir for two minutes.Added 120 μL seed solution to the growth solution, stirred for 1 minute, and then placed the solution in a water bath at 30 ℃ for 12 hours.
2.3 Preparation of SERS optical fiber probe
Three different optical fiber treatments were used.First, the cleaned optical fibers were soaked in piranha solution (H2SO4:H2O2=7:3), then rinsed with a large amount of deionized water and dried in an oven for 1 hour.The dried optical fibers were immersed in PDDA and PSS aqueous solutions in turn.Another part of the cleaned optical fibers was also treated with piranha solution, and then soaked in MPTMS ethanol solution with a volume fraction of 5%.The third method were to soak the cleaned fiber probes in a 0.5 mg/mL GO solution for 2 hours, then cleaned the fibers with deionized water and blew dry it with nitrogen.Finally, all the treated fibers were immersed in Au NRs solution to obtain SERS optical fiber probes.
2.4 SERS detection principle of optical fiber probe
The prepared SERS optical fiber probes were processed into an appropriate size for subsequent Raman detection.The SERS optical fiber probes were placed on a measuring table with one end attached to the nanomaterial facing down and the other end focused with an objective lens.Next, by setting the test conditions of the Raman spectrometer, 633 nm laser excitation was selected, the laser power was set to 100%, and the integration time was set to 4 s.During measurement,the laser was coupled through the objective lens into an optical fiber, transmitted through the optical fiber to the other end and interacted with the Au NRs.When the Au NRs were excited by laser, SPR and LSPR were generated on the surface, and the Raman signals generated by the adjacent molecules under test were amplified.The amplified Raman signals were sent back to the detector through the optical fiber, which was analyzed and processed by the computer to get the desired signals.As shown in Fig.1.
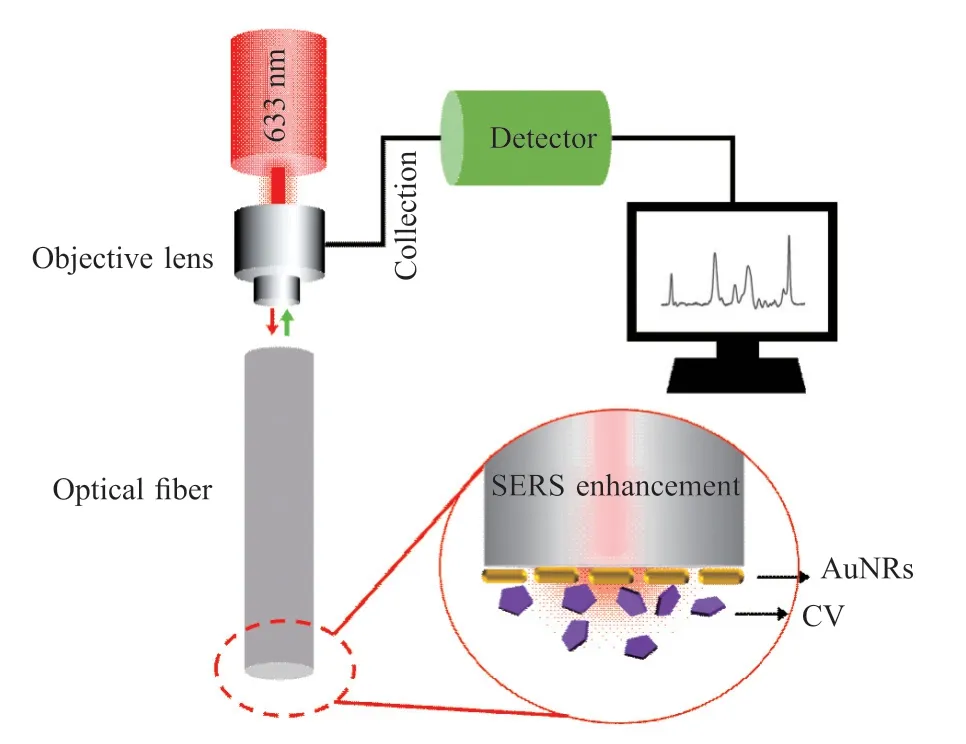
Fig.1 SERS detection and mechanism of SERS optical fiber probe
2.5 Characterization
Ultraviolet visible-visible spectrophotometer (UV-2600, Shimadzu, Japan) was used to characterize the ultraviolet-visible(UV-Vis) absorption spectra of different Au NRs.The adsorption of Au NRs on fiber end face was observed by scanning electron microscope(FE-ESEM).Tenia G2 F30 S-TWIN transmission electron microscopy (TEM) was used to characterize the morphology and size of Au NRs, and high-resolution images (HRTEM) was obtained to measure the lattice spacing.LABHRAN HR Evolution laser confocal Raman spectrometer was used to characterize the SERS properties of SERS optical fiber probes.
3 Results and discussion
3.1 Morphology regulation of Au NRs
During the synthesis of Au NRs, AgNO3was added to modulate the aspect ratio of the Au NRs.Au NRs with different aspect ratios were obtained by adding 300, 400, 500, 600, 700, and 800 μL AgNO3.Fig.2(a)showed the ultraviolet absorption spectra of the Au NRs with different aspect ratios.It can be seen that the Au NRs have two plasmon resonance absorption peaks.The transverse one located around 520 nm and the longitudinal one gradually red-shifted from 671 to 1 004 nm as the amount of AgNO3changes from 300 to 800 μL.
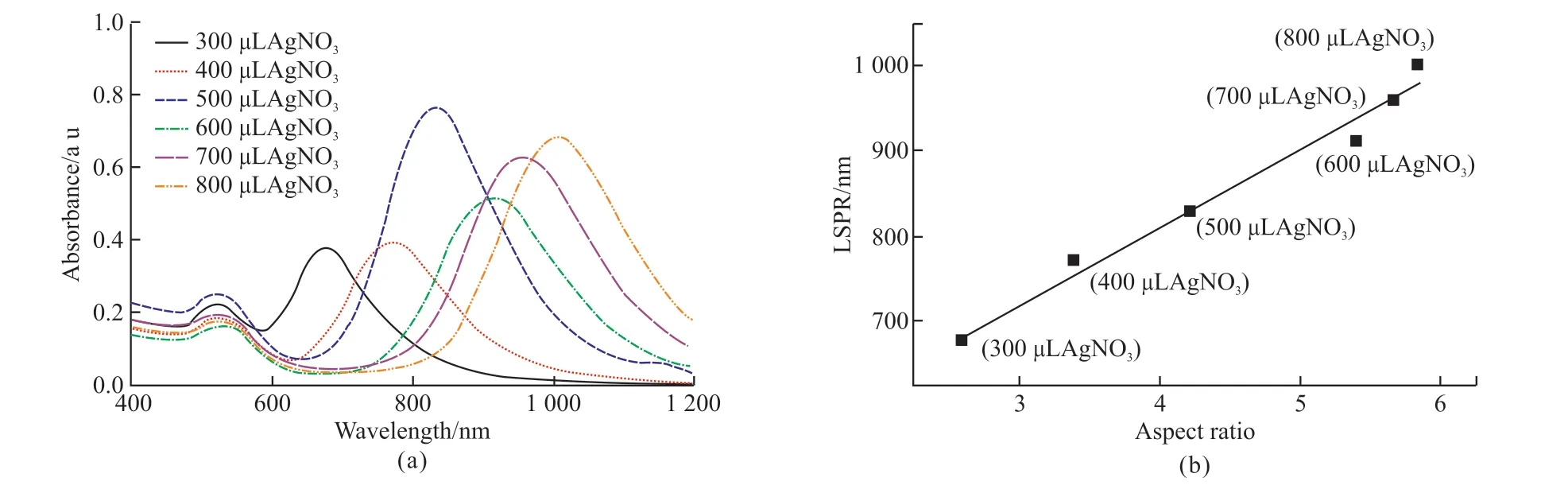
Fig.2 (a) Ultraviolet absorption spectra of Au NRs with different sizes; (b) Relationship between longitudinal LSPR and AR
The microstructures of Au NRs were characterized using TEM, revealing that the aspect ratio of the Au NRs increased in correlation with the red-shift of the longitudinal plasmon resonance absorption peak.A large number of Au NRs with various ratios were selected for dimensional measurements, and the relationship between longitudinal plasma resonance absorption peak and aspect ratio of Au NRs was obtained,as shown in Fig.2(b).
With the red-shift of the longitudinal plasma resonance absorption peak, the aspect ratio of the synthesized Au NRs also increases.AgNO3plays a key role in the longitudinal growth of Au NRs.Fig.3 shows the TEM images of Au NRs corresponding to 300, 400,500, 600, 700, and 800 μL of different AgNO3addition.Combined with the UV absorption spectra of Au NRs,it can be seen that the ratio of the intensity of the two absorption peaks reflects the good homogeneity of Au NRs, and the optimal addition amount of AgNO3is 500 μL

Fig.3 TEM images of Au NRs with different AgNO3 addition: (a)300 μL; (b) 400 μL; (c) 500 μL; (d) 600 μL; (e) 700 μL; (f)800 μL
Currently, the mechanism of AgNO3in the synthesis of Au NRs is still controversial[25].Firstly, The CTA-Br-Ag+complexes were proposed, which prevent the growth of Au NRs along the side, so the growth of Au NRs along the longitudinal is preferred[26].The second mechanism is Ag+underpotential deposition (UPD),which proposes that monolayers of Ag (0) are preferentially deposited in the longitudinal plane of growth,favoring anisotropic growth[27].In a third proposed mechanism, the CTAB micelle becomes cylindrical in the presence of silver ions, bromide ions, and gold seeds.The gold monomers are added to the seed and the CTAB micelles act as soft templates to control the synthesis of Au NRs (Fig.4).

Fig.4 (a) CTA+-Br-Ag+ complex capping agent; (b) Ag+underpotential deposition; (c) Ag+ assisted the formation of CTAB micelles
Furthermore, the impact of different components on the growth of Au NRs was further elucidated by adjusting other raw materials during the synthesis process.During the synthesis of Au NRs, HCl was added mainly to adjust the pH of the solution, which affects the aspect ratio of Au NRs[28].This phenomenon arises from the fact that the concentration of H+directly influences the size of CTAB micelles.With the increase of H+, the size of CTAB micelles become longer, and the longitudinal growth rate of Au NRs were faster than the transverse growth rate, thus increasing the aspect ratio of Au NRs.And the longitudinal plasmonic resonance absorption peak of Au NRs is red-shifted, as shown in Fig.5(a) and Fig.5(d).However, with the decrease of pH, the reducing ability of AA is constantly weakened,which slowed down the reduction rate of Au3+to Au+.As a result, Au+in solution is insufficient, resulting in the reduction of Au0content.Therefore, gold seeds do not have enough Au0to enable the growth of Au NRs,resulting in a large number of spheres in solution.

Fig.5 UV-Vis absorption spectra of Au NRs with different amounts of (a) HCl; (b) Seed solution; (c) AA; The relationship between (d) HCl; (e)Seed solution; (f) AA addition and Au NRs LSPR
Fig.5(b) shows the different UV-Vis absorption spectra obtained by changing the amount of AA added[29].As a relatively weaker reducing agent, AA plays a crucial role in controlling the growth rate of Au NRs and determining their final size.In the growth solution,AA first reduces Au3+to Au+, making the growth solution colorless.With the addition of seed solution, AA reduce Au+to Au0under the catalysis of the seed solution, and Au0make the gold seeds grow into Au NRs.As the AA content increases, the reduction rate of Au3+increases, which makes Au NRs grow along the longitudinal direction and the longitudinal plasma resonance absorption peak is red-shifted, as shown in Fig.5(e).However, when the reduction rate is too high, dog bone gold nanoparticles will be formed in the solution.
Fig.5(c) shows the UV-Vis absorption spectra of different Au NRs obtained by changing the amount of seed solution introduced[30].By manipulating the number of crystal nuclei introduced, the quantity of gold atoms deposited on each crystal nucleus decreases as the number of nuclei increases, resulting in smaller diameter Au NRs.As a result, the aspect ratio of Au NRs increases and the longitudinal plasmon resonance absorption peak is red-shifted, as shown in Fig.5(f).When the content of the seed solution is too high, the yield of the prepared Au NRs will be low and there will be many impurities because the content of gold atoms allocated to each gold seed is too small.
As shown in Fig.6, the nanomaterials were observed through TEM, and the rod-like morphology could be clearly seen.Under high resolution, the lattice spacing could be observed.The spacing of five crystal faces were measured, and the crystal face spacing was obtained and averaged.Standard PDF card was compared and identified as the (200) crystal group of Au NRs.
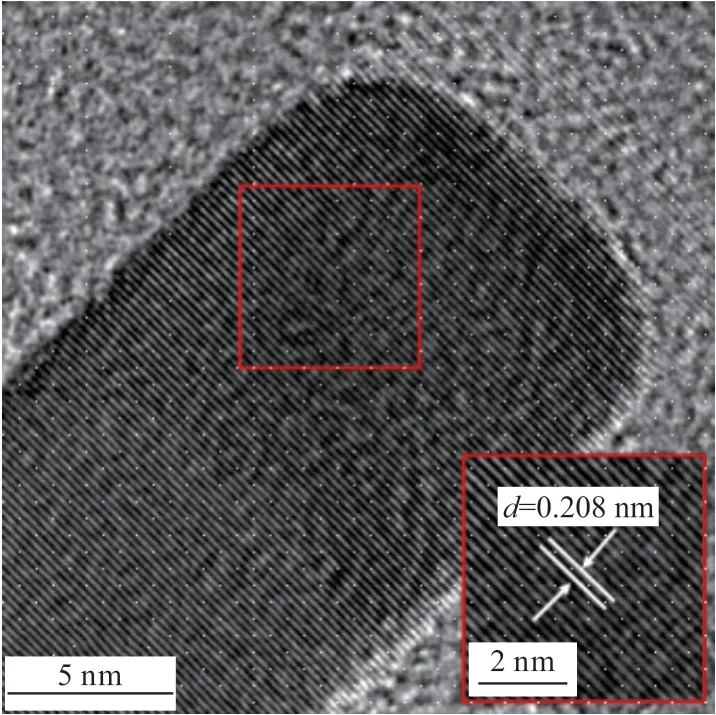
Fig.6 HRTEM image of Au NRs
3.2 Characterization of different processing methods SERS optical fiber probes
The preparation of SERS optical fiber probes involves two key steps.One of the most important steps is the stable adsorption of the nanomaterial onto the fiber end face, which directly determines the performance of the SERS optical fiber probe.Before preparing SERS optical fiber probes, the fibers were typically treated with piranha solution for half an hour to make the fiber cleaner and more easily retouched.
The preparation of SERS optical fiber probes can be achieved through various methods.The first one uses polyelectrolyte multilayers method.In this method, one end of the fiber was treated with PDDA solution to make it positively charged, and then treated with negatively charged PSS solution to make the end face of the fiber negatively charged.Electrostatic self-assembled SERS optical fiber probes were obtained by soaking negatively charged fiber in positively charged Au NRs solution.As shown in Fig.7, the optical fiber end face was observed by SEM.Au NRs were uniformly distributed at one end of the optical fiber, which was significantly different from the untreated optical fiber end face.
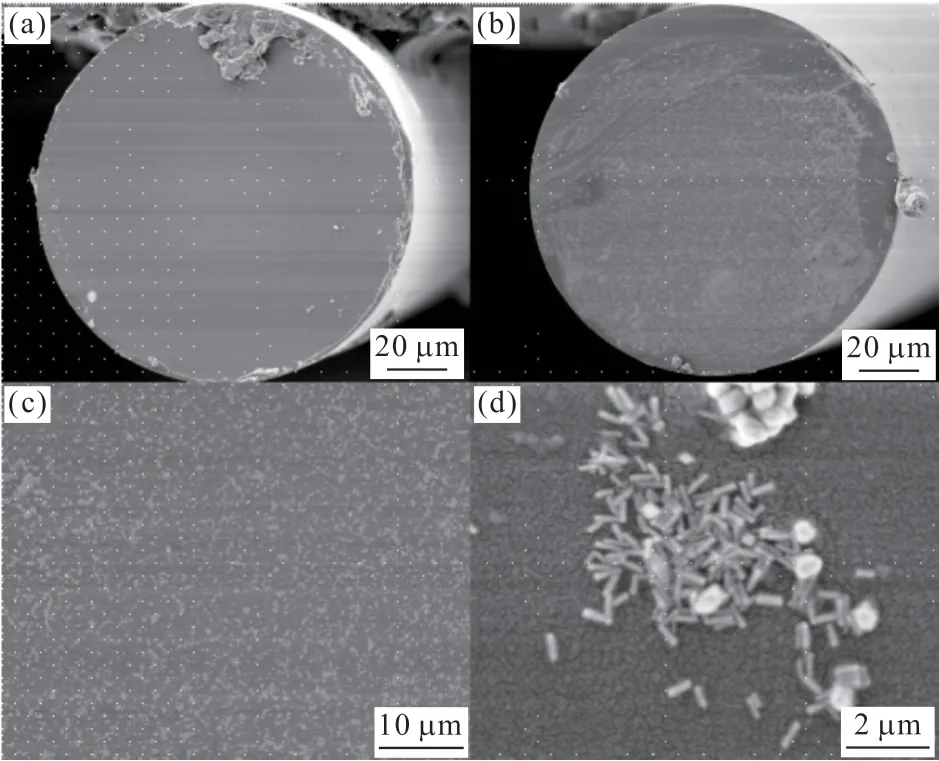
Fig.7 (a) Untreated optical fibers; (b-d) Polyelectrolyte multilayers method treated optical fibers
The second method is to use silane coupling agent to treat optical fiber, which are considered to be a very effective method of fiber treatment[31].One commonly used silane coupling agent is MPTMS, which is utilized to load the fiber end face with sulfhydryl groups for the adsorption of nanomaterials.As shown in Figs.8(a) and 8(b), the fibers were treated in a 5% MPTMS ethanol solution for two hours and then soaked in Au NRs solution to obtain SERS optical fiber probes.However, Au NRs distribution on the optical fiber surface was not uniform and the loading was small.Another method is to treat optical fibers with GO.And SERS optical fiber probes were synthesized by GO modified fiber end faces[32].Although GO provides additional chemical enhancement, SEM images of the fiber end surfaces reveal that only a small amount of Au NRs were absorbed, resulting in poor performance of the SERS optical fiber probe, as shown in Figs.8(c) and 8(d).

Fig.8 (a,b) Silane coupling agent treated optical fibers; (c,d) GO treated optical fibers
By comparing three different adsorption methods, the performance of SERS optical fiber probes was investigated according to the strength of SERS signal.The results showed that PDDA/PSS was significantly better than the other two methods, so the process method of PDDA/PSS was chosen to prepare the SERS optical fiber probes, as shown in Fig.9.

Fig.9 Raman spectra of different SERS optical fiber probes
3.3 Performance analysis of SERS optical fiber probes
In order to evaluate the SERS performance of SERS optical fiber probes, sensitivity, repeatability and stability of the optical fiber probes were tested.Only with good sensitivity, SERS optical fiber probes can have greater application potential, so sensitivity is a very important index of SERS optical fiber probes.
During the SERS test, a laser with a wavelength of 633 nm was chosen for excitation.The laser power was set to 100% intensity.As shown in Fig.10(a), CV was taken as the standard measured analyte, and SERS optical fiber probes were used to detect CV at concentration of 10-5, 10-6, 10-7, 10-8, and 10-9mol/L respectively.The measured SERS peaks corresponded to the characteristic peaks of CV.With the CV concentration decreased, SERS signal gradually weakened.When CV concentration was 10-9mol/L, the Raman signal could still be detected by the SERS optical fiber probe.Therefore, the prepared SERS optical fiber probe has excellent sensitivity, and the detection limit of CV was 10-9mol/L.Fig.10(b) shows the relationship between the logarithm of characteristic peak intensity and the logarithm of CV concentration.The correlation coeffi-cient is 0.96.

Fig.10 (a) Detected CV limit concentration; (b) CV signal strength and concentration relationship
It is evident that the SERS optical fiber probe possesses high sensitivity.To further compare its performance with that of other researchers, Table 1 presents a comparison.When utilizing a tapered optical fiber, the increased contact area enables a stronger SERS signal from the optical fiber probe.Some researchers have employed silver nanoparticles as the SERS active material, resulting in highly sensitive SERS optical fiber probes.However, stability remains a concern in these cases.Different optical fiber treatments determine the adsorption of nanomaterials, which in turn affects the final performance of the SERS fiber optic probe.In contrast, our SERS optical fiber probe employs gold nanoparticles as the SERS active material, offering certain advantages and significant potential for various applications.The use of gold nanoparticles enhances stability and improves the overall performance of the SERS fiber probe.This highlights the promising prospects and application potential of our SERS fiber probe compared to other research studies.

Table 1 SERS substrate and sensitivity
The reproducibility of the SERS fiber probe was evaluated by using six SERS optical fiber probes to detect CV at the same concentration.Fig.11 shows the SERS spectra obtained from the six optical fiber probes.It can be observed that the SERS spectra exhibit similar peak configurations and peak intensities.To quantify the reproducibility, the relative standard deviation (RSD) of the SERS signal was calculated using the characteristic peak at 1 173 cm-1as the reference.The RSD of six SERS optical fiber probes is 25%, so the SERS optical fiber probes have good repeatability.It avoids the situation that the performance of SERS optical fiber probes fluctuates greatly in practical application.
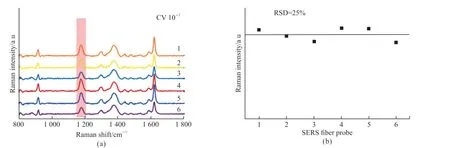
Fig.11 (a) SERS spectra by six SERS optical fiber probes with the same CV concentration; (b) Relative standard deviation of six SERS optical fiber probes
In practical applications, it is important for SERS optical fiber probes to exhibit good stability and maintain their SERS performance even after being stored for a period of time.As shown in Fig.12(a), the SERS optical fiber probes were stored in air and the SERS performance of the probes were tested at 3, 7, 10, 15,18 and 22 days.It was observed that the SERS signal gradually diminished with increasing storage time.However, even after 22 days, a significant SERS signal was still detectable.Since the plasma material on the end face of the SERS optical fiber probe is Au NRs, Au NRs are not easily oxidized in air, so there is not much loss of SERS signal.Therefore, the SERS optical fiber probe had good stability.Fig.12(b) shows the variation of the intensity of the characteristic peak of CV over time.
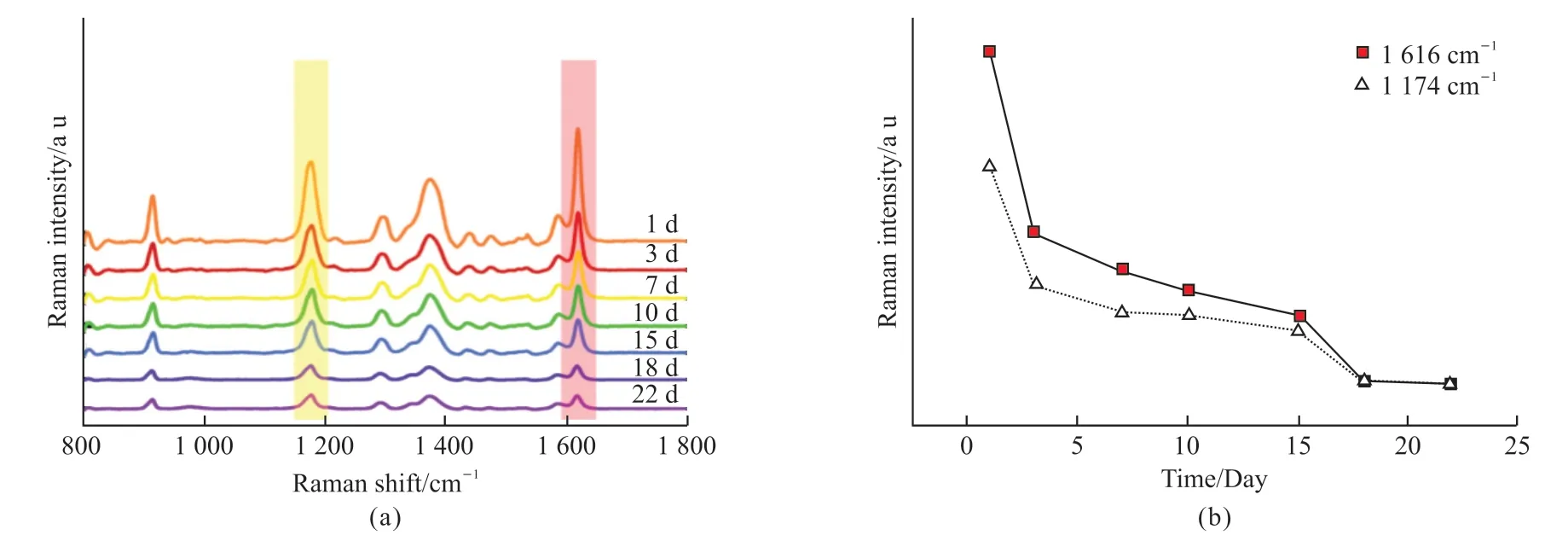
Fig.12 (a) The intensity of SERS optical fiber probes changed with time; (b) 1 174 cm-1 and 1 616 cm-1 peak intensity change
3.4 SERS optical fiber and Au NRs electromagnetic field simulation analysis
In order to explore the SERS intensity distribution of fiber end faces, a Mapping test was conducted on SERS optical fiber probes.The hot spot distribution was obtained by the SERS optical fiber probe from Mapping.As shown in Fig.13(a), the initial EM field distribution is different, which is caused by a combination of the LSPR of the plasma material and the optical waveguide propagation of the optical fiber.In Fig.13(b), it can be observed that the fiber core exhibits the strongest EM field when there are no Au nanorods(Au NRs) present.However, when the Au NRs interact with the incident light, the cores of the SERS optical fiber probes exhibit stronger electromagnetic fields.This enhancement is attributed to the interaction between the Au NRs and the incident light, which leads to the generation of localized surface plasmons and the amplification of the EM field in the fiber core.

Fig.13 (a) Mapping; (b) Fiber pattern analysis
The surface plasma distribution of Au NRs interacting with light was investigated using COMSOL Multiphysics[36].The finite element method was used for numerical simulation and the electromagnetic wave-frequency domain module of COMSOL Multiphysics was used for calculation.According to the size measurement of Au NRs by TEM, the length of Au nanorods was set to 40 nm and the diameter of Au nanorods to 13 nm.
As shown in Fig.14.The relative intensity distribution of the electromagnetic field on the surface of single and multiple coupled Au NRs was simulated.It was observed that a significant electromagnetic field was concentrated at the ends of the Au NRs.This phenomenon can be attributed to two factors: dipole plasmon resonance and the "lightning rod effect".It is well known that the high curvature associated with the ends of nanorods can lead to high electromagnetic fields and produce particularly high enhancement,called "lightning rod effect", which has an important contribution in SERS.In the case of multiple Au NRs coupling, stronger magnetoelectric fields are generated in the gaps between Au NRs.This is attributed to the tight coupling between the plasma nanoparticles, which leads to an enhancement of the maximum Raman signal of the molecules residing in the gap, resulting in a detection sensitivity close to a single molecule.Overall,these simulation results provide valuable insights into the distribution and enhancement of the electromagnetic field on the surface of Au NRs, highlighting their potential for applications in SERS-based sensing and detection.

Fig.14 Simulation of electromagnetic field distribution: (a) Single Au NRs; (b-d) Different combinations of Au NRs
4 Conclusions
Au NRs were controllable synthesized by seed growing method, and the growth mechanism of Au NRs was analyzed.The aspect ratio of Au NRs was found to increase with the addition of AgNO3, AA, HCl and seed solution.The highest yield of Au NRs was obtained when AgNO3was added in the amount of 500 μL.The self-assembled on polyelectrolyte multilayers method,MPTMS method and GO method were used to process optical fiber, combined with SERS signal and SEM image analysis to determine the self-assembled on polyelectrolyte multilayers method.The optimized SERS optical fiber probes exhibited a detection limit of 10-9mol/L for CV, excellent repeatability with a RSD of only 25%.The SERS optical fiber probes demonstrated good stability, as strong signals could still be measured after 22 days of exposure to air.Through simulation using COMSOL Multiphysics, the electromagnetic field is the strongest at the end of the Au NRs and reaches its peak when multiple Au NRs ends are coupled
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Effect of VEGF/GREDVY Modified Surface on Vascular Cells Behavior
- Evolution of Biofilm and Its Effect on Microstructure of Mortar Surfaces in Simulated Seawater
- Synthesis of Organic-Inorganic Hybrid Aluminum Hypophosphite Microspheres Flame Retardant and Its Flame Retardant Research on Thermoplastic Polyurethane
- Surface Metallization of Glass Fiber (GF) /Polyetheretherketone (PEEK) Composite with Cu Coatings Deposited by Magnetron Sputtering and Electroplating
- Effect of Size Change on Mechanical Properties ofMonolayer Arsenene
- Effects of Sinusoidal Vibration of Crystallization Roller on Composite Microstructure of Ti/Al Laminated Composites by Twin-Roll Casting
