Mo@PANI-catalyzed oxidative deoximation reaction
2024-04-05YiyangZhangWenLiZuofengHuXiaoiJingLeiYu
Yiyang Zhang ,Wen Li ,Zuofeng Hu,c ,Xiaoi Jing ,Lei Yu,*
a School of Mechanical Engineering,Yangzhou University,Yangzhou 225127,China
b School of Chemistry and Chemical Engineering,Yangzhou University,Yangzhou 225002,China
c Yangzhou Yangzi Auto Interior & Exterior Co.,Ltd.,Yangzhou 225119,China
Keywords: Molybdenum Polyaniline Oxidation Deoximation Carbonyl
ABSTRACT Deoximation is an important transformation in synthetic industry.It can be employed in protection,characterization and purification of the carbonyls,and in the synthesis of ketones from non-carbonyl molecules.In the field,oxidative deoximation reaction can utilize the driving force caused by the oxidation process so that the reaction can occur under relatively mild conditions.Recently,we designed and prepared polyaniline-supported molybdenum (Mo@PANI) just by immersing PANI into the EtOH/H2O solution of MoCl5.The material was successfully applied as the efficient catalyst for oxidative deoximation reactions,which were performed in ethanol using H2O2 as the clean oxidant.The substrate scope of the reaction was wide.It could be applied on heterocycle-containing substrates,making this protocol preferable for pharmaceutical intermediate synthesis.Since Mo is a necessary trace element for both animals and plants,this method is environment-friendly and is suitable for large-scale preparation.This work as the first example of Mo-catalyzed oxidative deoximation reaction may inspire novel ideas for both catalyst design and synthetic process development.
Deoximation is an important transformation in synthetic industry [1,2].Since oximes are usually crystals with stable melting points,the oximation-deoximation strategies can be employed to protect,characterize or purify carbonyls in the total synthesis of medicines or natural products.Early in 1979,Coryetal.have employed this protocol in the total synthesis of erthronolide A,a macro-cyclic antibiotic molecule with endo cyclic carbonyl[3].In industrial production of watermelon ketone,oximationdeoximation processes are employed for product purification [4].In addition,deoximation reaction can be employed in the production of ketones from non-carbonyl molecules.For example,the high-value-added spice carvone can be prepared from accessible limonene,and the processes include the addition of the endo cyclic C=C of limonene with NOCl,the elimination of HCl,and the last step deoximation reaction [5-7].Catalytic oxidative deoximation reaction is now the major deoximation method at present.In comparison with the traditional acidic deoximation method,it can utilize the driving force of the unit reaction of oxidation so that the transformation can occur under relatively mild conditions free of hazardous additives or halogen-containing solvents [8-11],and is in accordance with the developing trend of industrial synthesis[12-14].Currently,a series of elements such as Se,Te,Fe,have been employed to develop the catalysts for oxidative oximation reactions [15-19].The reaction can also be catalyzed by organic molecules,but suffer from the narrow substrate scope and high catalyst loadings [20,21].
On the other hand,polyanilines (PANIs) are recently found to be nice catalyst supports [22].Although anilines are toxic,their polymers are nontoxic and safe to the environments [23].The nitrogen groups in PANIs can well coordinate with catalytic metals,i.e.,PANIs are not only catalyst supports,but also ligands that may adjust the catalytic activities of the materials by using a varieties of easily available substituted anilines as their monomers [24-26].During the last decade,a variety of PANI-supported metal catalysts using Pd [27],Au [28],Pt [29],Ni [30],Cu [31],etc.,as the catalytic metals have been developed.These materials are phosphorus-free catalysts friendly to natural water sources,and the reactions can be performed under mild conditions with high catalyst turnover numbers (TONs).In our cases,we found that polyaniline-supported tungsten (W@PANI) could catalyze the oxidative deoximation [32].Owing to the strong coordination of nitrogen with tungsten,metal residues in the produced ketones were very low,and this feature might in accordance with the requirements of pharmaceutical industry.Recently,we designed and prepared the polyanilinesupported molybdenum (Mo@PANI) catalyst and successfully applied it in oxidative deoximation reactions.Since Mo is a necessary trace element for both animals and plants,this method is environment-friendly and is suitable for large-scale preparation.
PANIs were preparedviathe oxidative polymerization of the aniline monomers (Scheme 1).Three typical monomers such as aniline (R=H),p-anisidine (R=OMe) andp-fluoroaniline (R=F)were employed to produce the related PANIs marked as PANIH,PANI-OMe and PANI-F respectively.In this work,we employed H2O2as the clean oxidant so that the generation of solid wastes could be avoided [33].Mo@PANIs were prepared just by immersing the related PANIs into the 60 vol% aqueous ethanol solution of MoCl5.
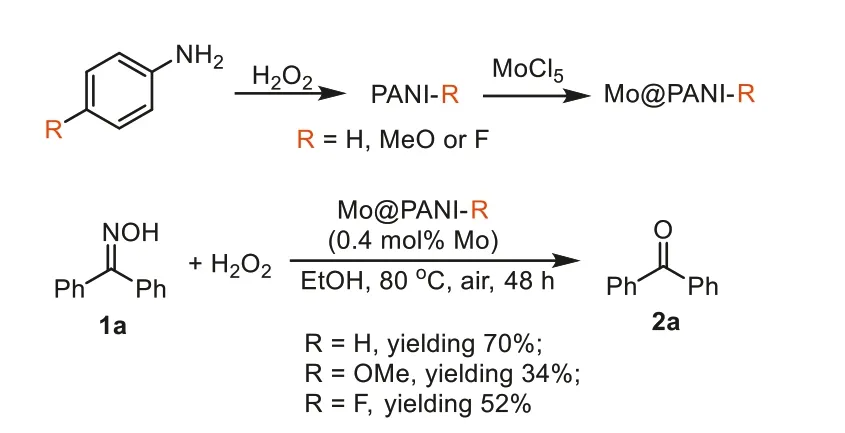
Scheme 1.Catalyst preparation & screenings.
The prepared Mo@PANIs were then employed in the oxidative deoximation reaction of diphenylmethanone oxime (1a) to evaluate their catalytic activities (Scheme 1).The deoximation reactions also employed H2O2as the clean oxidant and were performed in green solvent ethanol.Mo@PANIs containing 0.4 mol% of Movs.1awere employed.The reaction catalyzed by Mo@PANI-H afforded the desired ketone2ain 70% yield,while for the reactions being catalyzed by Mo@PANI-OMe and Mo@PANI-F,2awas produced in 34% and 52% yields respectively.Clearly,Mo@PANI-H was screened out to be the preferable catalyst.Introducing electron-donating or-withdrawing groups onto the aromatic ring of PANI could not improve the catalyst activity.
A series of parallel experiments were then performed to optimize the reaction conditions.Without catalyst,the blank reaction of1awith H2O2in ethanol at 80°C could afford2ain 31%yield (Fig.1a).The yield gradually increased along with the enhanced catalyst amount,and it could reach the peak at 77% when Mo@PANI-H containing 0.3 mol% of Movs.1awas employed (Fig.1a).Further increasing the catalyst amount led to decreased1ayield contrarily,possibly due to the fact that Mo might led to the decomposition of the oxidant H2O2(Fig.1a).Without H2O2,2awas produced in only 5% yield (Fig.1b).Elevated H2O2dosage could enhance the yield of2a,and using 100 mol% of H2O2vs.1awas screened out to be the most favorable condition (Fig.1b).Using excess H2O2resulted in the generation of over oxidation by-products and led to the decrease of the2ayield (Fig.1b).
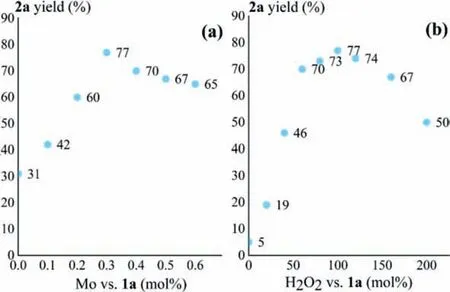
Fig.1.Screenings of the catalyst (a) and H2O2 (b) dosage for the reaction of 1a in ethanol at 80°C for 48 h.
Catalyzed by Mo@PANI-H containing 0.3 mol% of Mo,and using 100 mol% of H2O2as oxidant,parallel reactions of1aat different reaction temperatures were performed and the results were given in Table 1.It was found that 60°C was the preferable reaction temperature (Table 1,entry 2vs.entries 1,3 and 4).Low reaction temperature resulted in the incomplete reaction,while high reaction temperature might lead to the decomposition of H2O2,and all of them resulted in decreased product yields (Table 1,entry 2vs.entries 1,3 and 4).High polar solvents,such DMF and CH3CN,were not fit for the reaction (Table 1,entries 5 and 6).Reactions in 1,4-dioxane,DMC,EtOAc and toluene led to2ain 56%-70% yields,less than the yield of the reaction in EtOH (Table 1,entries 2vs.7-10).Thus,it could be concluded that performing the reaction in ethanol at 60°C should be the favorable reaction condition.

Table 1Reaction conditions optimizations.a
Under the optimized conditions described in Table 1,entry 2,a series of ketoximes1were employed as substrate to examine the scope of the reaction (Table 2).Besides1a,diaryl ketoximes with electron-withdrawing or -donating substituents were also suitable substrates for the reaction (Table 2,entries 1-8).Notably,in regardless of the oxidative reaction conditions,substrates with reductive functional groups,such as phenol (1i) and aniline (1j),were fit for the reaction,leading to the desired ketones2iand2jin moderate yields (Table 2,entries 9 and 10).Besides diary ketoximes,the reaction could employ ketoximes bearing aliphatic groups as substrates,affording the related ketones in moderate to excellent yields (Table 2,entries 11-21).Moreover,the reaction showed certain degree tolerance to heterocycles in substrates,such as pyridine,thiophene and furan,indicating that the method might be applicable for medicine intermediate synthesis (Table 2,entries 22-24).Reaction of (E)-1-(naphthalen-2-yl)ethan-1-one oxime (1y) could produce2yin 95% yield,in regardless of the steric hindrance of the substrate bearing naphthyl (Table 2,entry 25).Finally,cyclohexanone oxime (1z) was employed as the example of aliphatic substrate and its reaction led to2zin 40% GC yield(Table 2,entry 26).
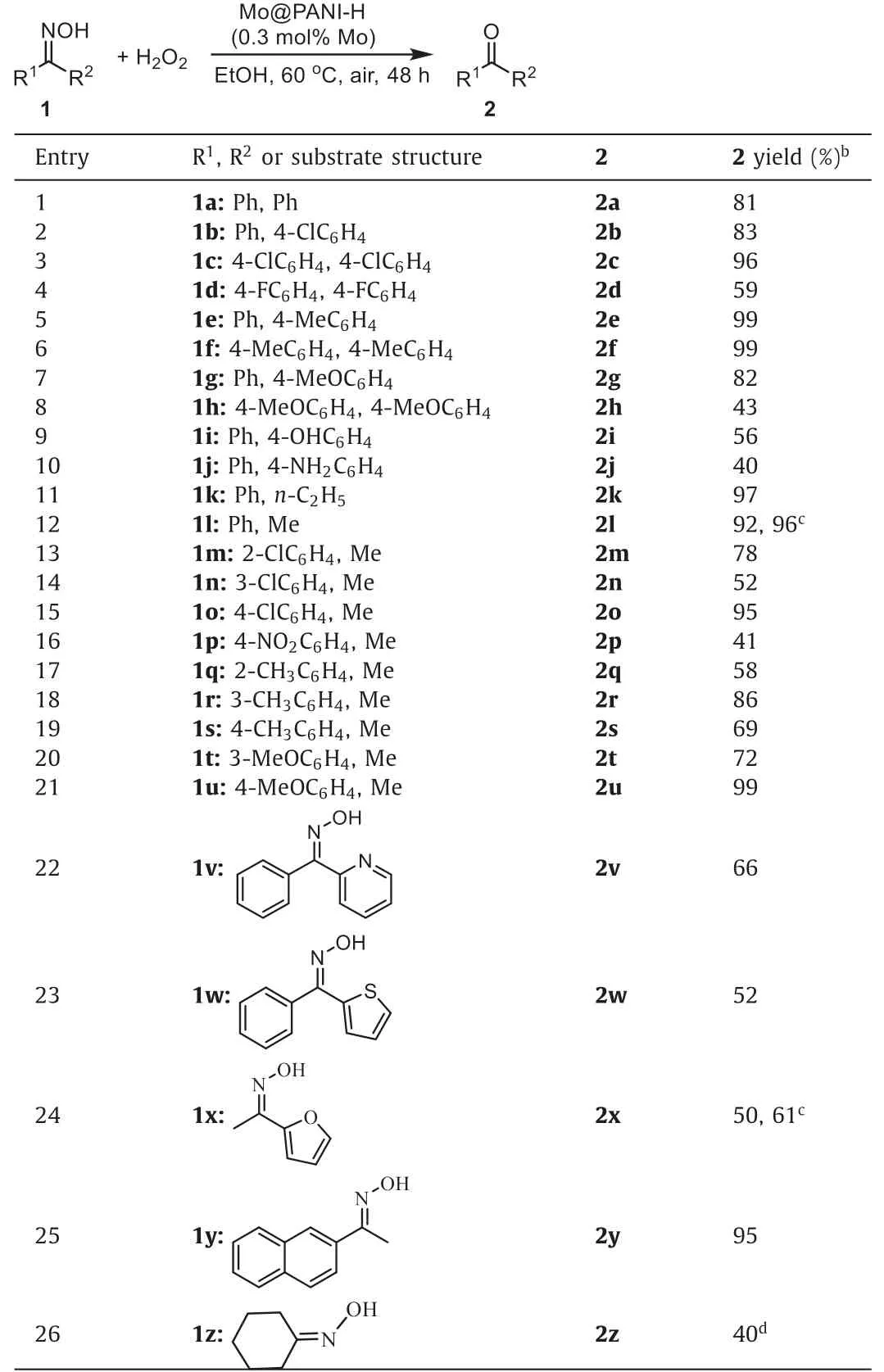
Table 2Reaction substrate scope extensions.a
Materials characterizations as well as control experiments were performed to get sufficient information for reaction mechanism study.X-ray photoelectron spectroscopy (XPS) analysis showed that Mo existed as Mo6+species in fresh Mo@PANI-H (Fig.2).The result indicated that the nitrogen-coordinated Mo was easily oxidized by air under ambient conditions.Moreover,in the XPS spectrum of the used catalyst,both Mo5+and Mo6+species could be observed,attesting that the high valent Mo species could be reduced by the organic substrates during the deoximation reaction process,i.e.,thevalent of Mo shifted between +5 and +6 during the catalysis process.

Fig.2.XPS spectra of the fresh and used catalysts.
MoCl5could also catalyze the reaction.However,in comparison with Mo@PANI-H,the catalytic activity of MoCl5was poor (Table 3,entry 2vs.1).Thus,it is supposed that PANI is a support that can enhance the activity of the metal owing to the coordination effect of its nitrogen group,and this has been discussed by our previous works [24-26].Performing the reaction in N2resulted in decreased product yield (Table 3,entry 3vs.1).Without H2O2oxidant,the reaction in air could also produce2ain 9% yield (Table 3,entry 4),and the product yield could be enhanced to 31% when O2was employed as the oxidant (Table 3,entry 5).In contrast,no reaction occurred when heating1aand Mo@PANI-H in N2without H2O2(Table 3,entry 6).The experimental results in Table 3,entries 3-6 could support the hypothesis that the Mo-catalyzed oxidative deoximation reaction could employ H2O2/air as the hybrid oxidant,so that no excess H2O2was required.Addition of free radical scavenger TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) into the system could restrain the reaction,showing that it proceededviaa free radical reaction mechanism (Table 3,entry 7) [34].Furthermore,the salicylic acid trapping experiment verified the existence of hydroxyl radical during the reaction progress (Fig.S1 in Supporting information).Cr@PANI can also catalyze the reaction,but the product yield is low (Table 3,entry 8).

Table 3Control experiments.a
On the basis of the experimental results as well as the literature reports,a plausible mechanism of the reaction was supposed in Scheme 2.Since the reaction was performed in organic solvents such as ethanol,the Mo(VI) species in catalyst could be reduced to Mo(V).Single electron transfer reaction of Mo(V) with H2O2could afford the hydroxyl radical,while Mo was oxidized into the Mo(VI)species bearing an addition hydroxyl group [35].The free radical addition of hydroxyl radical with substrate1led to the intermediate3[36],which rearranged to give the radical intermediate4[36].Decomposition of4produced the ketone2,i.e.the deoximation product,and led to the radical5[37].Shifting of the hydroxyl in Mo(VI) species to5led to HNO and H2O [38],and regenerated the Mo(V) species that could restart the catalysis circle.
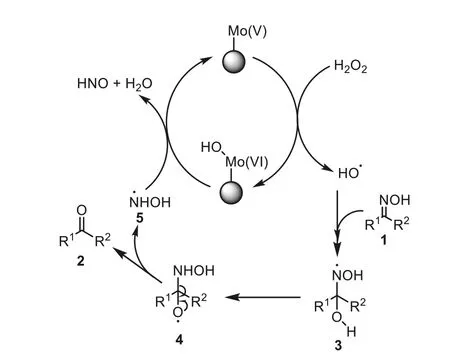
Scheme 2.Possible mechanisms of the reaction.
In conclusion,we designed and prepared Mo@PANI catalyst for the oxidative deoximation reaction.The material was easily prepared,while the deoximation process was clean.Since Mo is a necessary element for animals and plants,using this metal as catalyst is more environment friendly.Thus,this work could afford a practical method for deoximation reaction,which is an important transformation in synthetic industry.Continuous investigations are ongoing in our laboratory to expand the application scope of Mobased catalysts in more useful reactions.
Acknowledgments
We thank Priority Academic Program Development of Jiangsu Higher Education Institutions for support.This project was also financially supported by the fund of the joint-laboratory of Shanghai Dingya Pharmaceutical Chemical Technology Co.,Ltd.with Yangzhou University (No.2022-2027).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108938.
杂志排行
Chinese Chemical Letters的其它文章
- The 3rd Xihua Chemistry and Biomedicine Forum
- Professor Hualiang Jiang: A tribute to an esteemed visionary chemist and pharmacist
- Recent advances in visible light-mediated chemical transformations of enaminones
- Development of porphyrin-based fluorescent sensors and sensor arrays for saccharide recognition
- Recent advances of versatile reagents as controllable building blocks in organic synthesis
- Synthetic host-guest pairs as novel bioorthogonal tools for pre-targeting☆
