Development of porphyrin-based fluorescent sensors and sensor arrays for saccharide recognition
2024-04-05YubinDingJixinWngRuinnWngYongshuXie
Yubin Ding ,Jixin Wng ,Ruinn Wng ,Yongshu Xie
a Department of Chemistry,College of Sciences,Nanjing Agricultural University,Nanjing 210095,China
b Shanghai Key Laboratory of Functional Materials Chemistry,Key Laboratory for Advanced Materials and Institute of Fine Chemicals,School of Chemistry and Molecular Engineering,East China University of Science & Technology,Shanghai 200237,China
Keywords: Porphyrin Saccharide Fluorescent sensing Selective sensing Pattern recognition
ABSTRACT Saccharide sensing is a very meaningful research topic as saccharides are involved in many biological activities.However,it is challenging to design molecular sensors for saccharides because this family of compounds is hydromimetic in aqueous solutions and shares a similar chemical structure.In this review,research progress in the development of porphyrin-based saccharide sensors is described with representative examples.We focus on using porphyrin as the signal reporter because porphyrins exhibit unique advantages in high chemical stability,long emission wavelength,and multiple structural modification strategies.Reported literature results have been classified into mainly two sections according to the general working principles of the porphyrin sensor molecules.In the first section,recognition unit,design strategy and sensing performance of traditional porphyrin-based selective saccharide sensors are discussed.While in the second section,development of porphyrin-based sensor arrays for pattern recognition of saccharides has been summarized.Looking through the design strategy and sensing performance of reported achievements,it is reasonable to anticipate a bright future for designing practical porphyrinbased saccharide sensors.
1.Introduction
Saccharides are essential substances of life.They play many key roles in life activities by providing energy and carbon resources [1].The development of reliable and quantitative methods to measure and visualize target saccharides in biological systems is meaningful for early diagnosis of saccharide metabolism related diseases [2].For example,measurement of the blood glucose concentration is a key parameter to evaluate the healthy status of a diabetic patient[3].However,such an important family of compounds not only exists in many forms in our body but also shares a similar and complicated chemical structure [4].It is therefore a challenging work to design reliable and convenient tools for saccharide recognition[5,6].
In addition to expensive instrument-based analytical methods,the development of small molecule-based fluorescent sensors is meaningful due to their advantages of low cost,high accuracy and practical convenience [7].Small molecule sensors were proven to be suitable for sensing of versatile kinds of analytes [8,9].In this respect,the development of fluorescent sensors for saccharide sensing has also attracted much attention [10].Organic fluorophores including AIEgens [11,12],pyrene [13],coumarin [14],rhodamine [15],andN,N’-diphenyl-dihydrodibenzo[a,c]phenazine(DPAC) [16] have been used to design fluorescent sensors for saccharide sensing.
Among commonly used fluorophores,porphyrin can be considered as an outstanding one since it was selected as the “pigment of life” by the nature [17,18].To be specific,a tetrapyrrolic architecture can be found in the chemical structures of chlorophyll and heme,which were essential for corresponding life activities of plant and human being.Actually,porphyrin was also selected by scientists of different research fields due to its inherent advantages[19-21].Firstly,with a conjugated and aromatic structure,porphyrins are chemically stable,which is important for long-term usage of the material.Secondly,themeso-,β-positions and even inner NH of a porphyrin molecule can be functionalized to afford the desired structures.Thirdly,porphyrins exhibit a long fluorescence emission wavelength that can even extend into the near-infrared region.Such a long emission wavelength is beneficial for biological applications so as to avoid auto-fluorescence signals from living tissues [22].With these merits,porphyrin can be considered as a promising fluorophore for the development of small-molecule sensors for saccharide sensing.
In this review,the discussion on recent research progress in the development of porphyrin-based saccharide fluorescent sensors was divided into mainly two sections.The first section was focused on the design strategies,synthesis and sensing performance of porphyrin-based selective saccharide sensors.In the second section,porphyrin-based sensor arrays for differential saccharides sensing were discussed with representative examples.
2.Porphyrin-based selective saccharide sensors
Saccharides are all polyols and each of their hydroxyl group exhibits almost the same hydrogen bonding ability.The difficulty for recognition of a specific saccharide in non-polar organic solvents lies in how to arrange the hydrogen bonding sites in the sensor molecule [23].In comparison to saccharide recognition in nonpolar organic solvents,selective binding of saccharides in aqueous solutions became especially difficult.This was a result of the“hydromimetic” nature of saccharides,as the hydrogen bonding strength of water-saccharide was almost equal to that of sensorsaccharide [24].Even the nature occurring saccharide binding proteins,saying lectins,were just able to bind saccharides with binding constants of around 103L/mol [25].Obviously,it is very challenging to design molecular sensors for selective binding of a given saccharide in aqueous solutions.It is therefore easy to understand why glucose oxidase (GOx),an enzyme to oxidize glucose into gluconic acid,was still widely used in designing novel blood glucose sensors [26].In this respect,porphyrins were also used as building blocks to synthesize nano-materials with peroxidase-like catalytic activities (nanozymes [27]) and then combined with GOx enzyme and colorimetric substrates for glucose sensing [28-30].
In despite of the difficulties,there are mainly two approaches to achieve the goal of effective saccharide binding in water.One of them intends to arrange hydrophobic and CH-πinteractions in addition to hydrogen bonding to mimic the binding mode of lectins.The core idea is to build a full complementary binding interaction system between the target saccharide and the sensor molecule[31].For example,a bicyclic cage with a suitable cavity to mimic lectin was reported by Davis and co-workers,which could bind glucose effectively in water with a constant of about 18000 L/mol[32].For this approach,porphyrins have a relatively large molecule size,making them more suitable for binding of large saccharides such as oligosaccharides.Another approach was based on the interaction of the 1,2-diol moiety of saccharides with boronic acids to form dynamic five-membered boronate ester bonds [33,34].As the formation of boronate ester bonds can be easily achieved in water,boronic acid was considered as an ideal recognition unit to design saccharide sensors [35].In this regard,the introduction of boronic acid to the porphyrin fluorophore would be a promising way to afford selective saccharide sensors.
2.1. Boronic acid as the recognition unit
By introducing a boronic acid unit to the porphyrin core,the afforded boronic acid appended porphyrin would be able to interact with saccharides to form corresponding sensor-saccharide boronate complex,which should induce either aggregation state or photophysical property changes of the porphyrin fluorophore [36,37].
Research on the development of boronic acid appended porphyrin sensors for saccharide recognition was pioneered by the Shinkai group [38,39].As an early example,two phenylboronic acid units were functionalized atβ-positions of the porphyrin to afford the sensor moleculeP2B(Fig.1a),which was initially in the aggregation form with quenched fluorescence in DMSO-water mixed solvent.Upon addition of D-fructose,fluorescence emission intensity of the solution enhanced significantly.This was due to the formation of theP2B-sugar boronate complex,whose solubility in tested solvent was much better than that ofP2Bitself,resulting in deaggregation of the porphyrin molecule and recovery of its fluorescence.
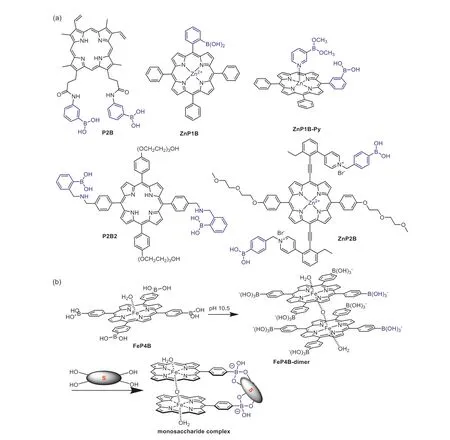
Fig.1.(a) Chemical structure of boronic acid appended porphyrins for saccharide sensing.(b) Formation of the μ-oxo porphinatoiron(III) dimer FeP4B-dimer and its saccharide binding mode.Some boronate units were omitted for clarity.
With a cooperative binding strategy,boronic acid appended zinc porphyrin sensorZnP1B(Fig.1a) was designed by the Shinkai group for selective binding of glucose-6-phosphate (G6P) [40].In this molecule,the boronic acid was incorporated to interact with the 1,2-diol moiety in saccharides,while an additional zinc ion coordinated at the inner core was used to bind the phosphate moiety in G6P.It was determined that sensorZnP1Bwas selective to G6P over glucose-1-phosphate,suggesting that an appropriate distance between the boronic acid and zinc recognition units was important.With a similar strategy,a diboronic-acid-based porphyrin sensorZnP1B-Py(Fig.1a) was synthesized taking the advantage of self-assembling property of zinc porphyrin with pyridine ligand at the axial position,which was able to form a ternary complex with different monosaccharides and resulted in significant spectral changes [41].
It was obvious that the distance between the two fixed binding sites determined the saccharide selectivity of the sensor.With a larger distance between two boronic acid binding sites,a porphyrin receptorP2B2(Fig.1a) was reported by Anslyn,Sessler and co-workers for the detection of five kinds of ginsenosides [42].Ginsenosides were used in clinic for treatment of a variety of diseases,whose structures share a gonane steroid nucleus with different sugar substitutions.It was believed that the glycosylation patterns of a ginsenoside significantly influenced its biological activity.Therefore sensing of ginsenosides was meaningful for their quality control.The titration ofP2B2with tested ginsenosides resulted in enhancement of the fluorescence emission intensity.The binding stoichiometry was determined to be 1:1 betweenP2B2and ginsenosides.It was discovered that the binding strength became apparently stronger by increasing the number of saccharide units and was influenced by the protopanaxtriol substitution pattern.The authors proposed a bifunctional binding mode betweenP2B2and the ginsenosides,which was consist of both the interactions between saccharide moiety and the boronic acid units and hydrophobic interactions between the porphyrin moiety and steroid core of ginsenosides.
Compared to porphyrin sensors with two position-fixed boronic acid units,the development of methods to modulate the distance between two boronic acids in a dynamical way was meaningful for improving the saccharide binding strength of the sensor [43].As an example,Shinkaietal.designed a porphyrin moleculeZnP2B(Fig.1a) with two boronic acid units modified at the axial position,which were linked to the porphyrin core through two ethynyl groups.Through rotation of the ethynyl group,distance between the two boronic acids could vary from 0.1 nm to 2.4 nm.This property enabled the sensor to adjust its conformation to bind saccharides in a dynamic way,as compoundZnP2Bcould bind both mono-and oligosaccharides to form corresponding 1:1 complexes with association constants in a range of 102-103L/mol.
In fact,manipulation of the spatial distribution of the boronic acids within a porphyrin sensor would also be an effective way the enhance the sensor’s ability to bind one kind of monosaccharide selectively over the others with only configuration differences[44].In this example,aμ-oxo porphinatoiron(III) dimerFeP4Bdimer(Fig.1b) was discovered to be act as a “sugar tweezer” for selective binding of D-glucose and D-galactose.Porphyrin dimerFeP4B-dimerwas obtained by simply adjusting the pH value of its monomer solution (FeP4B) to 10.5.The authors revealed thatFeP4B-dimerwas able to bind D-glucose with two of its eight boronic acid groups.The reason for the high selectivity was due to the fact that the distance between two porphyrin planes (3.8 ˚A)inFeP4B-dimerwas comparable to the size of monosaccharides(3.0 ˚A).The binding constants ofFeP4B-dimerwith D-glucose and D-galactose were calculated to be as large as 1.51×105L/mol and 2.43×104L/mol,respectively,which even reached the level of corresponding specific enzymes.
2.2. Monosaccharide sensing with other recognition units
As mentioned above that effective binding of saccharides in water requires complementary binding interactions between the sensor and saccharide molecules.From this point of view,groups that could afford corporative hydrogen bonding,metal coordination,and hydrophobic interaction sites could be used as an effective recognition unit for target saccharide.However,compared to the formation of boronate complex,hydrogen bonding and hydrophobic interactions were relatively weak.Therefore,the fluorescence signal output of a porphyrin sensor upon the addition of saccharide could also be ascribed to their altered aggregation states but not the formation of a new porphyrin-saccharide complex.As Davisetal.have reported that the titration of tetraphenylporphine tetrasulfonate (TPPS) with glucose was accompanied with vivid UV-visible spectral changes,while no1H NMR signal changes can be detected [45].Therefore,the in-deep mechanism for the spectral changes should be proved by all possible measurements.
By simply introducing phosphonate groups as the hydrogen bond acceptors,Kraletal.reported two water soluble compoundsP2PandP4P(Fig.2) for saccharide recognition [46,47].The titration ofP2PandP4Pwith monosaccharides such asα-D-glucose,α-D-arabinose,D-mannose,D-fructose,D-ribose,D-trehalose,Dmaltose andα-D-lactose in 95% water resulted in significant UV-vis absorption changes.The binding stoichiometry was determined to be 1:1 for bothP2PandP4Pto bind tested monosaccharides with the binding constants calculated to be 0.6×104~2.5×104L/mol.
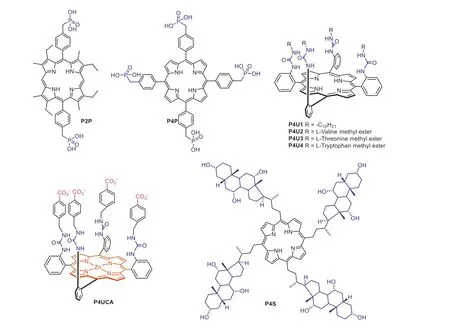
Fig.2.Chemical structures of porphyrin-based selective saccharide sensors with other recognition units.
A number of urea groups are rich in hydrogen bonding acceptors and donors,making them possible to be used as recognition sites to bind saccharide -OH groups.CompoundsP4U1-P4U4(Fig.2) were synthesized by Bonar-Law and co-workers through introducing four identical amino acid esters to themeso-positions of the porphyrin core using urea group as the linker [48].In this design,urea functionalized porphyrins could bind target saccharides through multiple hydrogen bonding interactions.Upon titration ofP4U1-P4U4with pyranosides such as galactoside,glucoside and mannoside in dichloromethane solution,vivid red-shift and changes in intensity of the porphyrin Soret absorption band can be detected.The binding constants betweenP4U1-P4U4with tested pyranosides were calculated to be in a range of 3×104~9×105L/mol.
Following this work,Hongetal.[49] reported another urea linked porphyrin receptorP4UCA(Fig.2) for saccharide recognition in aqueous solution [50].The structure of compoundP4UCAcan be divided into mainly three functional moieties.The zincinserted porphyrin plane served as both of the Lewis acid-base interaction site and a hydrophobic interaction site.The urea moieties acted as not only the linker but also were responsible for providing multiple hydrogen bonding sites.At last,the incorporation of carboxylate groups increased water solubility of the sensor and could participate in hydrogen bonding as well as electrostatic interactions.With this three-functional-moiety design strategy,compoundP4UCAwas able to bind amino saccharides including Dglucosamine,D-galactosamine and D-mannosamine with binding constants in a range of 38-430 L/mol in MeOH-H2O (v/v,1/3).
2.3. Oligosaccharide sensing
In addition to hydrogen bonding,the incorporation of hydrophobic interaction was also meaningful in designing saccharide sensors.Steroid-substituted porphyrinP4S(Fig.2) was synthesizedviaa condensation reaction of corresponding steroid aldehyde with pyrrole followed by deprotection of the hydroxyl groups [51].In this molecule,the steroid moiety was incorporated as the saccharide binding site.To avoid aggregation of the sensor molecule,a mixed solvent containing 50% aqueous 2-propanol was used as the test media.It was determined that sensorP4Swas able to bind saccharides and introduce vivid UV-vis spectral changes.In addition,sensorP4Sexhibited a higher selectivity toward oligosaccharides over monosaccharides.The binding mechanism was ascribed to hydrogen bonding and hydrophobic interactions between the steroidal part of the sensor and saccharide molecules.
Similar to steroid structures,bile acid could also provide both hydrogen bonding and hydrophobic interaction sites for saccharide recognition.In the work reported by Kralova,Kral and their coworkers,four bile acid appended porphyrinsP4BA1-P4BA4(Fig.3)were designed and synthesized for recognition of tumor expressed saccharides [52].In addition to the bile acid unit,since ammonium group was used as the linker,porphyrinsP4BA1-P4BA4were positively charged molecules.Therefore,porphyrinsP4BA1-P4BA4may bind negatively charged saccharides through hydrogen bonding,hydrophobic and electrostatic interactions.In this study,mixed solvent of MeOH-water (3:7,v/v) was used to investigate the binding interactions betweenP4BA1-P4BA4and saccharides including glucose,sialic acid,hyaluronic acid and heparan sulfate.It was determined that the binding ofP4BA1-P4BA4with heparan sulfate was much higher than that of the other tested saccharides.This was ascribed to additional electrostatic interactions between the porphyrin and heparan sulfate,since porphyrinsP4BA1-P4BA4were positively charged while heparan sulfate was negatively charged.Through binding to tumor expressed saccharides,porphyrinsP4BA1-P4BA4were used to image living cancer cells such as A431NS,HeLA,and 4T1 and applied for differentiating transformed (tumor) cells and their untransformed counterparts (Fig.4).To further investigate the binding mechanism of tetrakis(bile acid)-porphyrin conjugate with saccharides,ESI-FTICR(FTICR: Fourier transform ion cyclotron resonance) mass spectrometry and electron capture dissociation (ECD) experiments was carried out by Kalenius and co-workers [53].They revealed that at least one bile acid side arm and the porphyrin center in the tetrakis(bile acid)-porphyrin conjugate was responsible for saccharide binding.
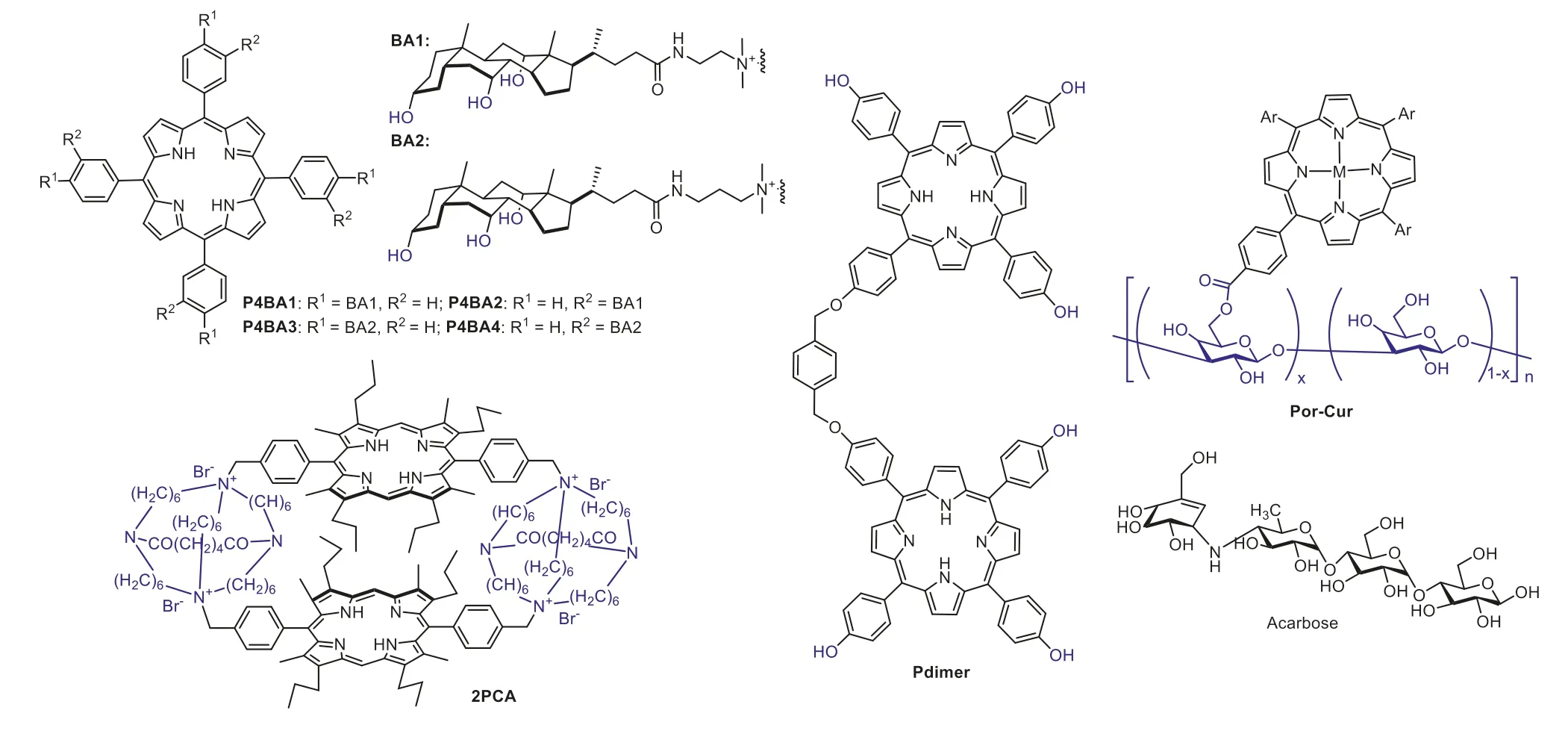
Fig.3.Chemical structures of porphyrin-based fluorescent sensors for oligosaccharides.
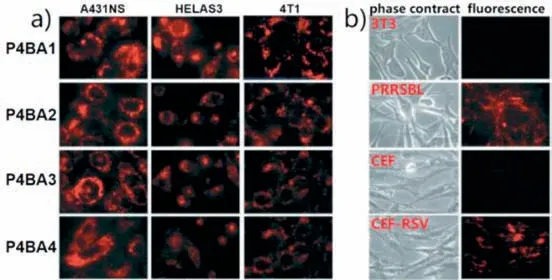
Fig.4.(a) Fluorescent imaging of living cancer cells (A431NS,HeLaS3,4T1) using P4BA1-P4BA4.(b) Imaging of transformed (tumor) cells (PRRSBL and CEF-RSV) and their untransformed counterparts (3T3 and CEF) using P4BA2.Reproduced with permission [52].Copyright 2008,The Royal Society of Chemistry.
In biology,natural carbohydrate-binding proteins usually provide a suitable “pocket” to meet the full complementarity saccharide binding requirement.Therefore,it is reasonable to design a molecular cage for effective saccharide binding [54].To provide a suitable cavity to encapsulate saccharides,bisporphyrinic sandwich compounds were synthesized by Kral and Schmidtchenetal.for effective saccharide sensing in highly competitive media (95%water) [55].For example,macrocyclic porphyrin sandwich compound2PCA(Fig.3) was synthesized by reaction between corresponding macrotricyclic amide and bis(bromomethyl)porphyrin.Investigations revealed that compound2PCAexhibited higher binding ability to a trisaccharide saying maltotriose than mono-and disaccharides.The binding stoichiometry between2PCAand maltotriose was determined to be 1:1 with a logKaup to about 4.72.The binding strength between sensor2PCAand maltotriose was a result of matched hydrophilic and hydrophobic host-guest interactions between the sensor and saccharide molecule.
Usingp-xylene as the linker,porphyrin dimerPdimer(Fig.3)was synthesized by Yeetal.for saccharide sensing [56].Different from a molecular cage with a cavity of fixed size,porphyrin dimerPdimerexposed a hydrogen bonding “cleft” for saccharide using its six phenolic hydroxyl groups.UV-vis and fluorescence emission titration experiments revealed thatPdimerwas able to bind a variety of monosaccharides and oligosaccharides in DMSO,accompanied with vivid spectral changes.Job plot data indicated a 1:1 binding stoichiometry betweenPdimerand D-glucose.The binding constant forPdimerwith maltotriose (~2000 L/mol) was determined to be much higher than that of monosaccharides (~170 L/mol) such as glucose and fructose.This oligosaccharide binding selectivity was possibly due to the large binding surface area of compoundPdimer.
Curdlan,making up with (1,3)-linked-β-D-glucose repeating units,is a linear glucan that usually forms random coils in DMSO but changed into triple-helices and globules in aqueous solution.By introducing porphyrin as the signal reporter to the curdlan molecule,a porphyrin-based sensorPor-Curfor acarbose (a drug for type-2 diabetes) was reported by Fukuhara,Inoue and coworkers (Fig.3) [57,58].In this work,the presence of acarbose resulted in significant circular dichroism (CD) signal changes through the formation of globule curdlan-saccharide co-aggregates.The sensitivity ofPor-Curto detect acarbose was determined to be 200 μmol/L.
From these limited examples,it can be concluded that porphyrin was a promising fluorophore for designing selective saccharide sensors.Through the incorporation of a suitable recognition unit,porphyrin-based sensor molecules can effectively bind target mono-and oligosaccharides in even aqueous solutions.
3.Porphyrin-based sensor arrays for pattern recognition of saccharides
From above examples it can be found that the design of a complementary receptor for mono-,di-and trisaccharides requires not only well manipulation of the hydrogen bonding,hydrophobic and C-H···πinteractions between the sensor and saccharide molecule but also incorporation of a suitable sized cavity to accommodate the target saccharide.However,this idea was only available to saccharides with a limited molecular size,as a polysaccharide should be too large to design a cavity that big enough to encapsulate it.
As a member of the saccharide family,glycosaminoglycans(GAGs) are a series of similar linear polysaccharides that exhibit versatile biological activities.Among various GAGs,heparin is much different from the others because it has a highly sulfated chemical structure and bears the highest negative charge density among all known large biomolecules [59].Heparin was able to bind antithrombin III and activate its anticoagulation activity.As a result,it is widely used in clinics as an anticoagulant,especially for anticoagulation treatment in surgery and blood dialysis [60].However,as the chemical structure of heparin was large and too complicated to be synthesized in a fully artificial way,production of such an important anticoagulant drug still requires extraction of it from biological tissues [61,62].Following the synthetic difficulty,the structural complexity of heparin also resulted in the challenge to design selective sensors for it [63].
As an alternative approach,pattern recognition can be applied for discrimination of a large number of similar analytes by mimicking the working principle of human olfactory systems [64].Since pattern recognition do not require the design of highly selective recognition units,it is very suitable to be applied for detection of analytes with similar and complicated chemical structures [65].Therefore the design of sensor arrays for pattern recognition of GAGs has attracted much attention [66-69].
Actually,literature reports on the design of porphyrin-based sensor arrays for pattern recognition of GAGs were still rare.Considering that the detection of GAGs should always be accomplished in biological samples such as serum,our group intended to develop saccharide sensor arrays that exhibit long emission wavelength to avoid auto-fluorescence interference from biological backgrounds.Thus five kinds of positively charged porphyrins (PP1,PP2a,PP2b,PP3andPP4) were designed and synthesized byN-methylation of the pyridine substituted porphyrin precursors (Fig.5a) [70].The afforded porphyrins were highly fluorescent in aqueous solutions,which can be almost fully quenched through the addition of graphene oxide (GO).The formation of the non-fluorescent porphyrin-GO complexes was ascribed toπ-πstacking,hydrogen bonding and electrostatic interactions.In the presence of different GAGs,the GAG molecules would bind the positively charged porphyrins through multiple hydrogen bonds and electrostatic interactions (Fig.5b).As a result of such multiple interactions,GAG molecules would compete with GO to remove porphyrin from its surface and recover the fluorescence of porphyrins (Fig.6a).It was determined that with different number and position of the 4-Nmethyl-pyridyl substituents,five porphyrin-GO complexes showed a cross-response behavior to tested GAGs (Fig.6b).The designed porphyrin-GO sensor array was successfully applied for discrimination of four kinds of GAGs in 10% serum media.Moreover,as low as 0.1% (wt%) of similar GAG contaminants in heparin can be detected with 100% accuracy.
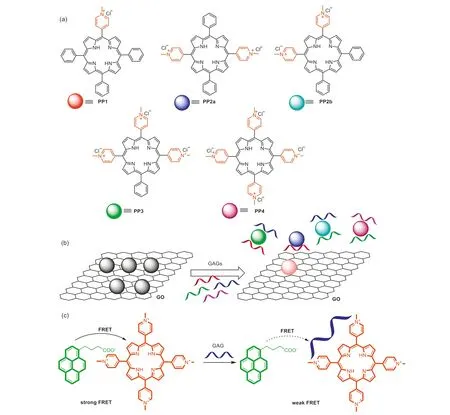
Fig.5.(a) Chemical structures of porphyrins PP1,PP2a,PP2b,PP3 and PP4.(b) Working principle of the porphyrin-GO sensor array.(c) Working principle of the pyreneporphyrin sensor array.(a,b) Reproduced with permission [70].Copyright 2020,American Chemical Society.

Fig.6.(a) Fluorescence titration profile of PP2b-GO nanocomposites with increasing amount of heparin in buffer.(b) Plots show the fluorescence emission intensity changes of the five nanocomposites upon addition of heparin.Reproduced with permission [70].Copyright 2020,American Chemical Society.
With the consideration that ratiometric signals could avoid the background interference from fluctuation of the sensor concentration and instrumental factors,above mentioned porphyrins (PP1,PP2aandPP4) were further used by our group as the FRET(Förster resonance energy transfer) energy acceptor to construct a pyrene-porphyrin based FRET sensor array for discrimination of GAGs (Fig.5c) [71].In this design,positively charged porphyrinsPP1,PP2aandPP4were combined with a negatively charged dye,saying pyrene-1-butyric acid (Py),to form three corresponding pyrene-porphyrin complexes.The driving force to form the pyreneporphyrin complexes wasπ-πstacking and electrostatic interactions betweenPyand the porphyrins.Upon the addition of GAGs to the solution of pyrene-porphyrin complexes,GAGs were able to bind porphyrins through hydrogen bonding and electrostatic interactions,which would influence the stability of corresponding pyrene-porphyrin complexes and result in changes in the FRET fluorescence signal.As porphyrinsPP1,PP2aandPP4were functionalized with different numbers of 4-N-methyl-pyridyl substituents,their binding strength with GAGs were different from each other.The pyrene-porphyrin ratiometric sensor array was determined to be cross-reactive toward tested GAGs.It was applied for accurate discrimination of GAGs such as heparin,chondroitin sulfate,dextran sulfate and hyaluronic acid in a concentration range of 2-100 μg/mL.Besides,it was worth pointing out that the ratiometric fluorescence signal of the pyrene-porphyrin sensor array response to GAGs can be even distinguished by naked eyes.
With a purpose to increase the discrimination sensitivity of the saccharide sensor array,our group designed a coumarin-porphyrin FRET complex named4Esculetin-BAAPfor pattern recognition of 7 kinds of monosaccharides (Fig.7) [72].Complex4Esculetin-BAAPwas synthesized through the formation of boronate ester bonds between esculetin and a boronic acid appended porphyrin.Looking into the chemical structure of4Esculetin-BAAPcomplex,four esculetin molecules were incorporated as the FRET energy donor while only one porphyrin acted as the energy acceptor.With an improved FRET energy donor to acceptor ratio,the FRET efficiency between esculetin and porphyrin within the complex was determined to be about 93.9%,apparently higher than the control molecule with only two esculetin energy donors.By dissolving4Esculetin-BAAPin PBS solutions with different pH values,a three-component sensor array4EBSAwas constructed for discrimination of 7 kinds of monosaccharides including glucose,fructose,mannose,galactose,glucose-6-phosphate,fructose-2-phosphate and fructose-6-phosphate even in a visualized way(Fig.7).Due to an enhanced FRET efficiency,sensor array4EBSAwas successfully applied for discrimination of tested saccharides in diluted serum samples with a sensitivity of 0.01 mmol/L.
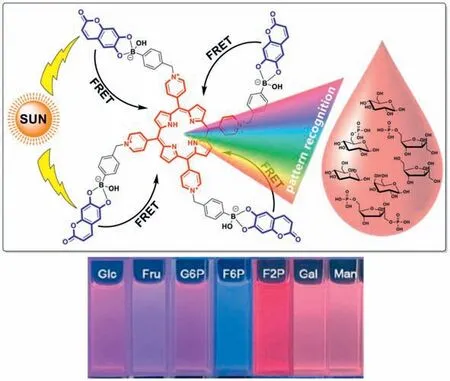
Fig.7.Top: working principle of coumarin-porphyrin complex 4Esculetin-BAAP for pattern recognition of monosaccharides;down: visual detection of monosaccharides using 4Esculetin-BAAP in pH 7.4 PBS buffer.Reproduced with permission [72].Copyright 2023,Elsevier B.V.
Looking through above examples,porphyrins can be considered as ideal building blocks to construct supramolecular fluorescent sensors for saccharide recognition.In addition,the example of4Esculetin-BAAPcomplex suggested that it is meaningful to increase the energy donor to acceptor ratio when designing FRET sensors,which could improve the energy transfer efficiency and sensitivity of the sensor.
4.Conclusions
In this review,the research progress in the development of porphyrin-based fluorescent sensors and sensor arrays for saccharide recognition was discussed with representative examples.We focused on using porphyrin as the fluorescence signal reporter because porphyrins exhibit some unique merits such as high chemical stability,long emission wavelength and multiple structural modification strategies.Generally,porphyrins were successfully applied for designing saccharide molecular sensors through two approaches.
One approach was to incorporate a suitable recognition unit to the porphyrin fluorophore to achieve highly selective fluorescent saccharide sensors.The difficulty for this approach lies in the fact that saccharides are hydromimetic in aqueous solutions and they share similar chemical structure.Therefore it was better to design a full complementary interaction system to increase the binding constant,where hydrogen bonding interactions should be integrated with hydrophobic,CH-πand electrostatic interactions.Among the commonly used recognition units for saccharide,boronic acid could be considered as an outstanding one,as it was able to form dynamic covalent bonds with the 1,2-diol moiety of saccharides.With the assistance of another saccharide bind unit substituted at a reasonable distance,boronic acid appended porphyrins could even exhibit a saccharide binding ability that equal to natural occurring saccharide binding proteins.
Another approach to achieve the purpose of practical saccharide sensing was to construct porphyrin-based sensor arrays for pattern recognition of saccharides.Since the working principle of a sensor array do not require the design of a highly selective recognition unit for the target saccharide,it is reasonable to pay more attention in the design of cross-reactive sensor arrays for saccharide recognition.However,the construction of a saccharide sensor array needs the design of several cross-reactive sensory units,which could significantly increase the production cost.It should therefore consider a balance between the cost and practical application needs.
In conclusion,porphyrins have been successfully applied for designing fluorescent sensors and sensor arrays for saccharide recognition.Considering that the number of literatures that focused on this topic was relatively limited,research on the development of porphyrin-based saccharide molecular sensors is still at an initial stage.It is obvious that the future development of both porphyrin-based saccharide sensors and sensor arrays will still rely on supramolecular approaches such as the indicator displacement assay.Therefore,one consideration is to use porphyrins as the building blocks to construct novel supramolecular structures such as molecular cages and metal-organic frameworks (MOFs)with suitable or even dynamic inner space to recognize and accommodate the target saccharide.We believe that these excellent literature results would inspire the further development of novel porphyrin-based fluorescent saccharide sensors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos.22278229,22131005 and 21904066) and 333 High Level Talent Project of Jiangsu Province.
杂志排行
Chinese Chemical Letters的其它文章
- The 3rd Xihua Chemistry and Biomedicine Forum
- Professor Hualiang Jiang: A tribute to an esteemed visionary chemist and pharmacist
- Recent advances in visible light-mediated chemical transformations of enaminones
- Recent advances of versatile reagents as controllable building blocks in organic synthesis
- Synthetic host-guest pairs as novel bioorthogonal tools for pre-targeting☆
- Functional nanoemulsions: Controllable low-energy nanoemulsification and advanced biomedical application
