Vinyltrimethylsilane as a novel electrolyte additive for improving interfacial stability of Li-rich cathode working in high voltage
2024-04-05BingJingHoLiBiLuoLehoLiuLihuChuQioboZhngMeichengLi
Bing Jing ,Ho Li ,Bi Luo ,Leho Liu ,Lihu Chu ,Qiobo Zhng ,Meicheng Li,*
a State Key Laboratory of Alternate Electrical Power System with Renewable Energy Sources,School of New Energy,North China Electric Power University,Beijing 102206,China
b State Key Laboratory of Physical Chemistry of Solid Surfaces,College of Materials,Xiamen University,Xiamen 361005,China
Keywords: Vinyltrimethylsilane Electrolyte additive Li-rich cathode Interfacial stability Capacity retention
ABSTRACT Boosting the interfacial stability between electrolyte and Li-rich cathode material at high operating voltage is vital important to enhance the cycling stability of Li-rich cathode materials for high-performance Li-ion batteries.In this work,vinyltrimethylsilane as a new type of organic silicon electrolyte additive is studied to address the interfacial instability of Li-rich cathode material at high operating voltage.The cells using vinyltrimethylsilane additive shows the high capacity retention of 73.9% after 300 cycles at 1 C,whereas the cells without this kind of additive only have the capacity retention of 58.9%.The improvement of stability is mainly attributed to the additive helping to form a more stable surface film for Li-rich cathode material,thus avoiding direct contact between the electrolyte and the cathode material,slowing down the dissolution of metal ions and the decomposition of the electrolyte under high operating voltage.Our findings in this work shed some light on the design of stable cycling performance of Li-rich cathode toward advanced Li-ion batteries.
In recent years,with the rapid development of energy storage power stations,electric vehicles and various electronic products,the market demand for lithium-ion batteries (LIBs) has also increased sharply [1-8].Therefore,the pursuit of high energy density lithium-ion battery has become the focus,especially for the cathode material.The development of lithium-ion battery cathode materials has gone through a long process,the traditional cathode materials cannot meet the current demand for energy density,such as LiCoO2(145 mAh/g),LiMn2O4(120 mAh/g) and LiFePO4(165 mAh/g) [9,10].Compared with these traditional cathode materials,Li-rich (xLi2MnO3·(1-x)LiTMO2(TM=Ni,Mn,Co),whenx=0.5,in the form of Li1.2Mn0.54Co0.13Ni0.13O2) cathode materials have attracted extensive attention due to their high reversible capacity(more than 250 mAh/g),high operating potential (2-4.8 V) and the lower cost [11-13].However,Li-rich cathode will be destroyed because the serious decomposition of electrolytic liquid under high cycle potential.Therefore,it is very important to improve the stability of Li-rich cathode materials under high cycle potential.
In order to improve the stability of Li-rich cathode materials,the researchers have done many works,in which two of the most traditional methods are doping [14,15] and surface coating [16-18].However,traditional modification methods such as doping and surface coating are complex and expensive.Therefore,introducing additives into electrolyte to form protective interface film on cathode surface is considered to be one of the effective and economical methods to enhance the electrochemical performance of layered lithium rich cathode materials [19,20].
Because of the excellent performance and economic benefits of electrolyte additives,the electrolyte additives introducing into lithium-ion batteries have aroused wide interest of many researchers.In recent years,a large number of electrolyte additives have been reported,such as boron additives [21,22],organophosphorus additives [23-26],carbonate additives [27],sulfur additive [28-30],ionic liquid additive [31] and some inorganic lithium salt additives [32-34].Besides,silicon compounds have high thermal and chemical stability,and become a hot research topic.Hieu Quang Phametal.demonstrated that methoxytriethyleneoxypropyltrimethoxysilane (MTE-TMS) electrolyte additive is able to stabilize the interface of both Ni-rich layered NCM851005 cathode and graphite anode in a full-cell [35].Bangwei Dengetal.used diphenyldimethoxysilane (DPDMS) as electrolyte additive greatly increased the capacity retention of Li/NCM622 half-cell [36].Seol Heui Jangetal.demonstrated that dimethoxydimethylsilane(DODSi) electrolyte additive can improve surface stability of Ni-rich cathode [37].
As a kind of silicon compound,comprising of a C=C double bond and a silicon-based functional group,vinyltrimethylsilane has good stability and has the unsaturated functional groups that are easy to be oxidized.In the other hand,compared to other silicon compounds,vinyltrimethylsilane has a much simpler structure and more practical values and economic benefits,because it is easy to be obtained.In this work,we propose to use vinyltrimethylsilane as a novel electrolyte additive in lithium-ion batteries,which could be oxidized prior to other components of the electrolyte to form a more stable interface film to inhibit the decomposition of the electrolyte and reduce side reactions with Li-rich cathode material.
The original electrolyte (DoDo Chem) is 1 mol/L LiPF6in ethylene carbonate (EC) and dimethyl carbonate (DMC) (3:7,v:v).As the electrolyte additive ALFA was added to the original electrolyte.The electrolyte with various concentrations of ALFA of 0.2%,0.5% and 1% in weight was prepared in argon-filled glove box(Mikrouna,China,H2O<0.1 ppm,O2<0.1 ppm).
The cathode slurry consisting of active material (LRNCM),acetylene black (Super-P) and polyvinylidene fluoride (PVDF) with the ration of 8:1:1 in weight were mixed inN-methyl pyrrolidone(NMP).Then the slurry was coated in the aluminum foil and dried in a vacuum oven at 120 °C for 12 h.The coin half-cells were assembled with Li metal disk as anode and glass fiber (Whatman)as separator.Two kinds of electrolyte were original and modified by additives.CR2032-type coin-cells were prepared in argonfilled glove box,where the content of H2O and O2were less than 0.1 ppm.
The cycle performance of LRNCM/Li half-cells were measured by multichannel battery test system (Land,China),which were charged to 4.8 V and discharged to 2.0 V (1 C=250 mAh/g).Linear sweep voltammetry (LSV),cyclic voltammetry (CV),chronoamperometry (CA) and AC impedance were performed by electrochemical workstation (CHI,China).LSV tests were measured at a scan rate of 1 mV/s from open-circuit voltage (OCV) of ≈3.0 V to 6.0 Vvs.Li+/Li,with stainless steel gasket as a working electrode,and lithium metal as the counter electrode.CV curves were measured at a scan rate of 0.1 mV/s in a voltage range of 2.0-4.8 V.CA was measured by maintaining the cells at 4.8 V after 3 cycles charge to 4.8 V at 0.1 C.The AC impedance was recorded during cycling and the data was fitted with the use of Z-View software.The frequency range is from 100 kHz to 0.01 Hz under 10 mV,
Crystal structure of the cathode material after cycling was analyzed by X-ray diffraction (XRD,BRUKER,D8 Focus,Germany),in which incidence angle is at a range of 10°-80° with the speed of 6°/min.X-ray photoelectron spectroscopy (XPS,ESCALAB 250Xi,overall resolution: 1 eV,Al peak) was used to investigate the surface of the cathode materials.The morphology of the cathode materials was investigated before and after cycling,by scanning electron microscope (SEM,Quanta 200F) and transmission electron microscope (TEM,Tecnai G2 F20).
The electrochemical stability of cells with original electrolyte without vinyltrimethylsilane and modified electrolyte with 0.5%vinyltrimethylsilane are measured respectively by linear sweep voltammetry (LSV) at a scan rate of 1 mV/s from open-circuit voltage (OCV) about 3.0-6.0 Vvs.Li+/Li (Fig.1a).Between 3.2 V to 3.7 V,an obvious oxidation peak is found in the curve with 0.5%vinyltrimethylsilane in the inset of Fig.1a,whereas the curve of original sample with original electrolyte is smooth,which indicates that vinyltrimethylsilane is oxidized in this interval.Above 4.0 V,the curve of the cell without additive rises abruptly,indicating that the electrolyte oxidizes and decomposes drastically.While through adding 0.5% vinyltrimethylsilane additive,the upward trend of the curve is slowed down and the oxidative current is distinctly reduced.These results show that oxidation is more influenced by vinyltrimethylsilane than other components of the electrolyte.Vinyltrimethylsilane can inhibit the oxidative decomposition of other electrolyte components under high operating potential [38-40].Fig.1b shows that the CV curves of LRNCM||Li half-cells without additive and with 0.5% vinyltrimethylsilane in the first cycle.The redox peak potential (ΔV) without additive is 0.746 V,whereas theΔVwith 0.5% vinyltrimethylsilane is 0.708 V,from which it is found thatΔVwas reduced about 40 mV because of vinyltrimethylsilane.The results show that the vinyltrimethylsilane probably improves the interface stability of the cathode material.In the CV curves,there are two significant oxidation peaks.One is the oxidation peak around 4.0 V associated with the oxidization of Ni2+and Co3+process.The other oxidation peak around 4.6 V is related to the partially reversible anion O2-→O2n-and the irreversible loss of oxygen during the first charge [41,42].With the addition of vinyltrimethylsilane,the oxidation current at 4.6 V decreased,indicating that the vinyltrimethylsilane inhibited the oxidation decomposition of electrolyte to a certain extent [40],which was almost consistent with the LSV test results.Fig.1c shows that the CV curves of LRNCM||Li half-cells without additive and with 0.5% vinyltrimethylsilane in the second cycle.We can find that two cells have similar curves and the cell with additive have closer REDOX peak difference,indicating that the surface film is more stable.CA is used to verify the oxidation stability of electrolyte at 4.8 V (Fig.1d).The two curves represent the residual current of LRNCM||Li half-cells with 0.5% vinyltrimethylsilane and without additive at 4.8 V.The cell without additive shows a larger residual current,which attributed to the more serious decomposition of the electrolyte [33,35,49].Whereas the lower residual current in the curve of the cell with 0.5% vinyltrimethylsilane indicates that additive can promote to form a more stable,dense protective CEI film on the cathode surface,and inhibit the oxidation and decomposition of the electrolyte.Figs.1e-g represent the charge and discharge performance of LRNCM||Li half-cells without additive and with 0.5% vinyltrimethylsilane additive.The cathode material also shows good cycling stability during long-term cycling at 1 C using the modified electrolyte (Fig.1e).The cell with original electrolyte presents an initial capacity of the cell of 239 mAh/g and a capacity retention of 58.9% after 300 cycles at 1 C.The cell with 0.5%vinyltrimethylsilane have the same initial capacity of 239 mAh/g and a final capacity of 177.2 mAh/g,indicating that the additive improves the capacity retention to 73.9% after 300 cycles at 1 C.Figs.1f and g show the voltage profiles of LRNCM||Li half-cells with and without vinyltrimethylsilane at different cycles.The cell with additive shows a better voltage platform.The average charging and discharging voltage is also slightly higher than the cell without additive (Fig.1h).
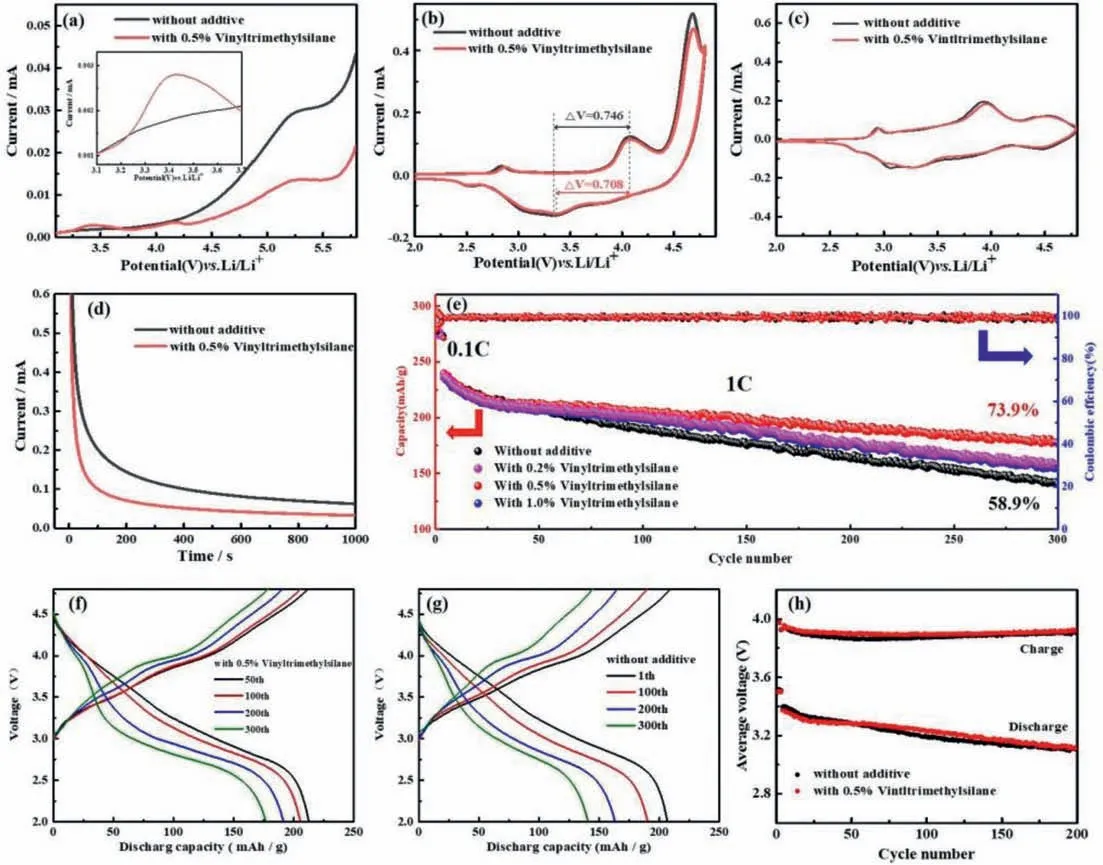
Fig.1.(a) LSV curves;(b) CV curves of LRNCM||Li half-cells at the first cycle.(c) CV curves of LRNCM||Li half-cells at the second cycle.(d) CA curves of LRNCM||Li half-cells after 3 cycles charge to 4.8 V at 0.1 C.(e) Cycling performance at 1 C of LRNCM||Li cells with and without vinyltrimethylsilane in a voltage range of 2.0-4.8 V.(f,g) Voltage profiles.(h) Average voltage.
In order to prove the results above,the electrochemical resistance was investigated after charging to 4.8 V.As shown in Fig.2,Nyquist plots are made of solution resistance (Rsol),the surface film resistance (Rsf),the charge transfer resistance (Rct),and Warburg impedance (Zw).Figs.2a-d show the impedance diagram of the cells with two different electrolytes at various cycles.At the beginning,Rsfof the cell with 0.5% vinyltrimethylsilane is slightly greater than the cell without additive,indicating that additives promote the formation of the denser interfacial film.During the cycles,the composition of the surface film changes,which causes the impedance value to change constantly.Therefore,Rsfof the cell with 0.5% vinyltrimethylsilane changed less than that of the cell without additive,indicating additives can promote to form a more stable interfacial film.In particular,Rctis obviously reduced.From Table 1,after 250thcycles,Rctof the cell without additive is 216Ω,whereas that of the cell with 0.5% vinyltrimethylsilane is only 103.6Ω.The results show that the stable interface film can prevent the cathode materials from electrolyte erosion during charging and discharging,so that the cycle life of cells is improved obviously [48].

Table 1Impedance fitting results of z view.

Fig.2.AC internal resistance of LRNCM||Li cells without additive and with 0.5%vinyltrimethylsilane after (a) 5th,(b) 50th,(c) 150th,(d) 250th cycles.(e) Relevant equivalent circuit,and the corresponding impedance parameters of (f) Rsf and (g)Rct.
Figs.3a-c show X-ray diffraction data of the cathode before and after 250 cycles at 1 C.Fig.3d shows the calculation results ofR-factor andI(003)/I(104).From previous reports,(I(006)+I(012))/I(101) is defined asR-factor,which ratio is related to hexagonal ordering and positive correlation (Smaller Rfactor corresponding the lower the hexagonal ordering).In addition,I(003)/I(104) is reported relevant to cation mixing of the cathode,in which the smallerI(003)/I(104) ratio,the worse cation mixing of the cathode [33,42-44].Therefore,the sample with 0.5%vinyltrimethylsilane shows better structural stability after 250thcycles (Fig.4d).Moreover,the diffraction peak of (003) without additive shifts slightly to lower 2θregion compared to the sample with 0.5% vinyltrimethylsilane after 250thcycles (Fig.4b),because of the loss of structural order and crystallinity [35].These results further evidence that vinyltrimethylsilane additive is effective to prevent the cathode material from structural destruction after long cycling at the high operating voltage.
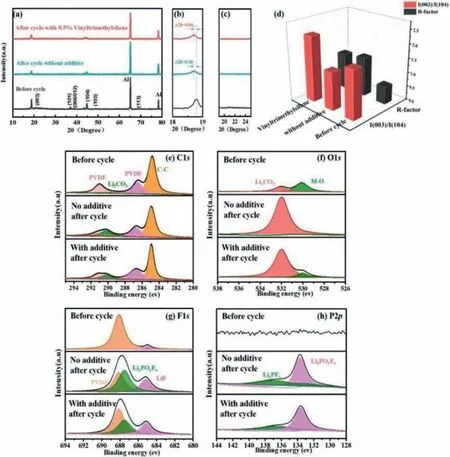
Fig.3.(a-c) The X-ray diffraction data and (d) calculated structural parameters of pristine and without additive and with 0.5% vinyltrimethylsilane after 250 cycles at 1 C.XPS spectra of the electrode: (e) C 1s,(f) O 1s,(g) F 1s,(h) P 2p.
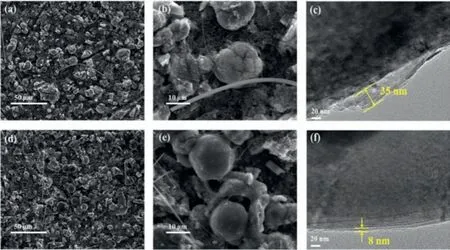
Fig.4.Morphology of the LRNCM cathode taken from LRNCM||Li half-cells after cycles.(a,b) SEM images of the LRNCM cathode without additive.(d,e) SEM images of the LRNCM cathode with 0.5% vinyltrimethylsilane.(c) TEM image of the LRNCM cathode without additive.(f) TEM image of the LRNCM cathode with 0.5% vinyltrimethylsilane.
The composition and chemical states of the surface films were characterized by XPS (Figs.3e-h).From C 1s,we can observe four peaks,the peak at 284.7 eV is corresponding to C-C,and the peak at 286.5 eV and 291 eV are corresponding to C-H and C-F of PVDF[33,35,42],respectively.The peak of C=O at 290 eV mainly corresponding to Li2CO3[42],which is mainly from the oxidation decomposition of electrolyte.More Li2CO3is observed in the cathode without additive than that with vinyltrimethylsilane,which is consistent with the result of the peak at 532 eV (C=O) in O 1s.It indicates that the oxidation decomposition of electrolyte is more serious in the cell without additive.Besides,we can make the same inference from F 1s and P 2p.The peak at 685.1 eV and 687.5 eV in F 1s are corresponding to LiF and LixPOyFz[45],respectively,which are mainly from the oxidation decomposition of electrolyte.The peak at 133.8 eV and 136.8 eV in P 2p have the same explanation as above,which are corresponding to LixPOyFzand LixPFy[46,47],respectively.From O 1s,the peak at 530 eV corresponding to transition metal M-O (M=Ni,Co,Mn) [35,40],the cathode with vinyltrimethylsilane contains more transition metal than that without additive.It shows that in the cell without additive,the cathode is more seriously corroded by electrolyte and more transition metals are dissolved.In summary,we can infer that with the addition of vinyltrimethylsilane,a more stable interface film forms in the cathode interface,which can inhibit the decomposition of electrolyte and the dissolution of transition metal ions,improving the cycle life of Li-rich cathode.
Figs.4a-f show the SEM and TEM of the LRNCM cathode taken from LRNCM||Li half-cells after cycles.The morphology of LRNCM cathode without additive (Figs.4a and b) and with 0.5% vinyltrimethylsilane (Figs.4d and e) after cycles were observed,respectively.There are some cracks in the cathode particles without additive after cycles,whereas the cathode with 0.5%vinyltrimethylsilane presents the better integrity.The results show the vinyltrimethylsilane additive could prevent the cathode materials from the erosion by electrolyte.The fragmentation of cathode particles without additive is more serious due to severe side reactions with the electrolyte,which leads to poor cyclic stability.Figs.4c and f show the TEM characterization,in which the cathode with additive shows the more stable,uniform and thinner interfacial film.This film is more conducive to the migration of lithium ions,protect the cathode material,and inhibit the side reaction with the electrolyte.Therefore,the cyclic stability of Li-rich cathode material is greatly improved.
Based on the above analysis results,Fig.5 shows briefly the surface modification mechanism of the vinyltrimethylsilane additive on Li-rich cathode.Vinyltrimethylsilane has good stability structure and is more easily oxidized and because of its C=C double bond and a silicon-based functional group.Therefore,it promotes the formation of more stable,uniform and thinner interface film on the surface of the cathode materials.Thus,the oxidation decomposition of electrolyte is inhibited and the erosion of cathode material by electrolyte could be slowed down.Because of the addition of vinyltrimethylsilane,most of cathode particles are intact and the transition metals are less dissolved after cycles,which are attributed to the more stable,uniform and thinner interface film.Therefore,the vinyltrimethylsilane additive improves the cyclic stability if Li-rich cathode.

Fig.5.The schematic for the surface modification mechanism of the vinyltrimethylsilane additive on Li-rich cathode (a) without additive and (b) with vinyltrimethylsilane.
In summary,the effects of the vinyltrimethylsilane additive in electrolyte on the stability of Li-rich cathode materials in Li-ion half-cells.The results show that the capacity retention of the cells is improved from 58.9% to 73.9% under 1 C after 300 cycles by vinyltrimethylsilane.Compared with the cells without additives,a more stable,uniform and thinner interface film is observed on the cathode surface because of vinyltrimethylsilane with the better oxidability.This CEI film inhibits the oxidation decomposition of electrolyte during the cycles and the dissolution of metal ions of cathodes at the high operating voltage.This paper presents vinyltrimethylsilane as a novel Si-based electrolyte additive,which can improve interfacial stability of Li-rich cathode working in high voltage.
Declaration of competing interest
The authors declare that they have no know competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is supported partially by State Key Laboratory of Alternate Electrical Power System with Renewable Energy Sources(Nos.LAPS21004,LAPS202114),National Natural Science Foundation of China (Nos.52272200,51972110,52102245 and 52072121),Beijing Science and Technology Project (No.Z211100004621010),Beijing Natural Science Foundation (Nos.2222076,2222077),Hebei Natural Science Foundation (No.E2022502022),Huaneng Group Headquarters Science and Technology Project (No.HNKJ20-H88),2022 Strategic Research Key Project of Science and Technology Commission of the Ministry of Education,China Postdoctoral Science Foundation (No.2022M721129) and the Fundamental Research Funds for the Central Universities (Nos.2022MS030,2021MS028,2020MS023,2020MS028),the NCEPU "Double First-Class" Program and the State Key Laboratory of Alternate Electrical Power System with Renewable Energy Sources (No.LAPS22005).
杂志排行
Chinese Chemical Letters的其它文章
- The 3rd Xihua Chemistry and Biomedicine Forum
- Professor Hualiang Jiang: A tribute to an esteemed visionary chemist and pharmacist
- Recent advances in visible light-mediated chemical transformations of enaminones
- Development of porphyrin-based fluorescent sensors and sensor arrays for saccharide recognition
- Recent advances of versatile reagents as controllable building blocks in organic synthesis
- Synthetic host-guest pairs as novel bioorthogonal tools for pre-targeting☆
