Anion cascade reactions III: Synthesis of 3-isoquinuclidone bridged polycyclic lactams☆
2024-04-05ZhiguoZhangBingbingShiXiyangCaoNanaMaHaoWuXingjieZhangGuishengZhang
Zhiguo Zhang ,Bingbing Shi,Xiyang Cao,Nana Ma ,Hao Wu,Xingjie Zhang,Guisheng Zhang
Key Laboratory of Green Chemical Media and Reactions,Ministry of Education,Collaborative Innovation Centre of Henan Province for Green Manufacturing of Fine Chemicals,NMPA Key Laboratory for Research and Evaluation of Innovative Drug,School of Chemistry and Chemical Engineering,Henan Normal University,Xinxiang 453007,China
Keywords: Anion cascade reactions 3-Isoquinuclidone Bridged polycyclic lactams Bis-Michael addition reaction Hemiaminalization
ABSTRACT Bridged polycyclic lactams are important structural units in organic functional materials,natural products,and pharmaceuticals.A flexible and efficient anion cascade reaction was developed for the preparation of bridged polycyclic lactams from readily available malonamides and 1,4-dien-3-ones.Various highly substituted bridged polycyclic lactams were synthesized in good to excellent yields by tandem nucleophilic sequences in the presence of tBuOK in commercially available EtOH solvent at 60°C.Notably,the simple reactions can be run on a gram scale.Mechanistically,bis-Michael addition reaction and hemiaminalization reactions are involved in the tandem transformation.
Bridged polycyclic lactams that incorporate the lactam nitrogen at a bridgehead position are important units in a variety of organic functional materials,natural products,and pharmaceutical agents,and have attracted much attention from organic and bioorganic chemists over the past several decades [1-8].In natural products,iboga-type alkaloids,which are well-known monoterpenoid indole alkaloids,share a characteristic isoquinu clidine unit (Fig.1) [9-12].These compounds are mostly isolated from plants of theTabernantheorTabernaemontanespecies belonging to the Apocynaceae family and show a wide range of pharmacological effects such as antiaddictive,anti-fungal or anti-lipase,anti-HIV-1,anti-cholinesterase,and leishmanicidal activities.
Over the past few decades,the medicinal and application potentials of these compounds have encouraged great efforts by synthetic chemists to develop simple and efficient synthetic methods to construct this type of bridged polycyclic lactams.However,bridged bicyclic lactams that incorporate a twisted amide are unable to achieve a standard planar geometry,making them less easy to synthesize than planar amides [13-16].Moreover,it has been reported that the twisted amide moiety embedded in the bridged ring causes these amides to undergo rapid hydrolysis[17,18].As a result,standard methods used for the construction of amide bonds often afford low yields or are accompanied by diffi-culties in product separation.A review of the literature shows that inverse-electron-demand Diels-Alder cycloaddition reactions of an electron-deficient 2-pyridones are the most common method to obtain this type of bridged polycyclic lactams (Scheme 1a) [2,19-21].Alternatively,the compounds can also be obtained by an intramolecular aminocyclization of 4-aminocyclohexanecarboxylates,starting from the 4-substituted cyclohexanoate derivatives(Scheme 1b) [12,22-26].Occasionally,catalytic hydrogenation of the aromatic ring moiety of 4-aminobenzoates affords the 4-aminocyclohexanecarboxylatesinsituin the presence of transition metal catalysis at high temperature [27-33],which can also be transformed to the bridged polycyclic lactamsviaan aminocyclization reaction (Scheme 1c).However,most synthetic routes have targeted simple representatives in the bridged polycyclic lactams with pre-functionalized starting materialsviatedious procedures under harsh conditions.Thus,efficient construction of structurally complex bridged polycyclic lactams bearing various functional groups in a minimal number of steps remains highly desirable.This ability might facilitate later derivatization for building a library of materials and biologically relevant functional organic molecules.Herein,we report a series of anion cascade reactions with important theoretical significance and practical application value for the synthesis of multi-substituted bridged polycyclic lactams with a broad range of substituents tolerance and high chemical conversion,starting from readily available malonamides and 1,4-dien-3-ones in the presence oftBuOK in commercially available EtOH solvent at 60°Cviaan intermolecular bis-Michael addition reaction and hemiaminalization cascade annulation reaction (Scheme 1d).

Scheme 1.Methods to bridged polycyclic lactams.
As versatile five-carbon building blocks,1,4-dien-3-ones can react with various bifunctional nucleophiles [34],such as malonic esters [35,36],malononitrile [37,38],cyanoacetates [39,40],Nbenzoylated glycines [41],nitromethane [42],and even oxindoles[43] and azlactones [44].Hence,these compounds have been extensively used for the preparation of functionalized organic compoundsviathe bis-Michael addition reaction,which is essentially an anion cascade reaction.This double-conjugate 1,4-addition process occurs through the continuous production of negative ions by the substrate under suitable conditions followed by nucleophilic attack on the bis-Michael acceptors,forming new chemical bonds.However,reports to date have mainly focused on the formation of chain [45],cyclic [46],fused-and spirocyclic analogues [47].In contrast,the generation of bridge polycyclic lactams has not been well documented,currently only starting from 1,4-dien-3-ones and active methylene compounds [48-53].
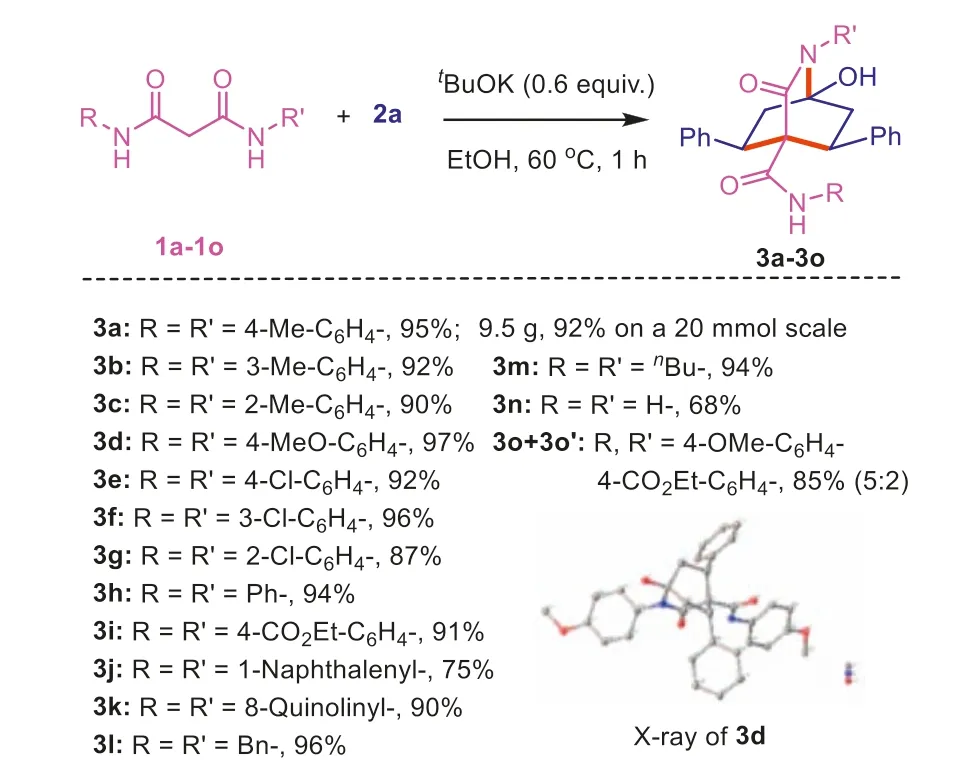
On the basis of our decades of work on the derivation of acetamides [54-58],we hoped to develop more convenient and practical methods for amide derivatization to enrich amide chemistry [59-65].Recently,we developed a highly efficient,selective,and flexible method for the straightforward synthesis of biologically valuable nonsymmetrical malonamides in excellent yieldsviacarbon-based regiospecific nucleophilic addition under mild conditions [66].A literature review indicated that readily available malonamides have not been used for the bis-Michael addition reaction with 1,4-dien-3-ones to assemblegem-dicarboxamidesubstituted cyclohexyl-4-one derivatives,although this approach may provide an intermolecular novel cascade of bis-Michael addition/bridging reactions that can be used to construct multisubstituted bridging polycyclic lactams (Scheme 1d).With this assumption in mind,readily available malonamideN1,N3-di-ptolylmalonamide (1a) and 1,5-diphenylpenta-1,4-dien-3-one (2a)were selected as model substrates.We attempted to achieve their transformation to 1-hydroxy-3-oxo-5,8-diphenyl-N,2-di-p-tolyl-2-azabicyclo[2.2.2]octane-4-carboxamide (3a).
Currently,green chemistry is one of the most powerful means of achieving efficient organic conversion that has been utilized in the synthesis of functional organic molecules with tailored properties [67-73].Many papers have reported that bis-Michael addition reactions can be carried out in the presence of base in eco-friendly EtOH media [40,42,48].Accordingly,we performed the reaction with 0.2 equiv.oftBuOK in commercially available EtOH at heating conditions.To our delight,we obtained product3ain 79% yield as a white solid when the reaction was heated at 60°C for 4 h(Table 1,entry 1).The conjugate 1,4-addition reaction proceeded as expected;however,the bridging reaction step also unexpectedly occurred smoothly under such mild conditions.Further investigations indicated that 0.6 equiv.oftBuOK afforded the highest yield of3a(95%),which is higher than that of 0.4 equiv.(86%) and 1.2 equiv.(89%) of base (Table 1,entries 2-4).In addition,neither lowering nor increasing the temperature increased the yield of the target compound3a(Table 1,entries 5 and 6).According to the literature,several bases,including inorganic basestBuONa,EtONa,KOH,NaOH,K2CO3,and organic base DBU,commonly used for bis-Michael addition have also been tested.As a result,they all showed a slightly lower reactivity than that oftBuOK (Table 1,entries 7-12).Traditionally,tBuOH andtBuOK are often used as a fixed pairing in the reaction.However,tBuOH media gave a slightly lower yield of3athan that of EtOH,and was therefore not as economical as EtOH (Table 1,entry 13vs.3).In consideration of the requirements of green chemistry,we chose ethanol as the reaction medium to explore the scope of the reaction (Schemes 2 and 3).Other solvents,such as THF,DMSO,and MeCN,provided less than 95% yield of3a(72%-88%) (Table 1,entries 14-16).Notably,the reaction performed in MeOH gave product3ain 8 h in the presence of 2.2 equiv.of2a(Table 1,entry 17).

Table 1Reaction optimization.a
With the optimal reaction conditions in hand (Table 1,entry 3),we explored the scope of this tandem reaction to access to various substituted 3-isoquinuclidones3,and the main results are listed in Schemes 2 and 3.First,various readily available malonamides1were carefully selected and reacted with 1,4-dien-3-one2a(Scheme 2).Experiments have shown that variousN-aryl substituted malonamides bearing electron donating groups (EDGs) (-OMe,-Me,-Cl),as well as the phenyl group,can react smoothly with2ato generate target products 2-azabicyclo[2.2.2]octanones3a-3hin yields of 87%-97% after 1 h.It was found that 9.5 g of3a(92%) was obtained after 1 h with treatment of 20 mmol of1aand 24 mmol of2awithtBuOK,demonstrating the flexibility and practicality of this strategy.Notably,the ethanol media could be recovered under reduced pressure after the reaction.Moreover,product3acould be obtained with higher purity from the above crude residue after simple workup.The structure of3dwas further confirmed by X-ray crystallography studies (CCDC: 2285766).Pleasingly,a starting material with an electron withdrawing group(-CO2Et) also gave a 91% yield of3i.Two kinds of malonamides substrates derived fromα-naphthylamine and 8-aminoquinoline,respectively,were well tolerated by the reaction.Additionally,Nalkyl-substituted malonamides gave high yields of3land3min 96% and 94%,respectively.In contrast,product3nwas isolated in a slightly lower yield.However,the free hydroxyl group,primary amide,and secondary amide moiety in the product3nprovide valuable derivatization sites for late-stage biological reactions.Interestingly,nonsymmetrical malonamides regioselectively generated two kinds of 3-isoquinuclidone derivatives3oand3o′in the mixture of 5:2 in 85% total yield,judging from the1H NMR spectrum.Of course,an ester exchange reaction occurred in this conversion because we used ethanol as the solvent.
Scheme 2.Extension of the reaction scope of malonamides 1a-1o with 2a.All reactions were conducted with 1 (0.3 mmol),2a (1.2 equiv.),and tBuOK (0.6 equiv.) in EtOH (3 mL) at 60°C in oil bath for 1 h.
Further transformation of1awith various 1,4-dien-3-ones showed that all reactions proceeded well (Scheme 3).Notably,1,5-diaryl substituted 1,4-dien-3-ones bearing a -OMe group at thepara-,meta-,andortho-position smoothly produced target products3p-rin yields of 85%-90%.However,the substrate with the-CO2Et group at thepara-position gave product3sin less than 72% yield owing to electronic effects.The introduction of the aryl bromine substituent also gave a good yield of3t(76%).As an important benefit,this product provides the possibility for further derivatization reactions including Buchwald-Hartwig [74-76],Suzuki-Miyaura [77-81],and Sonogashira coupling reactions [82-86].Furanyl-and thiophenyl-substituted divinyl ketones afforded new bridged lactams3uand3vin 87% and 90% yields,respectively.In addition,nonsymmetrical 1,4-dien-3-one2iyielded 3-isoquinuclidone derivative3win 90% yield.The tetrasubstituted 1,4-dien-3-one was also tolerated and gave the desired multisubstituted 2-azabicyclo[2.2.2]octanone3xin 84% yield after 1 h withca.8:1drvalue.To our delight,conformationally restricted dienones also provided structurally diverse bridged polycyclic lactam3yand3z,respectively,in 74% and 78% yields under the optimal reaction conditions [48].

Scheme 3.Extension of the reaction scope of 1,4-dien-3-ones 2b-2l with 1a.All reactions were conducted with 1a (0.3 mmol),2 (1.2 equiv.),and tBuOK (0.6 equiv.)in EtOH (3 mL) at 60°C in oil bath for 1 h.
On the basis of the above results and previous reports concerning the Michael addition reaction-based bridging reactions [48-50,53],obviously,bis-Michael addition reaction and hemiaminalization cascade process are involved in the transformation.Accordingly,a plausible mechanism for the tandem reaction is illustrated in Scheme 4.The overall transformation commences from atBuOKcatalyzed intermolecular Michael addition of malonamides1and 1,4-dien-3-ones2to provide intermediate4viaa 1,4-conjugated addition reaction of carbanion speciesA.Then,a successive intramolecular Michael addition reaction occurs again to form5,triggered by the carbanionCgeneratedinsituin the presence of base.Rowlandetal.has reported that thecisisomer is a more thermodynamically stable configuration than the trans isomer in this type of bis-Michael addition reaction [87].This is consistent with our observations of the final compound,as the single crystal structure of product3indicates that two aryl groups exist in acis-configuration.Then,bridging reaction occurs following the bis-Michael addition reaction.Theinsitugenerated nitrogen negative ions are spatially well suited for a rapid nucleophilic attack on the electrophilic carbonyl group [49,88,89],resulting in the generation of target compound3.

Scheme 4.Proposed mechanism.
To explore the potential applicability of these strategies in bioorganic and medicinal chemistry [90-97],some malonamides were synthesized and further attempts were made to introduce rigid bridge-ring structural units (Scheme 5).Rewardingly,five kinds of malonamides derived from cabozantinib (1p),kinase inhibitor (1q),amlodipine (1r),andS-ATBA(1s) all reacted with2ain short times,and gave corresponding biologically valuable bridged azacycles3aa-3adin 74%-96% yields (Scheme 5).These derivatives with a rigid bridging ring as a linker are ideal lead compounds with high affinity and selectivity for their targets,and have important application potential in medicinal chemistry.

Scheme 5.Derivatization of several bioactive molecules.
In conclusion,we developed a highly efficient and flexible method for the construction of multi-substituted bridged polycyclic lactam derivativesviaa Michael addition-based successive nucleophilic addition process in the presence oftBuOK in commercially available EtOH media and at 60°C,leading to excellent yields.The starting materials,malonamides and 1,4-dien-3-ones,and bases and solvents,are readily available and inexpensive.Their combination shows high reactivity,excellent functional group tolerance,stability to hydrolysis and alcoholysis,and easy workup processes,facilitating late-stage derivatizations to build large collections of functional organic molecules.Importantly,this strategy can be run on a gram scale and the solvent can be recovered after the reaction,showing potential applications that may be beneficial for materials chemistry,natural products chemistry,and medicinal chemistry.In addition,examples of the rapid synthesis of3yand3zfrom simple substrates demonstrate that our strategy may open a new and efficient route to structurally diverse bridged and spiro compounds,which are difficult to achieve by conventional methods or require multiple steps.Mechanistically,bis-Michael addition reaction and hemiaminalization reaction are involved in the tandem transformation,essentially,the carbon atoms of 1,4-dien-3-ones suffer from three nucleophilic attacks by the carbon and nitrogen anions producedinsituby malonamides.Further studies on the preparation of more structurally complex bridged polycyclic lactams from readily available acyclic substrates are currently underway in our laboratory,and the progress will be reported in due course.
Declaration of competing interest
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the National Natural Science Foundation of China(NSFC,Nos.21772032,21877206,and 22101074),the 111 Project(No.D17007),Excellent Youth Foundation of Henan Scientific Committee (No.222300420012),China Postdoctoral Science Foundation (No.2019M660173),the Natural Science Foundation of Henan Province (No.202300410233),and Henan Key Laboratory of Organic Functional Molecules and Drug Innovation for financial support.We thank Liwen Bianji for editing an English draft of this manuscript.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.109184.
杂志排行
Chinese Chemical Letters的其它文章
- The 3rd Xihua Chemistry and Biomedicine Forum
- Professor Hualiang Jiang: A tribute to an esteemed visionary chemist and pharmacist
- Recent advances in visible light-mediated chemical transformations of enaminones
- Development of porphyrin-based fluorescent sensors and sensor arrays for saccharide recognition
- Recent advances of versatile reagents as controllable building blocks in organic synthesis
- Synthetic host-guest pairs as novel bioorthogonal tools for pre-targeting☆
