A sequential continuous flow synthesis and purification process of calcium dibutyryladenosine cyclophosphate
2024-04-05LimingCoMolinSunChomingLingLeiYngYueyueRuihuChengYnxiongKeWeiYuJinxingYe
Liming Co ,Molin Sun ,Choming Ling ,Lei Yng ,Yueyue M ,Ruihu Cheng ,Ynxiong Ke,*,Wei Yu,c,Jinxing Ye,
a Engineering Research Center of Pharmaceutical Process Chemistry,Ministry of Education,School of Pharmacy,East China University of Science and Technology,Shanghai 200237,China
b School of Biomedical and Pharmaceutical Sciences,Guangdong University of Technology,Guangzhou 510006,China
c Shanghai No.1 Biochemical& Pharmaceutical Co.,Ltd.,Shanghai 200080,China
Keywords: Flow chemistry Calcium dibutyryladenosine cyclophosphate Continuous flow synthesis In-line purification 3D circular cyclone-type micromixer chip
ABSTRACT Calcium dibutyryladenosine cyclophosphate is a widely used cardiovascular drug.The traditional batch synthesis process suffers from long reaction times,tedious operations,and unstable yields.Herein,a sequential continuous flow synthesis combined with a multistage in-line purification process of calcium dibutyryladenosine cyclophosphate was developed.The acylation reaction was completed in a continuous coil reactor at 160 °C in 20 min.And the high toxic solvent pyridine was replaced by acetonitrile.Furthermore,the multistage in-line purification process was integrated into the homemade 3D circular cyclone-type micromixer chip.Combining with the membrane phase separators,the residence time of the purification step was 30 s.The isolated yield of this sequential continuous process was 92% with 99%purity.
Adenosine-3′,5′-cyclic cyclophosphate (Scheme 1,cAMP,1) is an essential intracellular second messenger controlling the critical cellular functions [1].The introduction of lipophilic side chains in positionN6and/or 2′-Oof cAMP enhances its membrane permeability and overcomes its defect to being easily destructed by cyclic nucleotide phosphodiesterase [2-4].Calcium dibutyryladenosine cyclophosphate (or calciumN6,2′-O-dibutyryl cyclic adenosine-3′,5′-monophosphate.Scheme 1,dbcAMP-Ca,2),derived from cAMP,is used in the clinical treatment of cardiovascular diseases,such as angina pectoris and acute myocardial infarction [5].

Scheme 1.Structure of cyclic adenosine-3′,5′-monophosphate (1) and calcium dibutyryladenosine cyclophosphate (2).
In 1960s,Posternak and co-workers developed a synthesis method for dbcAMP [6-8].This method typically consisted of three steps,the synthesis of cAMP-Et3N,the acylation reaction,and the purification process (Scheme 2a).It was widely used in industrial production because of the mild conditions and clear reaction mechanism [9-12].However,this process suffered from the long reaction time (4-10 days) and utilization of pyridine,which is an undesirable solvent in the pharmaceutical industry due to its high toxicity [13].In addition,tedious extraction and washing were time-consuming and unstable in the purification process,as the prolonged treatment of dbcAMP in aqueous could lead to hydrolysis [9,14].Therefore,an efficient,robust,and operationally convenient process for the synthesis of dbcAMP-Ca is greatly needed.

Scheme 2.Synthesis of dbcAMP-Ca (2).
In recent years,continuous flow chemistry has become a research hotspot both in academia and industry [15-21].Compared to the traditional batch synthesis,the continuous flow mode is more reliable and reproducible due to the accurate control of the reaction parameters,such as temperature,pressure,reagent dosage,and residence time [22].It is also characterized by the fast heat and mass transfer rates,significantly improving the overall safety of the organic synthesis process even under harsh reaction conditions [23,24].Besides,the scale-up continuous flow process could be achieved rapidly by changing the reactor volume with less process optimization [25,26].So far,many new continuous flow processes for drug synthesis were developed in decades [27-29].
Herein,we described a sequential continuous flow synthesis and purification process for dbcAMP-Ca (Scheme 2b).By combining the continuous acylation reaction in a coil reactor with the multistage in-line purification based on femtosecond laser-tailored 3D circular cyclone-type micromixer chips,the limitations of long production cycles,large amounts of highly toxic reagents,and unstable yields in traditional batch processes was eliminated.
The presence of a tertiary amine was necessary for the acylation reaction [14],and cAMP-Et3N could be easily prepared.Thus cAMP-Et3N was prepared on a large scale as the starting material (see Supporting information for details of the synthesis procedure).Then,the conditions of the acylation reaction were screened in batch (Table S2 in Supporting information).DMAP (4-(N,Ndimethylamino)-pyridine),the effective nucleophilic base for the acylation of amine and alcohols [30,31],could significantly improve the reaction efficiency (Table S2,entry 3).Acetonitrile was used as solvent for the acylation reaction replacing pyridine (Table S2,entry 4 and Table S3 in Supporting information).When 20 equiv.ofn-butyric anhydride and 1 mol% DMAP were added,the quantitative conversion was obtained on a 1 mmol scale at 100 °C for 3 h(Scheme 3).
Scheme 3.The acylation reaction of cAMP-Et3N in batch.General conditions:cAMP-Et3N (1 mmol,1 equiv.),n-butyric anhydride (20 mmol,20 equiv.),DMAP(0.01 mmol,1 mol%),and acetonitrile (1.4 mL) were stirred at 100 °C for 3 h.The yield was detected by HPLC area%.
In order to further enhance the acylation process,screening of the acylation conditions was carried out in continuous flow(Table 1).A 316L stainless steel coil was used as the flow reactor,with a gas chromatography oven and a back pressure regulator(BPR) to control the temperature and the pressure (Fig.S1 in Supporting information).It was worth noting that premixing cAMPEt3N,n-butyric anhydride,and DMAP in acetonitrile into a clear solution was necessary to prevent clogging.In the presence of 0.5 mol% DMAP,the conversion was almost quantitative at 140 °C with a residence time of 25 min (entries 1 and 2).Increasing the temperature further accelerated the reaction and thus shorten the required residence time (entry 3).Reducing the amount of DMAP to 0.3 mol%,a high yield was obtained as well by increasing the temperature to 160 °C with 20 min (entries 4-7).Further reduction in the amount of DMAP to 0.1 mol% lead to reduced conversion (68%,entry 8).When the temperature was increased to 170 °C,several impurities were generated and the yield decreased to 90% (entry 9).Moreover,a long run of the continuous acylation was carried out to demonstrate the process stability (entry 10).The flow process proceeded smoothly for 5 h with>99% yield of4within the entire period (Table S4 in Supporting information).
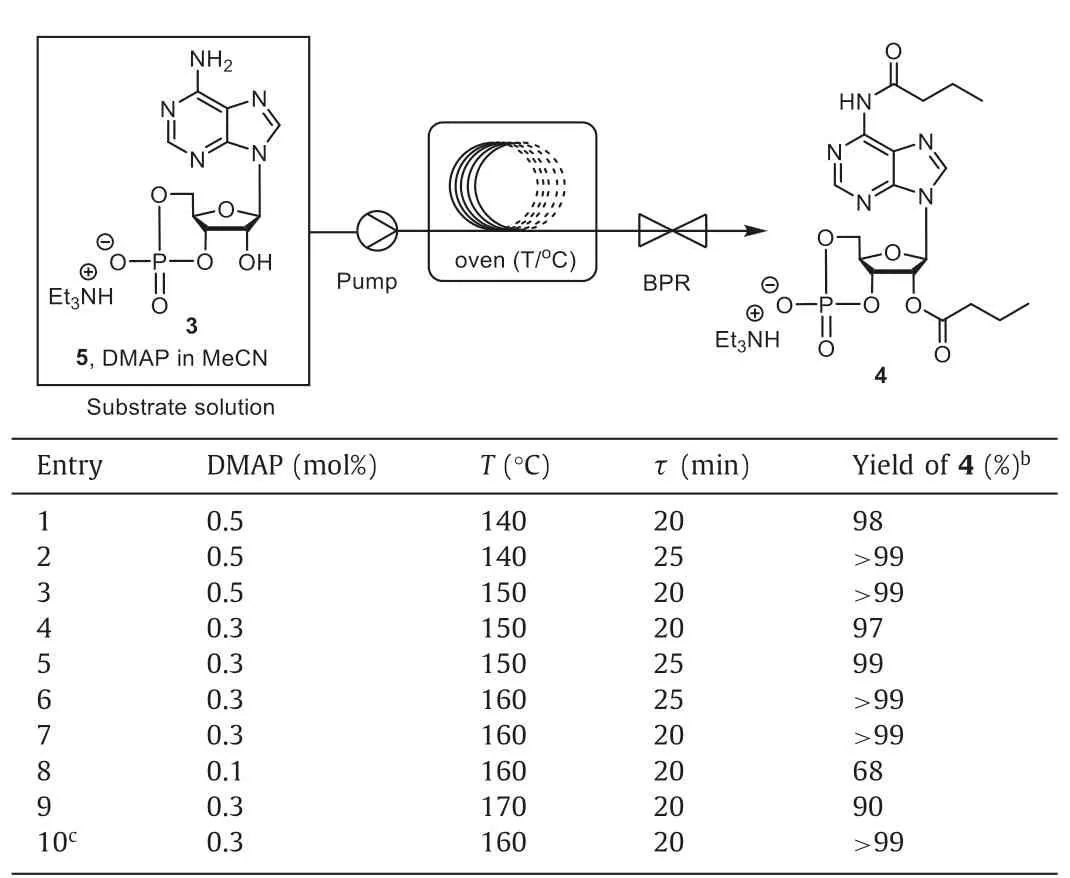
Table 1Optimization of the acylation reaction conditions in flow.a
In-line purification is an important tool to handle the unstable intermediates or multi-step sequential synthesis [32],and liquid-liquid continuous extraction is one of the common protocols [17,33-35].Recently,we designed and fabricated a glass fourmodule micromixer chip with 3D circular cyclone-type channel using femtosecond laser micromachining (Fig.1) [36-39].Multiphase fluids could be fully mixed with high mass transfer efficiency in the millimeter-level 3D cyclone passive microchannel (Fig.1a).This chip contains four individual modules,and separate temperature control channels were available for each module (Figs.1b and c),allowing operations to be performed independently in each module.Compared to traditional single-module mixer,this chip has the advantages of being highly efficient,space-saving,and versatile.

Fig.1.(a) Schematic view of the mixing mechanism of the single circular cyclonetype unit;(b) Schematic view of geometry of the four-module mixer chip;(c) Photograph of the mixer chip (contained heat transfer layers).Inset: the close view of the mixer units at the location of the red box.
Herein,we developed a multistage continuous purification process in the 3D four-module cyclone-type micromixer chip (Fig.S4 in Supporting information).As shown in Fig.2,the acylation residue was hydrolyzed by H2O in Module I,and the desired compound4could be extracted into the aqueous phase.MTBE(methylt-butyl ether) was added to dilute the reaction mixture in Module I to avoid blockage and facilitate phase separation (Table S5 in Supporting information for details).Zaiput membranebased liquid-liquid separators (SEP-10) were used for phase separation.The adjustable BPRs were fitted at appropriate locations to ensure the differential pressure of less than 0.35 MPa at the outlets of the membrane separators [40].Through Separator I,the aqueous phase of Module I flew directly into Module III,and the organic phase of Module I entered Module II for the secondary extraction.After adding H2O by Pump D and separating by Separator II,the aqueous phase of Module II also flew into Module III.The combined aqueous solution was acidified by 1 mol/L HCl (adjusted to pH 3-4),washed with extra MTBE to remove the resident fat-soluble impurities,such asn-butyric anhydride andn-butyric acid,and passed through Separator III.The aqueous phase of Module III was treated with CaHCO3in batch,followed by concentration and recrystallization to obtain the desired dbcAMP-Ca (2).The second extraction in Module II was necessary in this purification process as the yield could be improved effectively (Figs.S2 and S3,Tables S5-S7 in Supporting information).The isolated yield reached 92% (purity>99%) with a chip throughput of 16.4 g/h.Compared to the batch processes,this continuous purification process significantly simplified operations and reduced the residence time to approximately 30 s,while increasing product yield and purity.

Fig.2.The multistage in-line purification process.The holding volume of each module was 1.4 mL,and the allowable flow rate was 0-20 mL/min.The Zaiput membrane separators (SEP-10) ensured rapid separation of biphasic solutions using a hydrophobic membrane (OB-900).The BPRs were used to ensure the differential pressure was less than 3.5 MPa at the outlets of the separators.The yellow,blue and green lines represented the flow directions of the organic,aqueous and biphasic phases,respectively.The flow rate of each pump is: 3,2,4,2,5,0.2 mL/min for pumps A to F,respectively.
As shown in Fig.3,the sequential continuous synthesis and purification process for dbcAMP-Ca was established.Firstly,the substrate solution was pumped into the coil reactor with a holding volume of 60 mL to achieve the full conversion from3to4at 160 °C in 20 min.Then the acylation output was directly pumped into the in-line purification setup containing a four-module cyclone mixer chip and three Zaiput membrane separators with the residence time of 30 s.Finally,the resulting aqueous solution was subjected to batch workup to provide the desired dbcAMP-Ca as a white solid.
In addition,we implemented a scale-up of the continuous purification process,for which the circular cyclone-type micromixer chips with wider channels were manufactured (Fig.S5 in Supporting information).The holding volume of each chip was 6.5 mL and the allowable flow rate was 0-100 mL/min.The isolated yield was maintained at 93% (purity 99%),the throughput was increased to 123 g/h,where the total flow residence time was prolonged to approximately 6 min.
In summary,we have developed a sequential continuous flow synthesis and purification process for dbcAMP-Ca.The quantitative conversion of the continuous flow acylation reaction was achieved in 20 min and the high toxic solvent pyridine was replaced by acetonitrile.In the industrial application,the continuous acylation could be further optimized by using a screw pump for solid delivery.The multistage in-line purification process based on femtosecond laser-tailored 3D circular cyclone-type micromixer chips simplified the operation and improved the process stability.The yield of dbcAMP-Ca was up to 92% (purity>99%).Both the continuous acylation reactions and purification process could be expanded by increasing the reactor capacity.In addition,this multi-step continuous flow process overcomes the drawbacks of long reaction time,tedious operation,and unstable yields in the current synthesis process of dbcAMP-Ca and has the potential for further industrial development.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was partially supported by the National Natural Science Foundation of China (No.22278087).We acknowledge Prof.Ya Cheng and Dr.Miao Wu for their contributions to the manufacture of the glass micromixer chips using femtosecond laser micromachining,and Prof.Xuhong Qian and Prof.Weiping Zhu for their insightful guidance and discussion during the entire research.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108758.
杂志排行
Chinese Chemical Letters的其它文章
- The 3rd Xihua Chemistry and Biomedicine Forum
- Professor Hualiang Jiang: A tribute to an esteemed visionary chemist and pharmacist
- Recent advances in visible light-mediated chemical transformations of enaminones
- Development of porphyrin-based fluorescent sensors and sensor arrays for saccharide recognition
- Recent advances of versatile reagents as controllable building blocks in organic synthesis
- Synthetic host-guest pairs as novel bioorthogonal tools for pre-targeting☆
