B and Fe co-doped Co2P hollow nanocubes for nitrate electroreduction to ammonia
2024-04-05JingMioQinglingHongLipingLingGuominLiZhihongLiuShiinYinYuChen
Jing Mio ,Qingling Hong ,Liping Ling ,Guomin Li ,Zhihong Liu ,Shiin Yin ,Yu Chen,*
a School of Materials Science and Engineering,Taiyuan University of Science and Technology,Taiyuan 030024,China
b School of Materials Science and Engineering,Shaanxi Normal University,Xi’an 710062,China
c School of Chemistry and Chemical Engineering,Shaanxi Normal University,Xi’an 710062,China
d Guangxi Key Laboratory of Electrochemical Energy Materials,School of Chemistry and Chemical Engineering,Guangxi University,Nanning 530004,China
Keywords: Nitrate electroreduction reaction Ammonia synthesis Transition metal phosphides Hollow nanocubes
ABSTRACT Nitrate (NO3-) electroreduction reaction (NO3-RR) provides an attractive and sustainable route for NO3-pollution mitigation or energy-saved ammonia (NH3) synthesis.In this work,high-quality B and Fe co-doped Co2P hollow nanocubes (B/Fe-Co2P HNCs) are successfully synthesized though simultaneous boronation-phosphorization treatment,which reveal outstanding selectivity,activity,stability for the NO3- to NH3 conversion in neutral electrolyte because of big surface area,fast mass transport,superhydrophilic surface,and optimized electronic structure.B/Fe-Co2P HNCs can achieve the high NH3 yield rate (22.67 mg h-1 mgcat-1) as well as Faradaic efficiency (97.54%) for NO3-RR,greatly outperforming most of non-precious metal based NO3-RR electrocatalysts.
Ammonia (NH3) not only acts as a critical industrial raw chemical in the agriculture,textile,pharmaceutical and plastic industries,but also serves as an emissions-less and efficient energy carrier [1,2].Compared with the traditional Haber-Bosch process at high pressures and high temperatures,the electrochemical synthesis method can provide an environmentally friendly and energyefficient approach for large-scale NH3production [3].At present,nitrogen electroreduction reaction suffer from the highly stable N≡N triple bond (ca.941 kJ/mol) as well as unsatisfactory Faradaic efficiency [4].In contrast,the nitrate (NO3-) electroreduction reaction (NO3-RR) is considered as an attractive electrochemical method for NH3production because of the high solubility of NO3-in water and the small dissociation energy of N=O double bond(ca.204 kJ/mol) [5-7].In fact,NO3-pollution in drinking water causes severe problems to human health,which mainly originates from the excessive use of nitrogen-containing fertilizer and the improper discharge of wastewater [8,9].From the energy-saving and environmental protection perspectives,thus,NO3-RR is highly attractive to harness NO3-contamination to produce valuable NH3.However,the competing hydrogen evolution reaction (HER) and complex eight-electron transfer process may lead to the low NH3yield and selectivity [10,11].
Recently,transition metal phosphides (TMPs) are attractive electrocatalyst because of their low-cost,impressive activity and positive reduction potential for NO3-RR [12-14].To achieve high activity,selectivity,and durability of TMPs electrocatalysts for NO3-RR,many efforts have been devoted to exquisitely control their morphology,optimize their chemical composition,and modify their electronic structure [15,16].For example,Li and coworkers successfully synthesized Fe-doped CoP nanohoops,which revealed improved NO3-RR activity compared to CoP nanohoops due to electronic regulation function of Fe atoms [17].Among various nanostructures with different morphologies,hollow nanostructures can offer great benefits for boosting their electrocatalytic performance,which efficiently provide more exposed active sites and shorten the charge transport pathways [18-21].In additional,boron (B) doping is an efficient approach to boost the intrinsic activity of transition element-based nanostructures for various electrocatalytic reactions,such as B-doped Fe7S8/FeS2electrocatalysts for alkaline HER [22],B doped cobalt oxide nanocrystals for oxygen evolution reaction [23],and B,P co-doped Pd nanothorn arrays for NO3-RR [24].Mainly,B atoms can well rearrange the inhomogeneous spin and charge density of metal atoms,originating from the easy coordination with metal atoms [25].Inspired by this,it is highly desired to synthesize B-doped porous TMPs nanostructures for the NO3-RR.
In this work,we successfully synthesized B and Fe co-doped Co2P hollow nanocubes (B/Fe-Co2P HNCs) and further investigated the NO3-RR performance.The introduction of electron-deficient B atoms could efficiently rearrange the electronic structure of Fe-Co2P HNCs.Besides,the hollow and porous architecture could provide high surface area,abundant active sites and available mass transfer pathways.Benefiting from the hollow nanostructures,well-defined void space,and tunable chemical compositions,B/Fe-Co2P HNCs exhibited an outstanding selectivity,activity,stability for the NO3-to NH3conversion in neutral Na2SO4electrolyte.B/Fe-Co2P HNCs with long-term stability and high activity provide a facile strategy for synthesis NH3and elimination of NO3-contamination.
B/Fe-Co2P HCNs were synthesized though successive precipitation transformation and boronation-phosphidation methods,as illustrated in Scheme 1.Prussian blue analogues (PBAs) are recognized as popular precursor for TMPs synthesis because of their tunable chemical composition,large specific surface areas,and high porosity [26].
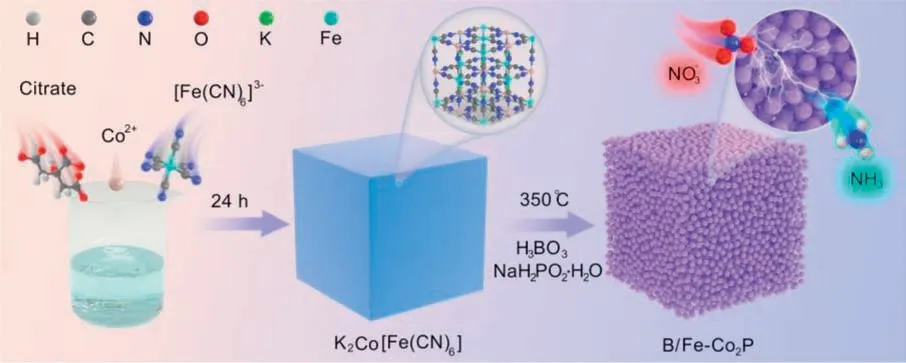
Scheme 1.The synthetic strategy of B/Fe-Co2P HNCs.
First,CoFe-PBA HNCs were easily synthesized through coprecipitation method (see experimental section in Supporting information for the detail).Both the scanning electron microscopy (SEM)and transmission electron microscopy (TEM) characterization show that CoFe-PBA HNCs have a well-defined cubic morphology,uniform size (ca.300-400 nm) and smooth surface (Fig.S1 in Supporting information).In X-ray diffraction (XRD) characterization,all diffraction peaks are well indexed to K2CoFe(CN)6crystal (JCPDS No.75-0038) (Fig.S2 in Supporting information).Thus,SEM and XRD characterization confirm the successful synthesis of CoFe-PBA HNCs.After simultaneous boronation-phosphorization process,the energy dispersive X-ray (EDX) characterization displays the coexistence of B,Fe,Co and P elements whereas K and N elements of PBA are not observed (Fig.1a).The XRD pattern of as-prepared sample matches well with Co2P crystal (JCPDS No.32-0306) and the characteristic peak of PBA precursor disappear completely (Fig.1b).Thus,we can be concluded that PBA is completely converted into B/Fe-Co2P HNCs.For comparison,Fe-Co2P HNCs without Bdoping were synthesized by phosphorization treatment (Fig.S3 in Supporting information).Compared with Fe-Co2P HNCs,the main diffraction peaks of B/Fe-Co2P HNCs shift positively,which can be attributed to B doping [27,28].According to nitrogen adsorptiondesorption test,the surface area of B/Fe-Co2P HNCs is 42.4 m2/g(Fig.1c),suggesting that the hollow porous B/Fe-Co2P HNCs can offer ample accessible active sites.Meanwhile,B/Fe-Co2P HNCs show a smaller contact angle compared to Fe-Co2P HNCs (Fig.1d),indicating that the introduction of B atoms can provide superhydrophilic surface.The superhydrophilic surface can facilitate electrolyte adsorption and electron transfer processes [24].

Fig.1.(a) EDX spectrum,(b) XRD pattern,and (c) nitrogen adsorption-desorption isotherm of B/Fe-Co2P HNCs,(d) the contact angle of B/Fe-Co2P HNCs and Fe-Co2P HNCs.
The hard template strategy can generally preserve the initial morphology of precursor.During the boronation-phosphorization process,CoFe-PBA HNCs are easily decomposed into lesser broken cubes with the rough and porous feature,indicating that CoFe-PBA HNCs are successfully converted into B/Fe-Co2P HNCs (Figs.2a and b).By adjusting the mass ratio of B and P,B/Fe-Co2P HNCs with different B/P mass ratio can also be obtained (Fig.S4 in Supporting information).TEM image clearly reveals the regular and large interior spaces within ultrafine nanoparticles embedded in the outer face (Fig.2c).High-resolution TEM image distinctly reveals the lattice fringes of 0.175 and 0.221 nm (Fig.2d),corresponding to (020)and (112) planes of Co2P [29,30].Elemental mapping characterization shows that Co,P,B,and Fe elements are homogeneously distributed on the nanocubes (Fig.2e),confirming the B and Fe co-doping,again.

Fig.2.(a,b) SEM images,(c) TEM image,(d) high-resolution TEM image,and (e)EDX elemental mapping images of B/Fe-Co2P HNCs.
The surface component and valence state of B/Fe-Co2P HNCs were examined by X-ray photoelectron spectroscopy (XPS).In the Co 2p spectrum (Fig.3a),the peaks at 795.5 and 780.1 eV correspond to 2p1/2and 2p3/2orbits of Co2+species,and the peaks at 797.2 and 782.1 eV are attributed to 2p1/2and 2p3/2orbits of Co3+species.The two broad peaks at 785.6 and 802.5 eV are assigned to the satellite peaks [31,32].In the Fe 2p spectrum (Fig.3b),two peaks at 724.5 and 710.7 eV can be ascribed to 2p1/2and 2p3/2orbits of Fe2+species,whereas the peaks at 727.6 and 713.3 eV correspond to 2p1/2and 2p3/2orbits of Fe3+species,along with two shakeup satellite peaks at 717.7 and 733.3 eV [33,34].Particularly,the binding energies of Co 2p and Fe 2p negatively shift after B doping (Fig.S5 in Supporting information),suggesting the electron interaction between metal atom and B atom because of the electron-deficient nature of B [35,36].In the B 1s spectrum (Fig.3c),the peak at 187.8 eV correspond to the peak of metallic B [37].The peaks at higher binding energy (194.8 eV,196.2 eV) correspond to the oxidic boron species [38,39].In the P 2p spectrum (Fig.3d),the huge peak of 133.9 eV belongs to P-O,and the two peaks at 129.3 and 130.4 eV can be ascribed to TMPs,which is constant with reported TMPs nanostructures [40,41].

Fig.3.(a) Co 2p,(b) Fe 2p,(c) B 1s,and (d) P 2p XPS spectra of B/Fe-Co2P HNCs.
The NO3-RR performance of B/Fe-Co2P HNCs and Fe-Co2P HNCs were investigated in 0.5 mol/L Na2SO4electrolyte with and without 50 mmol/L NaNO3.Both Fe-Co2P HNCs and B/Fe-Co2P HNCs display an obvious electroactivity for NO3-RR,reflecting the cathodic current markedly increases after the addition of NO3-into 0.5 mol/L Na2SO4electrolyte.Compared with Fe-Co2P HNCs without B doping,B/Fe-Co2P HNCs exhibit a more positive onset potential and a bigger reduction peak current density for NO3-RR,implying the outstanding NO3-RR catalytic activity (Fig.4a).This phenomenon further suggests that the intrinsic NO3-RR activity of Fe-Co2P HNCs is significantly enhanced after B doping.Since XPS measurements show the introduction of B atoms can effi-ciently regulate the electronic structure of Fe-Co2P HNCs,the electronic effect is responsible for intrinsic activity enhancement of Fe-Co2P HNCs due to B doping.Among B/Fe-Co2P HNCs with different B/P ratio,B/Fe-Co2P HNCs (B:P=2:1) has the highest NO3-RR(Fig.S6 in Supporting information),which in turn confirms that both B and P atoms have obvious influence on NO3-RR activity enhancement.To study the activity and selectivity of B/Fe-Co2P HNCs for NO3-RR,the reactant and reductive products were determined by the chronoamperometry tests at various applied potentials.After chronoamperometry tests for 3 h,colorimetric methods were used to quantity the NH3yields and Faradaic efficiency(Figs.S7 and S8 in Supporting information).Upon varying the applied potential from -0.5 V to -0.9 V (vs.RHE),the NH3production rate and conversion rate of NO3-RR at B/Fe-Co2P HNCs gradually increase,while the Faradaic efficiency of NH3at B/Fe-Co2P HNCs displays a volcanic shape (Fig.4b),similar to Fe-Co2P HNCs(Fig.S9 in Supporting information).Compared with Fe-Co2P HNCs,B/Fe-Co2P HNCs displays larger NH3production rate and Faradaic efficiency under same potential.For example,B/Fe-Co2P HNCs can achieve the NH3yield of 22.67 mg h-1mgcat-1and the Faradaic efficiency of 97.54% at -0.70 V potential (Fig.4c),much higher than that of Fe-Co2P HNCs,suggesting high activity and selectivity of B/Fe-Co2P HNCs for the NO3-to NH3conversion.The control experiment performed in 0.5 mol/L Na2SO4electrolyte only generates negligible NH3,excluding the interference of catalysts and environmental contaminants (Fig.S10 in Supporting information).Impressively,B/Fe-Co2P HNCs have higher NH3yield and Faradaic effi-ciency than most previously reported non-precious metal electrocatalysts for NO3-RR (Table S1 in Supporting information),further demonstrating the high electroactivity of B/Fe-Co2P HNCs.Electrochemical impedance spectroscopy (EIS) characterization displays that the charge transfer resistance of NO3-RR at B/Fe-Co2P HNCs(49.3Ω) is much smaller than that at Fe-Co2P HNCs (125Ω),suggesting that the B doping accelerates the reaction kinetics of NO3-RR (Fig.4d) [24].

Fig.4.(a) LSV curves of B/Fe-Co2P HNCs and Fe-Co2P HNCs in 0.5 mol/L Na2SO4 with and without 50 mmol/L NaNO3 at 5 mV/s.(b) Faradaic efficiency and NH3 yield rate of NO3-RR at B/Fe-Co2P HNCs at different potentials.(c) the NH3 yield rates of NO3-RR at B/Fe-Co2P HNCs and Fe-Co2P HNCs.(d) Nyquist plots of B/Fe-Co2P HNCs and Fe-Co2P HNCs.
Benefiting from the small particle size and porous structure,B/Fe-Co2P HNCs can provide a high surface area and expose affluent active sites,facilitating the NO3-to NH3conversion [42,43].The electrochemical double-layer capacitances (Cdl) are estimatedviacycling voltammetry (CV) curves under different scan rates (Fig.S11 in Supporting information).TheCdlvalue (22.41 mF/cm2) of B/Fe-Co2P HNCs is close to that of Fe-Co2P HNCs (21.87 mF/cm2),indicating that the electrochemical active surface area of B/Fe-Co2P HNCs is close to that of Fe-Co2P HNCs.Thus,the area effect is not a main factor for activity enhancement of B/Fe-Co2P HNCs.In fact,the doped B atoms can act as Lewis acid active sites to better adsorb NO3-,which accelerates the electroreduction of NO3-to NH3[23,44].Meanwhile,B/Fe-Co2P HNCs show a smaller contact angle compared to Fe-Co2P HNCs,indicating that the introduction of B atoms can provide superhydrophilic surface [45].Additionally,B doping can effectively optimize the charge density and redistribute the inhomogeneous spin,which is favorable for adsorption kinetics and reduce the reaction energy barrier during NO3-RR [46,47].As a result,B/Fe-Co2P exhibits extremely outstanding NO3-RR electroactivity.
Furthermore,the durability of B/Fe-Co2P HNCs was investigated by chronoamperometry test.Chronoamperometric curve reveals that B/Fe-Co2P HNCs can be sustained for 30 h with negligible decay during NO3-RR,revealing the excellent stability and practical application for electrochemical NH3production (Fig.5a).After long-time electrolysis,EDX,XRD and TEM characterizations display that chemical composition,morphology and structure remain nearly unchanged,revealing stability of B/Fe-Co2P toward NO3-RR(Figs.5b-d).
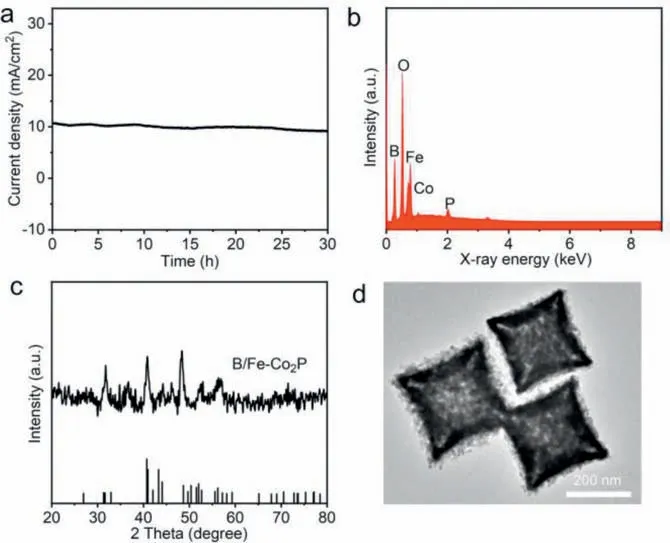
Fig.5.(a) Chronoamperometric curve of B/Fe-Co2P HNCs in 0.5 mol/L Na2SO4+0.05 mol/L NaNO3 electrolyte at -0.70 V potential.(b) EDX spectrum,(c)XRD pattern,and (d) TEM image of B/Fe-Co2P HNCs after chronoamperometry test.
In summary,we have rationally designed and synthesized B/Fe-Co2P HCNs with an open structure and unique hollow interior.Thanks to superhydrophilic surface and abundant active sites,the obtained B/Fe-Co2P HCNs show excellent electrocatalytic activity for NO3-to NH3conversion.B/Fe-Co2P HCNs show an impressive NH3production rate of 22.67 mg h-1mgcat-1and Faradaic effi-ciency of 97.54%,much superior to the most reported non-precious metal NO3-RR electrocatalysts in neutral media.This work not only highlights a new boronation-phosphorization way for the nanomaterials synthesis but also points out the high-performance of B/Fe-Co2P HCNs for NO3-removal and NH3synthesis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is supported by Natural Science Foundation of Shanxi Province (No.202203021222213),Taiyuan University of Science and Technology Scientific Research Initial Funding (No.20222091),National Natural Science Foundation of China (No.22073061),Science and Technology Innovation Team of Shaanxi Province (No.2023-CX-TD-27),Fundamental Research Funds for the Central Universities (No.GK202202001).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108935.
杂志排行
Chinese Chemical Letters的其它文章
- The 3rd Xihua Chemistry and Biomedicine Forum
- Professor Hualiang Jiang: A tribute to an esteemed visionary chemist and pharmacist
- Recent advances in visible light-mediated chemical transformations of enaminones
- Development of porphyrin-based fluorescent sensors and sensor arrays for saccharide recognition
- Recent advances of versatile reagents as controllable building blocks in organic synthesis
- Synthetic host-guest pairs as novel bioorthogonal tools for pre-targeting☆
