Harnessing bacteria for tumor therapy: Current advances and challenges
2024-04-05LinGuoJinsongDingWenhuZhou
Lin Guo ,Jinsong Ding,* ,Wenhu Zhou,b,c,*
a Xiangya School of Pharmaceutical Sciences,Central South University,Changsha 410013,China
b Changsha Medical University,Academician Workstation,Changsha 410219,China
c Key Laboratory of Biological Nanotechnology of National Health Commission,Changsha 410008,China
Keywords: Bacteria Tumor targeting Immunotherapy Combination therapy Genetic engineering Surface decoration
ABSTRACT After a century of standstill,bacteria-based tumor therapy has resurged recently benefiting from the revolution of tumor immunotherapy,which provides unique solutions to tackle the obstacles of traditional tumor treatments.Obligate and facultative anaerobes with active tropism can selectively colonize at tumor sites and suppress tumor growth via different mechanisms,serving as attractive tools for tumor treatment either as a monotherapy or combining with other therapies for synergistic anti-tumor effects.In this critical review,we introduce the recent advances of bacteria-based tumor therapy from the following aspects.First,the general properties of bacteria are reviewed emphasizing on their structural components related to tumor immunotherapy,and the main bacteria that have been used in tumor therapy are listed.Then,the benefits of bacteria for tumor therapy are illustrated,such as tumor targetability,deep penetration,and facile genetic engineering for attenuation,enhanced efficacy,as well as bioimaging.Next,anti-tumor mechanisms of bacteria are summarized,which refer to intrinsic tumoricidal activities,immune activation,bacteria metabolism,and their capability to regulate gut microbiota homeostasis.Moreover,bacteria could act as carriers to deliver various types of therapeutics to achieve combination therapy with improved efficacy.In addition,several challenges for anti-tumor applications of bacteria are discussed regarding the delivery,efficacy and safety issues,and potential solutions are also provided.Finally,the possible improvements and perspectives are discussed in the end,which provide a guideline for the design of advanced bacteria-based tumor therapeutics in the future.
1.Introduction
With the development of medical technology,a variety of diseases have been gradually conquered and the average human life expectancy has been prolonged.However,tumor remains a serious disease threatening public health that has high mortality worldwide.Although tremendous efforts have been made to treat tumor,the current therapies are still limited to surgery,radiotherapy,and chemotherapy,among which the chemotherapy capitalized on drugs is most widely used.These drug therapies,on the other hand,encounter problems like limited drug infiltration,drug resistance,non-specific toxicity to normal tissues and inefficiency against tumor metastasis and recurrence [1,2].In light of these issues,novel alternative therapy is urgently needed for the fight against cancer.The burgeoning immunotherapy which focuses on orchestrating the immune system of the host,such as chimeric antigen receptor T cells therapy and Food and Drug Administration(FDA)-approved immune checkpoint inhibitors,has revolutionized the tumor therapy [3,4].Compared to the traditional treatments,immunotherapy can elicit powerful immune responses against tumor cells with higher specificity and can efficiently suppress tumor metastasis and recurrence [5].
For better therapeutic efficacy and minimized side-effects,suitable drug delivery system is critically important.Although demonstrating promising results in the clinic,the current immunotherapy still suffers from low response rate in patients and severe immunerelated adverse events,which stem from the wide distribution of immunotherapeutics to result in systematic immune activation[6].Therefore,suitable delivery systems that can facilitate the tumor accumulation while minimizing side-effects are highly desired for the current immunotherapy drugs.Recently,Yang’s group proposed the “five features” principles for targeted delivery of antitumor drugs,including long circulation,tumor accumulation,deep penetration,cellular internalization and drug release [7].Based on the above principles,nanomedicine seems to offer considerable advantages [8,9].After systematic administration,these nanocarriers can protect the drugs from being cleared quickly and accumulate in tumor tissues through the well-known enhanced permeability and retention (EPR) effect [10].However,the efficiency of passive tumor targeting of nanoparticlesviaEPR is still under debate[11,12].The active targeting mediated by ligand-receptor recognition has been developed to improve the drug delivery,but the process of modification is always complicated [13].Thus,the delivery of present immunotherapeutic modalities is still unsatisfied.The precise and targeted delivery of drugs are going-on direction that needs our vigorous efforts.
The biochemotactic effects in nature such as neutrophils recruitment to the sites of infection provide new insights for targeted drug delivery [14].The chemotaxis-driven drug delivery mediated by neutrophils infiltration to the inflammatory tumor environment have been reported,which significantly improved the delivery efficiency [15-17].However,the tumor microenvironment must be primed by surgical resection,external photothermal or photodynamic therapy to create an inflammatory environment,which are too tedious to be used in the clinic.The biochemotactic effects of microorganisms provide an alternative method for drug delivery,among which the bacteria aroused great concern of researchers.The obligate and facultative anaerobes are found to selectively colonize in tumor tissues with distribution ratio against normal organs of more than 1000:1,and can exert anti-tumor effectsviavarious mechanisms including competition for nutrients,inducing tumor cells apoptosis and immune activation.Thus,bacteria possess natural advantages for acting as both therapeutic drugs and drug carrier,which contribute to resolving the above-mentioned problems and provide new opportunity for tumor treatment [18].
In fact,there is a long history of study that suggests bacterial infection can result in anti-tumor effects (Fig.1).The earliest documented record of bacteria therapy against cancer dates back to 1868,whenStreptococcuspyrogenes(S.pyrogenes) was used to infect sarcoma,and reduced tumor volume was observed on patient’s body.About two decades later,William B.Coley reported the successful eradication of sarcoma byS.pyrogenes,which reconfirmed the anti-cancer effect of bacteria.The subsequent “Coley’s toxins” withSerratiamarcescens(S.marcescens) stimulated a flourish of bacterial tumor therapy [19].As such,Coley has been regarded as a trailblazer for bacteria-based tumor therapy.However,with the rise of chemotherapy and radiotherapy,the further pursuit of using bacteria to treat tumor was curtailed.Until recently,with advances in microbiology and a deeper understanding of immunologic mechanisms,bacteria have entered a new era and once again attracted considerable attention for the treatment of tumor.The development of synthetic biology further expedites the engineering of bacteria with unique properties.Several of the bacterial strains have advanced to clinical trials,such as the use of Bacillus Calmette-Guerin (BCG) in the treatment of bladder cancer and attenuatedSalmonellabacterial strain VNP20009 in the treatment of melanoma [20,21].

Fig.1.(A) The history of bacteria-based tumor therapy.(B) The paper numbers of bacteria-based tumor therapy per year retrieving in Web of Science.
Over the past decades,the evidence has accumulated that obligate and facultative anaerobes can selectively colonize at both solid and metastatic tumors and penetrate to distal areas of blood vessels [22,23].A variety of bacteria,includingClostridium,Bifidobacterium,Escherichiacoli(E.coli) andSalmonellahave been explored for tumor treatment.These bacteria can be conveniently modified by genetic or chemical methods for specific needs and can be easily cultured to a large scale under appropriate culture conditions.Because of their unique characteristics,bacteria exhibit promising potential as both therapeutic drugs and drug carriers to deliver cargos.Attenuated bacteria with innate immunomodulatory effect can also be used to boost immune responses with immune cells activation and cytokines production,which synergize with other immunotherapy modalities for enhanced treatment efficacy.
Although several reviews regarding bacteria-based tumor therapy have been published in recent years [23-25],there still lacks a comprehensive summary and detailed discussion about the bacteria acting as intrinsic anti-tumor agent and drug carrier.In this review,we discuss the unique aspects of bacteria as therapeutic agents and the corresponding mechanisms.Additionally,we show that bacteria can be utilized as vehicles to deliver various therapeutic payloads including small molecules,biomacromolecules and nanomaterials,or subjected to surface functionalization (Fig.2).The challenges facing for bacterial clinical translation are also discussed.Finally,a brief summary and an outline of possible future research directions are provided,which can serve as a guideline for developing more intelligent bacteria therapeutics in the future.We hope that this in-depth review of bacteria-based targeted tumor therapy will benefit researchers for designing advanced bacteriabased tumor therapeutics and open new avenue for tumor precise therapy.
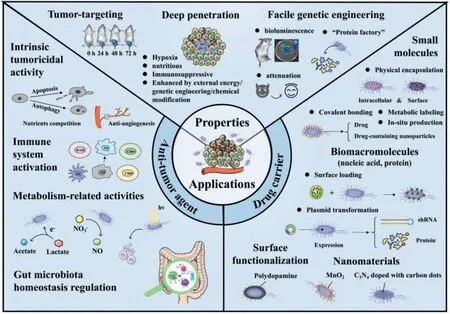
Fig.2.Schematic illustration of the properties and applications of bacteria for tumor therapy.
2.The bacteria for tumor therapy and their properties
2.1. General properties of bacteria
Bacteria are prokaryotes mainly composed of cell walls,cell membranes and cytoplasm,and they can be divided into two categories according to the Gram staining results,in which the purplestaining bacteria are called Gram-positive and the red-staining bacteria are called Gram-negative (Fig.S1 in Supporting information)[26].For more detailed discussion on their general structure,please refer to Part S1 (Supporting information).The above structural components of bacteria highlight their great potential for tumor immunotherapy,and many bacteria strains have been tested as discussed below.
2.2. Benefits of bacteria for tumor therapy
2.2.1.Tumor-targetingpropertiesandmechanisms
For tumor therapy,the nonspecific distribution of druginvivoafter administration not only reduces the efficacy but also brings serious side-effects.To address this issue,many researchers have been focusing on targeted drug delivery in the past decades,during which nanomedicine have been extensively studied and emerged as effective tool for tumor targeting [27].Specifically,nanomedicine can accumulate at tumor sites through both passive targeting known as EPR effect and active targeting based on the recognition of specific receptors by ligands [28].However,the targeting efficiency of nanoparticles is insufficient with only 0.7%of the intravenously injected nanoparticles reached the tumor site[11].Fortunately,it is reported that bacteria possess excellent intrinsic tumor-targeting ability with the distribution ratio of tumor to normal organs greater than 1000:1,which can be further enhanced by external energy,genetic engineering or surface chemical modification [25,29].For more detailed discussion,please refer to Part S2 (Supporting information).Note that the absolute targeting efficiency of bacteria as compared to injection dose is impossible to calculate,and thus it is usually to present the relative targeting effect by comparing the bacteria colonization in tumor and normal organs.
2.2.2.Deeppenetrationintumor
Due to the detrimental tumor microenvironment including abnormal tumor vasculature,elevated interstitial fluid pressure and dense extracellular matrix,the penetration of drugs and nanoparticles in tumors is extremely limited,along with the low accessibility to tumor cells and compromised efficacy,thus hinders the clinical application [30].Although several strategies such as rational design of nanoparticles for their physicochemical properties and tumor microenvironment remodeling have been proposed to improve the tumor penetration,they are too complicated with poor practicality and operability [31,32].In addition,the oxygen levels gradually decrease with depth and the highly hypoxia conditions inside tumor make it resistant to the conventional therapies including radiotherapy and chemotherapy [33].Thus,the effective killing of cells deep in tumor is still a great challenge for tumor treatment.
Unlike the traditional drugs,bacteria as living organisms possess the ability to perceive the surrounding environment and the motility propelled by flagella,which can spontaneously migrate away from the vasculature and penetrate deeply into the tumor tissues [34].For example,Salmonellahas been reported to form colonies near blood vessels in the early stage of infection and subsequently would migrate into the necrotic core of tumor gradually and proliferate within it [35,36].Our group has fluorescently stained the hypoxia biomarker carbonic anhydrase-IX (CA9)inSalmonella-infected tumor tissues.The merged image showed strong colocalization between green fluorescence of CA9 and bioluminescent red fluorescence ofSalmonella,demonstrating the colonization ofSalmonellain deep tumor [37].In addition,the effect of bacteria motility on the penetration depth has been investigated in aninvitrosolid tumor model,which showed that the more motile the bacteria the deeper they penetrated [34].Thus,bacteria show great potential in overcoming the diffusion resistance.Moreover,the anti-tumor effects of bacteria are not highly dependent on the oxygen and thus have less resistance [38].
Furthermore,Suhetal.constructed a nanoscale bacteriaenabled autonomous drug delivery system (NanoBEADS) by conjugating streptavidin-coated poly(lactic-co-glycolic acid) (PLGA)nanoparticles to the outer membrane of VNP20009 coated with biotinylated-antibody and then incubated with mPEG-biotin for polyethylene glycol (PEG) coating [39].After evaluation in various tumor spheroid modelsinvitroand in a breast cancer modelin vivo,they found that bacteria penetrated intratumorally mainly by intercellular translocation and their transport performance was not impeded by nanoparticle conjugation.It is worth noting that the retention and distribution of nanoparticles in solid tumors were enhanced by up to 100-fold in virtue of the bacteria as compared to traditional passive diffusing.Thus,this NanoBEADS platform provides a unique approach of delivering therapeutic agents by bacteria to sites difficult to be accessed by traditional preparations.
2.2.3.Facilegeneticengineering
Unlike the traditional tumor therapy,the bacteria can be genetically engineered to satisfy the need for tumor theranostics,including reducing the toxicity,improving the tumor-targeting capability,producing certain functional protein and realizing convenientinvivomonitoring.The genetic modifications for improving the tumor-targeting have been discussed in 2.2.1.For more detailed discussion on the engineering for attenuation,invivomonitoring and constructing “protein factory”,please refer to Part S3(Supporting information).
2.3. The main bacteria used in tumor therapy
Owing to the intrinsic tumor-targeting ability,various obligate anaerobes and facultative anaerobes have been investigated in tumor therapy.The representative bacteria are listed in Table S1(Supporting information).
3.Anti-tumor activities and mechanisms
Since the initial attempt of Coley to use bacteria for tumor therapy,such method has been silenced for a long time.However,with the development of immunology,bacteria-mediated tumor therapy rekindles the interest of researchers and is considered as a new paradigm.Up to now,many bacteria strains have been tested for tumor therapy in the animal models (Table S1),most of which exhibit prominent efficacy with some acting at tumor site while others acting somewhere else such as intestinal tract.However,even if playing their roles at the same place,different bacteria strains may exert anti-tumor effects through different mechanisms.
3.1. Intrinsic tumoricidal activities
As metabolically active live organisms,lots of nutrients are required for bacteria to grow and so as the tumor cells,and thus they compete for the limited extracellular nutrients at tumor site with each other and the proliferation of tumor cells is inhibited to a certain extent [40].Meanwhile,the bacteria can secrete some substances,such as bacteriocins and specific toxins,which have strong toxicity and show anti-tumor effects [41].For example,tetrodotoxin (TTX) produced byShewanellaalgaecan selectively bind and block the transmembrane glycoprotein-forming sodium channel,thus inhibiting the proliferation and invasion of tumor cells and inducing cell apoptosis through increasing the oxidative stress [42].The cytolysin can trigger caspase-mediated apoptosis signaling pathways by creating pores on the cell membrane and disrupting the barrier function and the diphtheria toxin can exert anti-tumor effect by inhibiting protein synthesis [43].In addition to the roles playing extracellularly,some bacteria can even enter tumor cells to exert the functions.Listeriamonocytogeneshave been reported to kill tumor cells through producing high levels of reactive oxygen species (ROS) [44].Interestingly,Salmonellaare found to induce cell apoptosis after intracellular accumulation as evidenced by cleaved caspase-3 activity and nuclear condensation probably through activating the related signal transduction pathways [35].Besides,Salmonellahave been demonstrated to induce tumor cells autophagyviaregulation on protein kinase B(PKB/AKT)/mammalian target of rapamycin (mTOR) pathway which showed dependence on both the dose and time and efficiently controlled the tumor growth [45].Apart from direct killing tumor cells through induction of apoptosis and autophagy,Salmonellacan inhibit the tumor angiogenesis by downregulating the related factors which can promote the formation of blood vessels including hypoxia inducible factor-1α(HIF-1α) and vascular endothelial growth factor (VEGF) either through AKT/mTOR pathway or through upregulating connexin 43 (Cx43),thus reducing the supply of oxygen and nutrients necessary for tumor growth and inhibiting tumor growth [46-48].In addition,the formed tumor vasculature may be disrupted by the pro-inflammatory cytokines produced post bacterial infection in tumors,with blood cells exudating out of blood vessels and thrombosis occurring,which cut off the nutrient supply to tumor cells and enable potent photothermal therapy and photoacoustic imaging on account of the darkened color along with strong near-infrared absorbance [49,50].Such bacteriainduced blood coagulation also provides opportunities for antitumor drugs to specifically localize at tumor site capitalized on the tropism of platelets for blood coagulation [51].
3.2. Immune system activation
As discussed in 2.1,bacteria can activate the immune system through their special components including LPS,lipoprotein,flagellin,CpG DNA and teichoic acid.Owing to the excellent targeting performance,intrinsic tumoricidal activity and fully sequenced genomes convenient for genetic manipulation,Salmonellaare the most widely studied strain among the bacteria playing their roles at the tumor site [18].As Gram-negative bacteria,Salmonellacan induce both the innate and adaptive immune responses,along with the recruitment of host immune cells including DCs,macrophages,neutrophils,B cells,T cells and natural killer (NK) cells into tumor tissues,through which the solid tumors can be eliminated[18].Specifically,after the tumor cells are killed bySalmonella,the tumor-associated antigens can be released and further taken by the antigen presenting cells like DCs and macrophages [52].Alternatively,Salmonellacan directly promote the formation of gap junction between tumor cells and DCs through upregulation of Cx43 and then promote the tumor antigen transfer [47].Then the antigens are presented to T cells and tumor-specific immune responses are awaken [53].This cross-presentation pathway is more effective since the traditional process of antigens presenting in DCs proteasome always generate different antigens from tumor cells and initiate immune responses that will not recognize tumor cells.In addition,Salmonellacan suppress tumor metastasis through NK cells activation dependent on interferon-γ(IFNγ) [54].Furthermore,Salmonellacan ameliorate the tumor microenvironment to be immune-permissive.Specifically speaking,tumor associated macrophages (TAMs),the most abundant immune cells inside tumors,usually exhibit the protumoral M2 phenotype which can promote the proliferation and immune escape of the tumor cells and play an essential role in the tumor progression [55].In contrast,M1 macrophages can exert tumoricidal effects through phagocytosis,antigen presentation and proinflammatory cytokines release [56,57].Thus,TAMs have been an attractive therapeutic target and re-polarization of TAMs to the M1 phenotype shows great potential for tumor therapy [58].It has been reported that administration ofSalmonellacould induce the macrophage re-polarization with immunostimulatory factors expression capitalized on the bacterial O antigen [59].The macrophage re-polarization effects ofE.coliandListeriamonocytogeneshave also been demonstrated [60,61].We recently verified the M2-to-M1 phenotype switch of macrophages uponSalmonellatreatment,which was further strengthened by flagellin B (FlaB) excreted from the engineered bacteria strainS.t-ΔpGFlaB[62].Moreover,the function of T cells can be restricted by the regulatory T (Treg) cells which serves as another obstacle for tumor immunotherapy,and therefore various complicated Treg-targeting strategies including neutralizing and destabilizing Treg cells or inducing resistance of T cells towards Treg cells have been proposed to enhance anti-tumor immunity [63].Surprisingly,the numbers of Treg cells in tumors were decreased afterSalmonellacolonization and the tumor growth was efficiently suppressed [64].The expression of immune checkpoint molecules,such as indoleamine 2,3-dioxygenase (IDO) and programmed death ligand 1 (PD-L1),may explain the immune escape of tumor from another way [65].IDO is an enzyme overexpressed in several tumors and mediates the transformation from tryptophan (Trp) to kynurenine (Kyn).With Trp depletion and Kyn accumulation,the Treg cells are activated while T cells are suppressed [66].The infection ofSalmonellacan reduce the IDO expression and impede its immunosuppressive effects through upregulating Cx43 [67,68].Similarly,systematic administration ofSalmonellacan reduce the PD-L1 expression,enhance T cell infiltration,and significantly inhibit the tumor growth [69].Capitalized on these effects,Salmonellacan initiate intense immune responses towards tumor cells,thus achieving tumor elimination.For more detailed discussion on the immunological effects of other bacterial strains,please refer to Part S4 (Supporting information).
3.3. Metabolism-related activities
In addition,the unique metabolic activity of some specific bacteria strains can be employed for tumor therapy,which is mainly mediated by specific enzymes.Zhengetal.used a nonpathogenic bacteriumE.coliMG1655 to metabolize endogenous NO3-into antineoplastic NO,with nano-photocatalyst carbon nitride to strengthen their metabolic activities,achieving opticallycontrolled bacterial metabolite therapy [70].Chenetal.usedShewanellaoneidensisMR-1 to catabolize the produced lactate,which plays a critical role in tumor progression,and decorated manganese dioxide (MnO2) nanoflowers on the surface to generate oxygen by catalyzing the decomposition of hydrogen peroxide,which could prevent lactate production by downregulating the glycolysis-related transporters/enzymes,achieving tumor inhibition by combining bacterial respiration and tumor metabolism [71].Some photosynthetic bacteria are also utilized for tumor therapy taking advantage of their oxygen-generating characteristics [72].Liuetal.utilizedSynechococcus7942 for targeted delivery of photosensitizer andinsituoxygen generation for enhanced photodynamic therapy (PDT),which further evoked robust anti-tumor immune responses and prevented tumor recurrence and metastasis[73].Similarly,Huoetal.integrated cyanobacteria with photosensitizer for photosynthesis-boosted PDT [74].
3.4. Gut microbiota homeostasis regulation
Some bacteria can enhance the efficacy of tumor therapy by colonizing in the intestinal tract.The disruption of gut microbiota homeostasis and changes of interactions between gut microbiota and host immune systems are associated with tumorigenesis,progression and drug resistance [75,76].Manipulations of the gut microbiota might improve the efficacy of anti-tumor chemo-,radio-,immune-and target therapy,such as gemcitabine,CpG oligodeoxynucleotide,tyrosine kinase inhibitors and immune checkpoint inhibitors [77,78].The probiotics such asBifidobacteriumandE.colican regulate the gut microbiota [79].For example,Chenetal.demonstrated that combined treatment of probiotics and gemcitabine achieved a low degree of pancreatic intraepithelial neoplasia formation,while the introduction of probiotics could make the standard chemotherapy of pancreatic cancer more effective [80].A different study suggested that probiotic supplementation could enhance the anti-tumor effect of 5-fluorouracil[81].In addition,Sivanetal.reported that oral administration ofBifidobacteriumcould inhibit the tumor progression by augmenting DCs function which led to enhanced T cells accumulation in the tumor microenvironment,and synergistic effect was achieved by combination with PD-L1-specific antibody,suggesting that manipulating the microbiota may improve the cancer immunotherapy[82].
4.Bacteria as drug carrier
Although various mechanisms to combat tumor,the therapeutic effects of bacterial monotherapies may not be satisfied.On the one hand,the dose of bacteria is largely restricted to avoid the dose-limiting toxicity.On the other hand,bacteria only thrive in necrotic and hypoxic tumor regions but not in the highly perfused areas.The heterogeneity of tumors makes it difficult to achieve cure with bacterial monotherapies.Capitalized on the excellent tumor-targeting ability and facile functionalization,bacteria have been employed as carriers for various kinds of drugs,including small molecules,biomacromolecules and nanomaterials,and engineered for surface functionalization,which can enhance the therapeutic effect of bacteria as monotherapy.
4.1. Small molecules
Small molecules like chemotherapy drugs and photosensitizers can be loaded by bacteria through physical encapsulation,covalent bonding,metabolic labeling orin-situproduction.
4.1.1.Physicalencapsulation
For the physical encapsulation,the drugs can be loaded in the cytoplasm or on the surface of the bacteria,in which the toxicity of drugs to the bacteria must be taken into consideration.Xiangetal.internalized doxorubicin (DOX) in the magnetotatic bacteria MSR-1 through simple short-time incubation,which efficiently prevented the toxicity of DOX,with no effect on the viability and mobility of the bacteria [83].The drug-internalized bacteria could navigate towards the tumor site under the guidance of external magnet field,rendering enhanced anti-tumor efficacy compared with dead bacteria or DOX alone and holding promise for precise therapy.In fact,only the positive charged molecules like DOX can penetrate the bacterial membrane easily since the bacterial membrane is negatively charged [84].For the anionic drugs,modifications for charge reversal are necessary.Huoetal.modified the anionic photosensitizers including chlorin e6 (Ce6) and protoporphyrin (Ppix)with dual-amide-terminated PEG,which endowed them with positive charge by the amide end groups and enabled potent internalization by cyanobacteria [74].To completely avoid toxicity of small molecule drugs to the bacteria,Zoabyetal.developed autonomous tumor-targeting nanoswimmers with DOX-containing liposomes loaded into bacteria through electroporation [85].Only when the drug-loaded bacteria invaded the cancer cell,the drug would be released from the liposomes and killed the bacteria,after which the chemotherapeutic activity against the tumor cells was executed.This resulted in a site-specific therapeutic activity,demonstrating the potential of tumor-targeting bacteria to deliver drugs for tumor therapy.In addition,Chuetal.demonstrated that indocyanine green (ICG) loaded in maltodextrin (MD)-conjugated nanoparticles could be internalized into bacteria through bacteria specific MD transporters [86].All these internalization strategies for small molecule drugs can endow high payloads and good loading stability with no premature releaseinvivo,thus achieving effi-cient targeted delivery.
Decorating on the bacterial surface is an alternative strategy for loading small molecules.Metal-organic frameworks (MOFs) have attracted much attention for drug delivery owing to their large pore volume,high drug loading capacity,tunable composition and facile functionalization for improved biostability and targetability[87-89].Recently,Yanetal.proposed that MOFs was a powerful tool for loading tumor therapeutics on bacteria,in which a photosensitizer and a chemical drug wereinsituencapsulated during the formation of zeolitic imidazolate framework-8 (ZIF-8) [90].The viability of bacteria was preserved in as-prepared bacteria@MOFs hybrid,with tumor-targeted therapeutic delivery and combined chemo-photodynamic therapy achieved.This study engineered the bacteria with MOFs for the first time,demonstrating the potential of biomineralization of living organisms in combinational therapeutic applications.
4.1.2.Covalentbonding
It has been reported that there are high levels of intrinsic functional groups on the surface of bacteria,such as amino,carboxyl,hydroxyl and phosphate groups [91].Thus,in addition to physical encapsulation,the small molecule drugs can also be immobilized on the bacteria through covalent bonding,either with drug alone or with drug-containing nanoparticles.Xieetal.directly conjugated DOX ontoE.coliNissle 1917viaacid-labilecis-aconitic anhydride linkers,with the bacterial motion profiles and viability maintained,achieving tumor-targeted drug delivery and responsive drug release [92].The DOX accumulation in tumor was much higher than that of nanocarriers,thus resulting in improved antitumor efficiency and spatiotemporal controllability.Chenetal.first loaded photosensitizer ICG into solid lipid nanoparticles and then covalently attached the nanoparticles toSalmonellatyphimuriumYB1viaamide bonds [93].After reaching the tumor siteviahypoxiatargeting and photothermal-assisted accumulation,highly efficient photothermal therapy was achieved under the near-infrared (NIR)laser irradiation,with large solid tumors eradicated without relapse.This work provided a valuable strategy for large solid tumors therapy with high efficiency and low toxicity.In addition,the bacteria can be functionalized with other special groups for further conjugation.Cu(Ⅰ)-catalyzed azide-alkyne cycloaddition,generally known as “click reaction”,has been widely used in molecular connection owing to its advantages of mild conditions and high efficiency and selectivity [94].Recently,Gaoetal.functionalizedE.coliwith positively charged oligo(phenylene-vinylene)-alkyne and anchored the azide-bearing paclitaxel on their surface through intracellular click reaction,which enriched the therapeutic drugs in drug-resistant tumor cells and selectively induced apoptosis,overcoming the drug resistance of tumor cells [95].
4.1.3.Metaboliclabeling
Metabolic labeling,a technique which employs substrate analogs containing diminutive tags to infiltrate biosynthetic pathways and generate labeled product in cells,has led to advances in the means by which drugs can be loaded on the bacteria [96].For example,Shietal.conjugated a photosensitizer with a bacteria metabolic substrate D-alanine (D-Ala) and constructed engineered bacteria with photosensitizer decorated through metabolic incorporation into bacterial peptidoglycan,providing a promising regime for eradicating malignant melanoma [97].Liuetal.also incorporated photosensitizer on bacteria through metabolic labeling,which generated ROS under light irradiation and triggered the trapped plasmid release into cytoplasm through destructing the bacteria cell membrane to sufficiently suppress tumor growth [98].
Notably,Salmonellacan decrease the drug efflux transporter Pglycoprotein (P-gp) by the type III secretion effector SipA through a caspase-3-involved pathway,which can overcome multidrug resistance and recover the sensitivity of tumor cells to the conventional chemotherapeutics [99].Capitalized on this fact,we recently reported a bacteria-based chemo-sensitization strategy,resulting enhanced glioma therapy [62].Therefore,Salmonellacan maximize the efficacy of small molecule drugs such as DOX and 5-Fluorouracil by the combinational effects of targeted delivery and intracellular accumulation [100].
4.1.4.In-situproduction
In addition to the strategy of directly loading drugs described above,small molecule drugs can also be producedin-situby bacteria or with the assistance of genetically engineered bacteria,thereby maximizing efficacy and minimizing systemic toxicity.TTX is a neurotoxin which can exert anti-tumor effects by acting on the active ionic channels that promote tumor cell proliferation and invasion.Shewanellaalgaehave been found to be natural TTXproducing bacteria through their intracellular metabolic reactions,which can be boosted by exogenous energy of electrons.Wangetal.deposited photocatalytic gold nanoparticles (AuNPs) onShewanellaalgaesurface,which produced photoelectrons under light irradiation and transferred them into bacteria to enhance the production of TTX for tumor therapy (Figs.S2A-C in Supporting information) [42].This optimal controlled and material-assisted bacterial biosynthesis provided a novel antitumor strategy with artificial controllability and high yields.Besides,the nontoxic prodrugs can be converted to toxic drugs at the tumor site by combination of prodrugs and bacteria expressing prodrugs converting enzymes.For example,a supramolecular complex CPPDI based on perylene diimide (PDI) derivative was selectively reduced to radical anions by oxygen-sensitive hydrogenase expressed on the surface ofE.coliin hypoxic tumors.These radical anions could adsorb NIR light and act as effective photothermal agent to achieve selective photothermal therapy for highly precise tumor suppression(Figs.S2D and E in Supporting information) [101].Mesa-Pereiraetal.engineered aSalmonellastrain to express cytosine deaminase,which could convert 5-fluorocytosine (5-FC) into antineoplastic 5-fluorouracyl (5-FU),and meanwhile,deleted the upp gene sequence to prevent the bacteria from being killed by accumulated 5-FU [102].They demonstrated that thisSalmonellastrain exhibited increased cytotoxic effects towards tumor cells.Besides,the antiviral drug ganciclovir could be converted by herpes simplex virus thymidine kinase (HSV-TK) into a deoxyguanosine triphosphate analog and then induced tumor cells apoptosis by incorporated in DNA.TheSalmonellaengineered to express HSV-TK showed efficacy in human lymphoma xenografts with ganciclovir co-administered intratumorally or intravenously,while few adverse effects were detected [103].The purine nucleoside phosphorylase,which can convert 6-methylpurine 2′-deoxyriboside and 6-methoxypurine 2′-deoxyriboside into 6-methylpurine and 6-methoxypurine,has also been investigated [104,105].Apart from the cytotoxic drugs mentioned above,some critical amino acids can also be produced at the tumor sites.The L-arginine content is the key factor determining the efficiency of anti-tumor T cell responses,and increasing intratumor L-arginine levels can potentiate the immune responses.P.Canaleetal.had developed anE.coliNissle 1917 strain expressing enzymes for arginine biosynthesis with arginine repressor gene deleted and exhibited feedback-resistant,which could efficiently convert the metabolic waste product ammonia into Larginine (Fig.S2F in Supporting information).The intratumoural L-arginine concentrations were significantly increased by the colonization of these bacteria and synergistic effects with PD-L1 antibodies for restricting tumor growth were observed accompanied by increased T cells infiltration (Figs.S2G-I in Supporting information)[106].This was the first report of metabolic modulation of tumors by engineered bacteria to enhance the efficacy of immunotherapies.
4.2. Biomacromolecules
4.2.1.Surfaceloading
The biomacromolecules including nucleic acid and protein can also be delivered by bacteria.In consideration of that most of these biomacromolecules are negatively charged,electrostatic adsorption seems to be the easiest way for their loading.However,the surface of bacteria is also negatively charged,hence some other positively charged materials are necessary to load the biomacromolecules onto the surface of bacteria.For this purpose,Huetal.assembled the plasmid DNA encoding vascular endothelial growth factor receptor 2 (VEGFR2) with cationic polymers to form cationic nanoparticles and then adsorbed them onto the bacteria as a coating layer.The obtained hybrid system could overcome the biological barriers during the vaccination process including escaping from phagosomes and tolerating against the acid in stomach and serve as oral DNA vaccines which efficiently activated the T cell and inhibited the tumor growth [107].
4.2.2.Plasmidtransformation
In fact,the delivery of various functional RNA and protein is frequently realized by transformation of a plasmid DNA containing encoded genes into the bacteria by electroporation taking advantage of the gene-expressing factory nature of bacteria [108].Interestingly,the kinetics of gene expression including transient,sustained and regulated expression can be realized by choosing appropriate promoters which contain the bind region for RNA polymerase and associated factors to control transcription [109].Phanetal.used attenuatedSalmonellatyphimuriumto deliver IDOtargeting small hairpin RNA (shDNA) plasmid,which significantly reduced the expression of immune checkpoint protein IDO and efficiently delayed the tumor progression by robust activation of innate immunity [110].Fanetal.transformed the plasmids expressing the therapeutic protein TNF-αwith a thermally sensitive promoter to the noninvasive bacteriaE.coliMG1655 and decorated with AuNPs on the surfaceviainsituspontaneous reduction by bacteria (Fig.S3A in Supporting information) [111].When orally administrated,the bacteria could invade the internal microcirculation by means of M cells and then target tumor regions.The expression of TNF-αcould be controlled by NIR laser irradiation,which could selectively induce the apoptosis of tumor cells (Figs.S3B and C in Supporting information).This strategy not only improved the controllability on biomacromolecules expression but also overcame the challenges for oral delivery of biomacromolecular drugs including limited adsorption and poor tumor targeting.Similarly,Wangetal.established photothermal bacteria with AuNPs decorated and plasmids encoding pore-forming cytolysin A(ClyA) transformed,which expressed ClyA under the activation of heat produced through photothermal effects and under the control of NIR laser irradiation [112].This triggering strategy by incorporating specific promoter sequences in the plasmids enables precise control of gene expression,which can maximize the therapeutic efficacy while minimize the systemic toxicity.The common triggers include small molecules (L-arabinose,tetracycline,salicylate,etc.),tumor microenvironment (hypoxia,acidic),γ-irradiation,ultrasound and the above-mentioned heat [49,113-117].In addition to the cytotoxic protein,immuno-stimulating protein or antibodies can also be delivered by the bacteria.Leventhaletal.engineered a living biotherapeutic of non-pathogenicE.coliNissle producing a stimulator of interferon genes (STING) agonist,resulting in efficient anti-tumor immunity and immunological memory[118].Zhengetal.engineered attenuatedSalmonellastrain to secrete FlaB,the tumor-suppressive effect of which depended on the TLR4 signaling and could be augmented by TLR5 signaling [119].
Notably,the release of the expressed protein from bacteria is sometimes inefficient.To solve this problem,the strategy of fusing target protein with secreted protein has been developed.The following secreted protein are most widely used: Lpp,LamB,LTB,MalE,OmpA,OmpC,OmpF,OmpT,PelB,PhoA,PhoE and SopA[120].For example,the type III secretion system (T3SS) has been constructed with secreted protein SopA fused with the target protein,which was activated upon contact with eukaryotic cells to direct the protein secretion [121].IFN-γwas fused with the secreted protein SipB for secretion from attenuatedSalmonella[122].In addition,several strategies have been developed to promote the lysis of bacteria.Gurbatrietal.engineered the probiotic bacteria to locally release nanobodies of PD-L1 and cytotoxic T lymphocyteassociated protein-4 (CTLA-4) by coupling to an optimized lysing mechanism known as quorum-sensing,which could lyse when grow to a critical density and efficiently release the nanobodies within the tumor microenvironment (Figs.S3D-F in Supporting information) [123].Wuetal.coatedE.coliwith photosensitizercontaining nanoparticles,which could generate ROS under light irradiation and break the bacteria,promoting the protein release(Figs.S3G and H in Supporting information) [124].In addition,the antibiotic-sensitive strain was used to release the cargo upon the administration of antibiotic [125].These studies presented strategies to optimize bacteria-mediated intracellular biomacromolecules delivery.
4.3. Nanomaterials
The rapid development of nanotechnology has brought new approaches for tumor treatment [126].As described above,diverse functional materials can also be delivered by the bacteria,such as photocatalytic materials and metal oxide materials.Zhenget al.loaded the photocatalytic materials C3N4onE.coliby electrostatic interactions,which could promote the metabolic activities ofE.coliby transferring the produced photoelectrons and thus enhanced the anti-tumor efficacy [70].In addition,MnO2nanoflower was decorated on the bacteria to catabolize lactate at tumor sites by accepting the electron and inhibit lactate production by catalyzed oxygen production and HIF-1αdownregulation,thus exhausting the extracellular lactate and inhibiting tumor progression[71].Bi2S3nanoparticles (BNPs) were transported to tumor region by tumor-tropic bacteria and elicited radiosensitizing effects under X-ray irradiation based on the high atomic number of Bi element[127].Based on the former facts,the combination of bacteria and nanomaterials will certainly revolutionize the tumor therapy.
4.4. Surface functionalization
In addition to serving as carriers for small molecules,biomacromolecules and nanomaterials as mentioned above,the bacteria could also be surface modified for enhanced efficacy,especially through the combination with photothermal therapy.Chenetal.coated VNP20009 with polydopamine through the selfpolymerization of dopamine under alkaline condition,which exhibited strong NIR laser absorption and high photothermal conversion efficiency,realizing combined biotherapy and photothermal therapy [128].Liu’s group further conjugated tumor-specific antigens and checkpoint blocking antibodies on the polydopamine coated bacteria,generating immunotherapeutic with triple immune activators [129].The tumor-associated macrophages could be repolarized into a pro-inflammatory phenotype by the photothermal effect of polydopamine,which synergized with DCs maturation by antigens and cytotoxic T lymphocytes activation by immune checkpoint antibodies to generate robust tumor-specific immune responses.To optimize the photothermal efficiency,they further modified the bacteria both inside and outside to express the photothermal melanin by means of genetic engineering and polymer chemistry [130].Upon the light irradiation,moderate heat could be repeatedly generated,which stimulated immune activation and reversed the tumor immunosuppressive microenvironment.Given the facility of the bacterial modification strategy mentioned above as well as the controllability and noninvasiveness of photothermal therapy [131,132],these studies have proposed a promising direction for optimizing anti-tumor efficacy.
5.Clinical translation
Based on the in-depth preclinical research,several bacterial strains have been advanced to be tested in human patients.Many clinical trials with tumor-targeting bacteria have been reported or registered in recent years (Table S2 in Supporting information),most of which were conducted with attenuatedSalmonellastrains or their engineered derivatives to express specific payloads.For more detailed discussion on these clinical trials,please refer to Part S5 (Supporting information).Although the outcomes were not satisfactory,the results have revealed some obstacles that must be overcome for bacterial clinical translation.We will discuss it comprehensively in the next section.The bacterial therapeutics still have a long way to go before they can be applied in the clinic.
6.Problems and current solutions
As discussed above,the bacteria can exert anti-tumor effects through diverse mechanisms,including direct killing of tumor cells,metabolizing in the tumor microenvironment,regulating the gut microbiota and activating the immune system.Despite bacteriamediated tumor therapy has many advantages and holds promise for overcoming the limitations of traditional therapies,the failure in the clinical trials suggests that it still faces many challenges [21].
6.1. Delivery barrier in body
6.1.1.Immuneclearance
As an extraneous live microorganism,bacteria invaded into the blood would be recognized by the host immune system and eliminated from the body before reaching the tumor site,which severely inhibit their anti-tumor efficacy [133,134].As seen in the phase Ⅰclinical trial of attenuatedSalmonellaVNP20009 for patients with metastatic melanoma,no evident bacteria colonization and objective tumor responses were observed [21].Thus,reducing the elimination of bacteria in the blood and increasing their tumor colonization are of great significance for enhanced efficacy.
6.1.2.Gastricdestructionandtoxicity
For bacteria administrated orally,the first barrier they face is the stomach,an extremely acidic environment in which many bacteria strains cannot tolerate and survive [135].The structure of bacteria is always destroyed and they cannot move forward to the intestinal tract to exert therapeutic effects [136].For some bacteria which are strong enough to resist the harsh environment in the stomach [137],however,they will colonize,proliferate rapidly and even produce toxins [138].As thus,the epithelial barrier is damaged by direct invasion or interfered by the metabolites,which bring health risks of severe infections [139].
Based on the problems mentioned above,the surface modulation strategy has been developed to improve theinvivoperformance of bacteria (please refer to Part S6 in Supporting information for more detailed discussion).
6.2. Heterogeneous distribution/poor dissemination inside tumors
In addition,the heterogeneous distribution of bacteria in the tumor is another reason for compromised anti-tumor efficacy of bacteria.It was found that the tumor-targeting bacteria shown poor dissemination inside the tumors and mainly accumulated in the central necrotic areas,with outer viable tumor cells unaffected.The necrotic region and viable tumor region were separated by a barrier of neutrophils which have been recruited to the tumor site by the colonized bacteria [140].Thus,selective depletion of neutrophils at the tumor site is expected to promote the homogeneous distribution of bacteria and achieve complete tumor eradication.Bacteria-based combinational tumor therapy has become an inevitable trend.To this end,our group used sialic acid-decorated silver nanoparticles to selectively recognize the neutrophils and achieve tumor-homing based on the neutrophil infiltration under the recruitment ofSalmonella(Figs.S5A-C in Supporting information).The tumor-targeting silver nanoparticles locally depleted the neutrophils and boostedSalmonellaefficacy (Fig.S5D in Supporting information) [37].Chenetal.demonstrated that diterpenoid triepoxide compound triptolide could improveSalmonellatherapy by suppressing the infiltration of neutrophils and inhibiting angiogenesis [141].
6.3. Immunosuppression
The immunosuppressive microenvironment is a major feature of malignant tumors.One of the reasons for the failure of bacteria in clinical trials is that highly immunosuppressive tumor microenvironment attenuates the immunological effect.As is known to all,tumors can evade immune surveillance through various mechanisms,such as reducing the immunogenicity through downregulation of tumor-associated antigens,secreting immunosuppressive factors by altering metabolic pathways and thus recruiting suppressor immune cells [142].Although bacteria have strong immune-stimulatory ability,only weak immune responses can be induced at the dose limited by the dose-dependent toxicity.In addition,they cannot achieve comprehensive regulation on multiple pathways and will even cause a series of compensatory responses which promote the immune escape.Therefore,combination with other immune activators acting on diverse pathways is necessary to achieve stronger immune responses and better anti-tumor effects.To this end,various strategies have been investigated for enhancing bacterial immunotherapy (Table S3 and Part S7 in Supporting information).
6.4. Safety
Safety is the major concern of bacteria application,with the risk of clinical infection or sepsis,especially in the immunocompromised individual.Several strategies have been developed to improve the biosafety of bacteria-based therapy.
6.4.1.Geneticengineering
The most widely studied method for bacteria attenuation is genetic modification,such as the deletion of virulence-related genes and the genes encoding the essential components of bacteria,as discussed in detail in Section 2.2.3.
6.4.2.Bacterialderivatives
Some bacterial derivatives with similar immunogenicity as bacteria are used in order to improve the biosafety.Bacterial outer membrane vesicles (OMVs) are spherical,lipid bilayer structures produced during the normal growth of Gram-negative bacteria,which contain many bacterial components,including DNA,LPS,proteins and enzymes,like exosomes derived from different cells[143,144].Kimetal.demonstrated that OMVs could specifically accumulate in the tumor site and induce specific anti-tumor immune responses without apparent adverse effects,having the potential as an effective and safe immunotherapeutic agent [145].In fact,a kind of OMV-based vaccine,meningococcal group B (MenB) vaccine,whose trade name was Bexsero,has been used for children in the clinic to provide protection against MenB disease [146].In addition,the bacterially derived nonviable nano-cells with diameter of 400 ± 20 nm has been demonstrated to be able to package with cytotoxic drugs or nucleic acids for tumor targeted delivery.Such system could induce both innate and adaptive immune responses to elicit anti-tumor effects,and its safety has been demonstrated in three clinical trials with various tumor patients [147].
7.Conclusions and future perspective
Many researchers have found that bacteria-based tumor therapy is a promising therapeutic candidate.The obligate and facultative anaerobes exhibit intrinsic advantages over traditional tumor therapy,such as the capacity of precisely navigating and penetrating in tumor tissues using their own environmental perception and driving forces.In addition,they can be genetically or chemically modified in a facile manner to possess unique properties meeting the clinical needs.Capitalized on their facile functionalization and outstanding drug loading capacity,bacteria can be utilized as carriers for various types of drugs including small molecules,biomacromolecules and nanomaterials,thus achieving combination therapies with therapeutic efficacy improved.Although considerable progress has been made in the field of bacteria-based tumor therapy,there are still many hurdles restricting the development and application of bacteria to be solved.(1) The intrinsic bacterial immunogenicity and toxicity pose safety risk for patients.Bacteria are living organisms which can proliferate in the human body.Although the virulence of bacteria can be reduced through genetic engineering,the excessive attenuation can affect the capacity of tumor colonization and immune responses induction.Meanwhile,the potential residual toxicity may still be horrible for tumor patients.For example,the systemically administrated bacteria with general immunogenicity may induce systemic inflammation and even cause severe septic shock.Developing “smart” bacterial therapeutics which hide their immunogenicity in circulation and then reactivateinsitumay be a promising strategy to avoid systemic toxicity,which can be accomplished by surface modification with materials responsive to the tumor environment.(2) How to clear bacteria after the tumor has been cured remains a question.Some antibiotics may be used to kill the bacteria.However,several new problems arise such as the selection of proper antibiotics and optimization of the administration dosage.Besides,long-term use of antibiotics may destroy the microbiota balance of host.The excessive use of antibiotics,known as “antibiotic abuse”,and the resulting bacteria drug resistance even poses a serious threat to human health and is a severe problem that needs to be faced by the whole world.(3) The current animal tumor models used to assess the anti-tumor effects of bacteria cannot adequately simulate tumors in clinical patients.Briefly,the growth of tumors in human’s body usually takes years while the murine tumor models grow much faster which can be established within days or weeks,resulting in the formation of hyperpermeable blood vessels and lack of pathological signs mimicking the real disease conditions.More reliable animal tumor models such as patient-derived xenograft (PDX) model should be considered in future research.Despite these unresolved problems,bacteria-based cancer therapy is an emerging research frontier that deserves further exploration for precise tumor treatment and expanding application for various disease treatments.In summary,bacteria-based tumor therapy is a type of long-history therapeutic modality,which has great potential to combat tumor.With significant fundamental understanding to illustrate the anti-tumor mechanisms,bacteria have entered clinical trials for modern scientific studies.While the clinical outcomes are somehow frustrated,it also indicates the room for future improvement.The main research direction is to improve therapeutic efficacy of bacteria,and this can be achieved by either bioengineering of bacteria,or rational design of combinatorial systems for synergistic tumor therapy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Nos.U1903125,82071986),Natural Science Foundation of Hunan province in China (No.2021JJ20084),the Science and Technology Innovation Program of Hunan Province (No.2021RC3020).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108557.
杂志排行
Chinese Chemical Letters的其它文章
- The 3rd Xihua Chemistry and Biomedicine Forum
- Professor Hualiang Jiang: A tribute to an esteemed visionary chemist and pharmacist
- Recent advances in visible light-mediated chemical transformations of enaminones
- Development of porphyrin-based fluorescent sensors and sensor arrays for saccharide recognition
- Recent advances of versatile reagents as controllable building blocks in organic synthesis
- Synthetic host-guest pairs as novel bioorthogonal tools for pre-targeting☆
