眼底血管改变与脑白质高信号的相关性分析
2024-03-16花金萍徐博伦詹建梅熊俊峰童毓华
花金萍 徐博伦 詹建梅 熊俊峰 童毓华


[摘要] 目的 探討眼底血管改变与脑白质高信号(white matter hypertensity,WMH)的相关性。方法 选取2022年7月至2023年7月于衢州市人民医院神经内科确诊的87例WMH患者作为实验组,同期随机选择80例年龄相匹配的无WMH的健康体检者作为对照组。应用光学相干断层扫描(optical coherence tomography,OCT)及光学相干断层扫描血管成像(optical coherence tomography angiography,OCTA)技术测量两组入选者的视网膜血管结构、微血管密度和神经纤维层(retinal nerve fiber layer,RNFL)厚度等眼底血管参数,比较两组的差异并分析其相关性。结果 与对照组比较,实验组患者的视网膜血管结构和微血管密度参数与WMH无相关性。平均、颞上部、鼻下部RNFL厚度减少(P<0.05)。结论 OCT与OCTA检测WMH均具有可行性,颞上部、鼻下部视网膜神经纤维层厚度可作为预测WMH发生风险的指标之一。
[关键词] 光学相干断层扫描;光学相干断层扫描血管成像;假定血管源性脑白质高信号;视网膜血流;视网膜神经纤维层
[中图分类号] R816.97;R770.43 [文献标识码] A [DOI] 10.3969/j.issn.1673-9701.2024.06.011
Correlation analysis between retinal vascular changes and white matter hyperintensity
HUA Jinping1, XU Bolun2, ZHAN Jianmei3, XIONG Junfeng4, TONG Yuhua2
1.The Second Clinical Medical College, Zhejiang Chineese Medicine University, Hangzhou 310000, Zhejiang, China; 2.Department of Ophthalmolog, Quzhou Peoples Hospital, Quzhou 324000, Zhejiang, China; 3.Department of Neurology, Quzhou Peoples Hospital, Quzhou 324000, Zhejiang, China; 4.Department of Radiology, Quzhou Peoples Hospital, Quzhou 324000, Zhejiang, China
[Abstract] Objective To investigate the correlation between retinal vascular changes and patients with white matter hyperintensity (WMH). Methods A total of 87 patients diagnosed with WMH in the Department of Neurology, Quzhou People's Hospital, from July 2022 to July 2023 were selected as experimental group. Concurrently, 80 age-matched healthy individuals without WMH undergoing regular health check-ups were randomly selected as control group. Optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA) technologies were applied to measure retinal vascular parameters, including vascular structure, microvascular density, and retinal nerve fiber layer (RNFL) thickness. Compare the differences between two groups and analyze their correlation. Results Compared with the control group, the experimental group exhibited no correlation between retinal vascular structure and microvascular density parameters with WMH. However, there was a significant decrease in average, temporal upper, and nasal lower RNFL thickness (P<0.05). Conclusion OCT and OCTA are feasible methods for detecting WMH, and the thickness of the temporal upper and nasal lower retinal nerve fiber layers can serve as indicators for predicting the risk of WMH occurrence.
[Key words] Optical coherence tomography; Optical coherence tomography angiography; White matter hyperintensities of presumed vascular origin; Retinal blood flow; Retinal nerve fiber layer
腦白质高信号(white matter hypertensity,WMH)是脑小血管疾病的影像学表现之一。2013年,国际神经影像学血管性改变报告标准明确WMH的定义为双侧大脑白质T2加权成像(T2 weighted imaging,T2WI)或液体抑制反转恢复序列(fluid attenuated inversion recovery sequence,FLAIR序列)上表现为点、片、融合状或对称分布高信号,T1WI序列上呈等信号或低信号,不包括深部灰质或脑干的病变[1]。WMH在64岁左右人群中的患病率达11%~21%,在82岁左右人群中的患病率高达94%[2]。WMH与脑卒中、认知障碍、痴呆及死亡的风险增加密切相关[3]。根据2016年美国心脏协会(American Heart Association,AHA)及美国卒中协会(American Stroke Association,ASA)的共同声明,脑白质高信号可能与衰老和血管危险因素相关的动脉硬化性微血管疾病有关[4]。目前MRI是检测脑小血管疾病最重要的工具,然而MRI无法检测到<500μm的细微退行性变和微血管变化。视网膜作为中枢神经系统的一部分,其发育起源与大脑相似,并具有类似的胚胎学、解剖学和生理学特征[5]。眼底直径为100~300μm的小动脉和小静脉具有与脑小血管相似的解剖学和生理学特征。因此,视网膜的异常变化可反映脑血管情况,有助于识别高危人群并促进早期干预。
1 资料与方法
1.1 一般资料
选取2022年7月至2023年7月于衢州市人民医院神经内科确诊的87例WMH患者作为实验组,同期随机选择年龄相匹配的无WMH的80名健康体检者作为对照组。实验组中男40例,女47例,平均年龄(64.60±6.03)岁;对照组中男39例,女41例,平均年龄(63.58±6.48)岁。纳入标准:①年龄50~84岁;②WMH符合神经影像学血管性改变报告标准[1];③入选者均行头颅磁共振检查且自愿配合做眼底检查;④入选者均知情同意。排除标准:①近期有皮质下小梗死者;②既往有腔隙性脑梗死者;③脑出血者;④有非血管源性的白质高信号,如多发性硬化、CO中毒性脑病等;⑤神经退行性疾病,如帕金森、阿尔茨海默病、痴呆;⑥有严重的眼底病变疾病,如糖尿病视网膜病变、严重的老年性白内障等。本研究经衢州市人民医院伦理委员会审批通过(伦理审批号:衢州市人民医院伦审2022研第033号)。1.2 方法
1.2.1 光学相干断层扫描检查 采用德国海德堡光学相干断层扫描(optical coherence tomography,OCT)扫描仪(型号Spectralis OCT)对入选者右眼颞上方距离视盘边缘0.5~1.0倍视盘直径距离的动静脉血管进行线性扫描。操作者将扫描环的内圆与视盘边缘等距,手动调整扫描线以确保垂直血管走形。每条血管至少获取2张及以上清晰的图像。操作完成后将图像垂直水平比率调整为1∶1μm,放大8倍后保存血管的横截面图像。使用ImagJ软件(美国国立卫生研究院)和半峰宽算法对血管进行测量并计算视网膜动脉外径及内径、静脉外径及内径,动脉血管壁厚度=(动脉外径-动脉内径)/2,静脉血管壁厚度=(静脉外径-静脉内径)/2,动静脉管径比值=动脉内径/动脉外径,见图1。
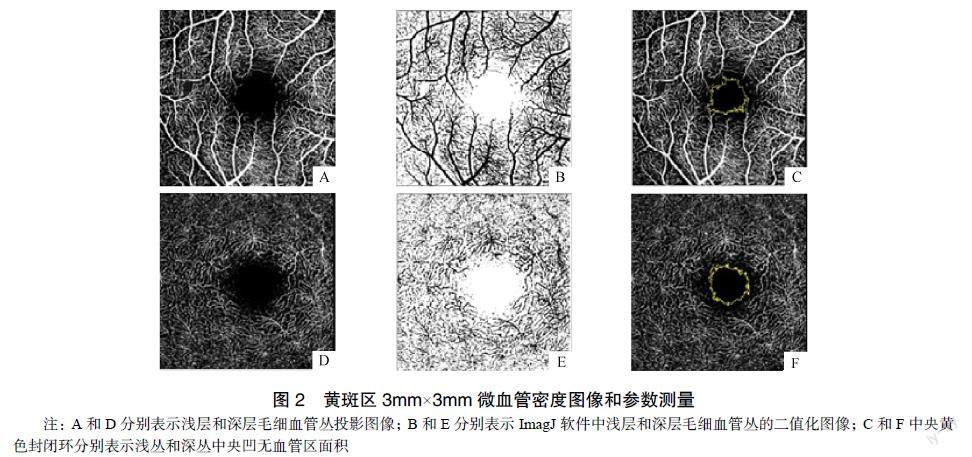
1.2.2 光学相干断层扫描血管成像检查 扫描系统切换至Spectralis光学相干断层扫描血管成像(optical coherence tomography angiography,OCTA),采集黄斑区3mm×3mm范围内的浅层毛细血管丛、深层毛细血管丛(deep capillary pleus,DCP)和中央凹无血管区面积(foveal avascular zone,FAZ)图像,受试者固视正前方的指示灯完成操作。保存没有明显运动伪影、血管连续清晰的图像。ImageJ软件计算图像中的白色像素点与血流信号占图像总像素点的百分比得到血管密度参数和自动识别获得FAZ面积参数,见图2。
1.2.3 OCT测量神经纤维层厚度 扫描视盘时将中心聚焦于视杯的中心,扫描直径为3.4mm,自动获得视盘周围视网膜神经纤维层(retinal nerve fiber layer,RNFL)厚度。上述眼科检查均由一名经验丰富的眼科医生完成,见图3。
1.3 统计学方法
采用SPSS 26.0统计学软件对数据进行处理分析。计数资料以例数(百分率)[n(%)]表示,比较采用c2检验;符合正态分布的计量资料以均数±标准差(![]() )表示,比较采用独立样本t检验,不符合正态分布的计量资料以中位数(四分位数间距)[M(Q1,Q3)]表示,比较采用Mann-Whitney检验;采用二元Logistic回归,默认enter方法分析眼底血管参数与WMH的相关性。P<0.05为差异有统计学意义。
)表示,比较采用独立样本t检验,不符合正态分布的计量资料以中位数(四分位数间距)[M(Q1,Q3)]表示,比较采用Mann-Whitney检验;采用二元Logistic回归,默认enter方法分析眼底血管参数与WMH的相关性。P<0.05为差异有统计学意义。
2 结果
2.1 两组入选者的一般资料比较
两组入选者的一般资料比较,差异无统计学意义(P>0.05),见表1。
2.2 两组入选者的眼底血管结构参数比较
实验组患者的右眼颞上动脉外径、内径小于对照组,静脉外径、内径大于对照组,差异有统计学意义(P<0.05),见表2。
2.3 两组入选者的黄斑区微血管密度参数比較
实验组患者的深层毛细血管丛血流密度小于对照组,浅从与深丛中央凹无血管区面积大于对照组,差异有统计学意义(P<0.05),见表3。
2.4 两组入选者的神经纤维层厚度比较
实验组患者的视网膜周围平均、颞上部、鼻下部的RNFL厚度小于对照组,差异有统计学意义(P<0.05),见表4。
2.5 两组眼底血管参数的二元Logistic回归分析
以是否患有WMH为因变量(有WMH=1,无WMH=2),将单因素筛选有意义的10个眼底血管参数作为自变量进行二元Logistic回归,结果发现视网膜周围颞上部、鼻下部神经纤维层厚度减少与WMH发生风险增高相关,见表5和表6。
3 讨论
WMH病理机制较为复杂,内皮功能障碍、脑血管反应性受损、静脉损伤和微栓塞等均为关键因素[6-9]。这些因素导致微血管受损,随后出现白质脱髓鞘和轴突损伤等病理改变。了解微血管损伤,对于识别WMH病理变化的潜在机制至关重要。以往研究通过眼底照相方法测量视网膜微血管变化,表明小动脉变窄和小静脉变宽与白质微结构损伤有关[10]。而本研究应用半峰宽算法测量视网膜血管可获得视网膜血管内外径、血管壁厚度、小动静脉比值,显著降低重复测量的误差,提高血管测量的准确性[11]。本研究显示与对照组相比,WMH患者血管壁厚度、小动静脉比值差异尚未达到统计学意义,眼底血管结构参数与WMH无相关性,可能是由于样本量小所致。
本研究中笔者使用OCTA测量所有入选者的黄斑区微血管密度,结果显示,与对照组相比,WMH患者的DCP血流密度降低,浅丛和深丛FAZ面积扩大。?evik等[12]研究结果显示DCP血流密度下降有统计学意义,与本研究结果一致。笔者推测,DCP血流密度下降可能与大脑中线粒体功能障碍和高耗氧有关[13]。本研究中WMH患者FAZ面积扩大,这一结果与Gao等[14]研究结果一致。视网膜内FAZ面积扩大可能继发于视网膜内神经元和胶质细胞的损伤及其对血流密度的影响[15]。
本研究通过OCT测量各象限RNFL厚度,结果显示WMH患者视盘颞上部、鼻下部的RNFL厚度变薄;而正常情况下,视网膜周围RNFL下象限和上象限较厚,鼻象限和颞象限较薄。这反映视网膜、视神经、视束损伤导致的视神经轴突损失,这与WMH的病理机制相似。此前的研究也揭示了WMH与视网膜周围RNFL厚度相关[16-17]。
综上,眼底血管变化对WMH的发生具有一定的预测价值,OCT和OCTA为WMH患者的早期诊断提供更多可能的成像靶点,可预测脑血管的风险,有利于WMH患者早期干预和治疗。
利益冲突:所有作者均声明不存在利益冲突。
[参考文献]
[1] WARDLAW J M, SMITH E E, BIESSELS G J, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration[J]. Lancet Neurol, 2013, 12(8): 822–838.
[2] SUN L, HUI L, LI Y, et al. Pathogenesis and research progress in leukoaraiosis[J]. Front Hum Neurosci, 2022, 16: 902731.
[3] DEBETTE S, BEISER A, DECARLI C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The framingham offspring study[J]. Stroke, 2010, 41(4): 600–606.
[4] SMITH E E, SAPOSNIK G, BIESSELS G J, et al. Prevention of stroke in patients with silent cerebrovascular disease: A scientific statement for healthcare professionals from the american heart association/american stroke association[J]. Stroke, 2017, 48(2): e44–e71.
[5] 謝新晖, 陈浜, 赵一天, 等. 眼脑联动与神经精神疾病[J]. 中华精神科杂志, 2020, 53(6): 546–552.
[6] SAM K, CONKLIN J, HOLMES K R, et al. Impaired dynamic cerebrovascular response to hypercapnia predicts development of white matter hyperintensities[J]. Neuroimage Clin, 2016, 11: 796–801.
[7] KEITH J, GAO F Q, NOOR R, et al. Collagenosis of the deep medullary veins: An underrecognized pathologic correlate of white matter hyperintensities and periventricular infarction?[J]. J Neuropathol Exp Neurol, 2017, 76(4): 299–312.
[8] ARBA F, GIANNINI A, PICCARDI B, et al. Small vessel disease and biomarkers of endothelial dysfunction after ischaemic stroke[J]. Eur Stroke J, 2019, 4(2): 119–126.
[9] AMMIRATI E, MORONI F, MAGNONI M, et al. Progression of brain white matter hyperintensities in asymptomatic patients with carotid atherosclerotic plaques and no indication for revascularization[J]. Atherosclerosis, 2019, 287: 171–178.
[10] MUTLU U, CREMERS L G, DE GROOT M, et al. Retinal microvasculature and white matter microstructure: The Rotterdam study[J]. Neurology, 2016, 87(10): 1003–1010.
[11] ZHU T P, TONG Y H, ZHAN H J, et al. Update on retinal vessel structure measurement with spectral-domain optical coherence tomography[J]. Microvasc Res, 2014, 95: 7–14.
[12] ?EVIK S G, BA?L? B S. Change in the foveal avascular zone and macular capillary network density after hyperbaric oxygen therapy in healthy retina[J]. J Ophthalmic Vis Res, 2021, 16(3): 393–399.
[13] CRINGLE S J, YU D Y, YU P K, et al. Intraretinal oxygen consumption in the rat in vivo[J]. Invest Ophthalmol Vis Sci, 2002, 43(6): 1922–1927.
[14] GAO Y, KWAPONG W R, ZHANG Y, et al. Retinal microvascular changes in white matter hyperintensities investigated by swept source optical coherence tomography angiography[J]. BMC Ophthalmol, 2022, 22(1): 77.
[15] PELLEGRINI M, VAGGE A, FERRO DESIDERI L F, et al. Optical coherence tomography angiography in neurodegenerative disorders[J]. J Clin Med, 2020, 9(6): 1706.
[16] MUTLU U, BONNEMAIJER P W M, IKRAM M A, et al. Retinal neurodegeneration and brain MRI markers: The Rotterdam study[J]. Neurobiol Aging, 2017, 60: 183–191.
[17] KIM M, PARK K H, KWON J W, et al. Retinal nerve fiber layer defect and cerebral small vessel disease[J]. Invest Ophthalmol Vis Sci, 2011, 52(9): 6882–6886.
(收稿日期:2023–11–10)
(修回日期:2024–02–16)
![]()
