Alternative splicing of the PECTINESTERASE gene encoding a cell wall-degrading enzyme affects postharvest softening in grape
2024-03-12HainanLiuMaosongPeiCharlesAmpomahDwamenaYaxinShangYiheYuTongluWeiQiaofangShiDalongGuo
Hainan Liu ,Maosong Pei ,Charles Ampomah-Dwamena ,Yaxin Shang ,Yihe Yu,Tonglu Wei,Qiaofang Shi,Dalong Guo#
1 College of Horticulture and Plant Protection, Henan University of Science and Technology, Luoyang 471000, China
2 Henan Engineering Technology Research Center of Quality Regulation and Control of Horticultural Plants, Luoyang 471000,China
3 The New Zealand Institute for Plant &Food Research Limited (PFR), Auckland 1010, New Zealand
Abstract The firmness of table grape berries is a crucial quality parameter. Despite extensive research on postharvest fruit softening,its precise molecular mechanisms remain elusive. To enhance our comprehension of the underlying molecular factors,we initially identified differentially expressed genes (DEGs) by comparing the transcriptomes of folic acid (FA)-treated and water-treated (CK) berries at different time points. We then analyzed the sequences to detect alternatively spliced (AS) genes associated with postharvest softening. A total of 2,559 DEGs were identified and categorized into four subclusters based on their expression patterns,with subcluster-4 genes exhibiting higher expression in the CK group compared with the FA treatment group. There were 1,045 AS-associated genes specific to FA-treated berries and 1,042 in the CK-treated berries,respectively. Gene Ontology (GO) annotation indicated that the AS-associated genes in CK-treated berries were predominantly enriched in cell wall metabolic processes,particularly cell wall degradation processes. Through a comparison between treatment-associated AS genes and subcluster-4 DEGs,we identified eight genes,including Pectinesterase 2 (VvPE2,Vitvi15g00704),which encodes a cell wall-degrading enzyme and was predicted to undergo an A3SS event. The reverse transcription polymerase chain reaction further confirmed the presence of a truncated transcript variant of VvPE2 in the FA-treated berries.Our study provides a comprehensive analysis of AS events in postharvest grape berries using transcriptome sequencing and underscores the pivotal role of VvPE2 during the postharvest storage of grape berries.
Keywords: grape,postharvest softening,folic acid,alternative splicing,Pectinesterase 2,alternative 3´ splice site(A3SS)
1.Introduction
Berry softening is an important postharvest quality attribute in table grapes (Wang Jet al.2018;Romeroet al.2020;Wang Het al.2020). While softening enhances fruit flavor and mouthfeel,it concurrently diminishes fruit disease resistance and shortens shelf life. Consequently,berry firmness represents a pivotal aspect in table grape breeding programs (Correaet al.2016). Recent decades have witnessed substantial progress in the study of fruit softening,which is a multifaceted and regulated process underpinned by physiological and biochemical changes(Correaet al.2016;Wonget al.2016) that include cell wall degradation (Paniaguaet al.2014;Niet al.2020),alterations in inclusions (Castellarinet al.2016;Liuet al.2021,2022),respiration rate shifts,and various metabolic transformations (Nasseret al.2022). The softening phenomenon primarily results from the breakdown of the intercellular layer within the cortical parenchyma cell wall,leading to increased pectin release (Santiago-Doménechet al.2008;Luoet al.2009;Paniaguaet al.2014).Throughout fruit softening,the solubilization of cell wall pectin coincides with the dissolution of the adhesive layer in the cell wall and disruption of the primary wall (Castillejoet al.2004). The alterations in the cell wall structure are due to the degradation of the cell wall which is catalyzed by a diverse array of enzymes,including pectinesterase(PE),polygalacturonase (PG),pectin methylesterase(PME),pectate lyase (PL),β-galactosidase (β-Gal),cellulase (CX) (Cosgrove 2005;Payasiet al.2009). In essence,the degradation of the cell wall polysaccharides constitutes the crux of fruit softening (Paniaguaet al.2014). A considerable body of research has been dedicated to the pectinolytic enzymes,given that pectin degradation is a primary determinant of fruit firmness(Santiago-Doménechet al.2008;Atkinsonet al.2012;Paniaguaet al.2014;Wang D et al.2018). For instance,PE catalyzes pectin demethylation to render the cell wall more susceptible to PG degradation (Brummell and Harpster 2001;Luo 2006);hence,PE is regarded a pivotal control point in the assembly and disassembly of cell wall networks (Castillejoet al.2004;Phanet al.2007;Wang Xet al.2020). Although the differential expression ofPEgenes across different species is generally associated with postharvest fruit softening,the molecular mechanisms underpinning this differential expression remain elusive (Chenet al.2013;Zhouet al.2021;Liuet al.2022).
Alternative splicing (AS) denotes the transcriptional process in which a single mRNA precursor generates two or more mature mRNAsviadistinct splicing mechanisms(Reddyet al.2013). In essence,AS yields a pool of transcriptional variants which,in turn,give rise to proteins with varying structures and functions,so it serves as a vital regulatory mechanism (Filichkinet al.2015;Niklaset al.2015). AS plays a pivotal role in the plants’capacity to respond adaptively to both environmental and endogenous stimuli (Staiger and Brown 2013). As a post-transcriptional regulatory mechanism,AS exhibits rapid responsiveness when plants detect changes in their external environment (Laloumet al.2018;Dantaset al.2019),thus warranting greater attention. The analysis of expressed sequence tags and transcriptome data has identified AS events in various plant species (Syedet al.2012;Clarket al.2019;Maillotet al.2021).
Previous research has demonstrated that up to 70%of multi-exon genes produce at least one splice variant in nine significant plant species,includingArabidopsis thaliana,Amborellatrichopoda,Glycinemax,Medicago truncatula,Oryzasativa,Phaseolusvulgaris,Populus trichocarpa,Solanumlycopersicum,andVitisvinifera(Chamalaet al.2015). The widespread prevalence of AS and its influence on the functional genome underscore its importance in plants (Kalynaet al.2012;Syedet al.2012;Clarket al.2019;Maillotet al.2021). It is worth noting that not all AS events result in functional proteins,as some may yield truncated proteins with potentially positive or negative regulatory effects on the plant. In general,AS also regulates gene expression by generating isoforms that are subject to degradation through the nonsensemediated decay (NMD) pathway (Kalynaet al.2012;Syedet al.2012). Despite the extensive exploration of AS in regulating functional gene expression and producing proteins with diverse functions,only a limited number of studies have delved into the regulatory impact of AS on grape berry softening.
To explore the AS patterns associated with grape berry softening,we applied folic acid (FA) treatment to postharvest grapes and employed RNA sequencing for a comprehensive analysis of genome-wide AS events by comparing the FA-treated and control samples. This study unveiled specific AS-related genes in both FAtreated and non-FA-treated berries,with alternative 5´first exon (transcription start site;TSS) and alternative 3´last exon (transcription terminal site;TTS) emerging as the dominant AS types among the treatments. A closer examination of thePectinesterase2(VvPE2) gene found that it undergoes a specific AS event (A3SS) in the FAtreated group,suggesting that AS events may account for the differential expression ofVvPE2between in the FA-treated and non-FA-treated groups. In summary,this study provides a comprehensive understanding of the AS events in postharvest grapes and underscores the crucial role of AS in postharvest softening.
2.Materials and methods
2.1.Plant materials and treatments
Details on the postharvest FA treatment of ‘Kyoho’ (Vitis viniferaL.×VitislabruscaL.) grapes were described in our previously published report (Peiet al.2023). Briefly,grape clusters were immersed in a FA solution (1 mg L–1)or sterile water (non-FA treatment,CK),both containing 0.01% Silwet L-77. We collected 30 berries at random every third day until 9 days after treatment (3,6,and 9 DAT). Building upon our prior research on postharvest FA treatment and the associated transcriptome findings,we conducted additional analyses to unveil the regulatory mechanisms governing postharvest softening.
2.2.PCA analysis and functional annotation of DEGs
To investigate the changes in expression patterns following the FA and CK treatments,we performed principal component analysis (PCA) on the data from 18 samples (i.e.,the samples on the 3rd,6th,and 9th days after FA and CK treatments,with three biological replicates each) using BMKCloud (www.biocloud.net).Volcano plots built with the R package “ggplot2” (3.3.3)(https://www.rdocumentation.org/packages/ggplot2/versions/3.3.0) were applied for visualizing the expression of the differentially expressed genes (DEGs) between FAand CK-treated berries. Gene Ontology (GO) enrichment analysis of the DEGs was conducted using the GOseq R package,which corrects for the gene length bias in DEGs by using the Wallenius non-central hyper-geometric distribution (Younget al.2010).
2.3.Cluster analysis of DEGs expression patterns in the transcriptome
To identify DEGs exhibiting similar expression patterns during postharvest storage under the FA and CK treatments,we subjected the expression data of these DEGs to cluster analysis and gene expression trend analysis. For cluster analysis,we used the R package(heatmap3),which categorized the DEGs based on their expression pattern changes. Hierarchical clustering heat maps and expression trend line graphs were employed to visualize the expression trends across different samples.
2.4.Transcriptome-based AS analysis
In gene expression processes,specific exons in the premRNA can be included or excluded from the mature mRNA. To identify AS events,we employed StringTie(Perteaet al.2015) to assemble the mapped reads generated by Hisat2. Subsequently,ASprofile (Cingolaniet al.2012) was used to predict the AS events in each sample and categorize them into different typical types.
2.5.ldentification of specific AS events and GSEA analysis
AS is not uniform across different treatments,tissues,or developmental stages. To identify and analyze specific AS events,we performed an integrated analysis using ASprofile (Cingolaniet al.2012) and DEGs analysis,where the differential expression analysis was conducted using DESeq2 (Loveet al.2014). Additionally,we conducted gene set enrichment analysis (GSEA) (Ge and Boris 2016),which can detect weak alterations in gene expression and was processed on the specific AS-associated genes based on their expression levels. The gene sets of each specific ASassociated gene were used as the background gene set.
2.6.PCR-based sequence validation of AS events
For validation of the selected AS events,we performed reverse transcription PCR (RT-PCR) and quantitative real-time PCR (qRT-PCR) using RNAs extracted from the berries treated with FA and CK at 3,6,and 9 DAT.The primers for RT-PCR (Appendix A) were designed using Primer 5.0 software,and RT-PCR was conducted as per our previous studies (Liuet al.2021). The qRTPCR procedures and reaction systems were prepared according to the TransStart Top Green qPCR SuperMix Kit instructions (TANSGEN,Beijing,China). For each transcript,qRT-PCR reactions were conducted with four biological replicates. The relative expression of different transcripts was normalized using the reference geneVvUbiquitin1(NCBI accession number CA808925) and calculated using the 2-ΔΔCtmethod (Xueet al.2019).Statistical analysis of the results was performed using SPSS through Tukey’s multiple comparison test.
Open reading frame (ORF) finder (https://www.ncbi.nlm.nih.gov/orffinder/) was used to search the obtained sequences for potential protein encoding segments and the corresponding amino acids were deduced,followed by multiple sequence alignments using DNAMAN sequence analysis software. The conserved domains and functions of the corresponding proteins were analyzed using National Center for Biotechnology Information Conserved Domain Search (NCBI’s Conserved Domain Database,https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
3.Results
3.1.Differential expression analysis of genes in the FA-treated grape berries
A total of 18 mRNA samples,representing both FA and CK treatment groups at 3,6,and 9 DAT,underwent sequencing,yielding approximately 114.44 Gb of clean reads. Each sample produced about 5.77 Gb of clean data,with at least 90.32% of the data achieving a quality score of Q30. The GC contents ranged from 46.79 to 47.97% (Appendix B). PCA was conducted on the transcriptome dataset to assess the correlation among biological replicates and to identify the primary sources of gene expression variation. The PCA plot revealed consistency across the biological replicates. The first two principal components (PC1 and PC2) explained 83.5% of the total variability in gene expression. PC1 contributed 59.8% of the variance,clearly distinguishing the FA and CK groups. PC2 accounted for 23.7% of the variance and differentiated between different storage stages. The different treatments were the main contributors to gene expression variability over the nine-day storage period(Fig.1-A).
In total,2,559 differentially expressed genes (DEGs)were identified between FA and the CK treatments at 3,6,and 9 DAT (Fig.1-B). Specifically,507,684,and 250 genes exhibited differential expression between the FA and CK groups at 3,6,and 9 DAT,respectively. A total of 39 DEGs were shared among the three treatment vs control comparisons (Fig.1-B). GO enrichment analysis revealed that DEGs were implicated in“response to reactive oxygen metabolism”,“cell wall organization”,and “xyloglucan metabolic process”within the “biological process” and “molecular function”categories,respectively (Fig.1-C). GSEA further affirmed that specific AS-associated genes were closely associated with macromolecule catabolic processes and cell wall metabolism (Appendix C). Among the enriched GO terms,those related to reactive oxygen species (ROS) and cell wall metabolism played a significant role in the deterioration of postharvest berry quality.
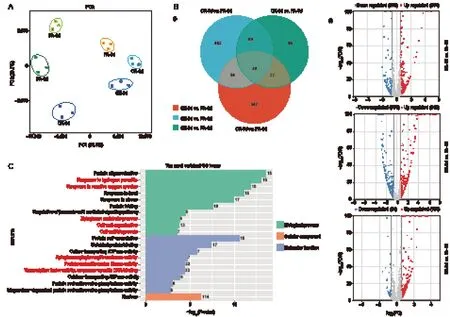
Fig. 1 Identification and functional annotation of the differentially expressed genes (DEGs) induced by folic acid (FA) treatment. A,principal component analysis (PCA). B,Venn diagram depicting DEGs between the samples treated with FA and water (control)at 3,6,and 9 days after treatment (DAT) (i) and Volcano plots illustrating the DEGs (ii). Cutoffs are indicated by lines;highly upregulated genes are mapped in red,and highly downregulated genes are mapped in blue. C,Gene Ontology (GO) enrichment analysis of the DEGs in “biological process”,“cellular component”,and “molecular function”. The GO terms-associated with reactive oxygen species (ROS) and cell wall metabolism are highlighted in red.
3.2.Cluster analysis of DEGs based on gene expression profiles

Fig. 2 Clustering analysis of differentially expressed genes (DEGs). A,hierarchical clustering graph of the 2,559 DEGs based on the averaged log2(FPKM+1) values of all genes in each cluster. B,differential gene module expression trends shown by the line chart. The 2,559 DEGs were clustered into four subclusters. The number of genes for each cluster is shown at the top right of each cluster.
Cluster analysis of the DEGs under the FA and CK treatments (Fig.2-A) categorized them into four subclusters with distinct expression profiles (Fig.2-B).Notably,one subset of DEGs (subcluster-4) exhibited continuous and high expression during the initial stages of the nine-day storage under CK treatment (CK-3d,CK-6d,and CK-9d) compared to the FA treatment. In contrast,genes from the other subclusters demonstrated differential expression at early,middle,or late storage,indicating a pattern of fluctuating expression (Fig.2-B). As reported by Peietal(2023),the berry firmness decreased in both the CK and FA treatment groups,and the berry firmness in the FA treatment declined significantly more slowly between days 3,6,and 9 compared with the firmness of the corresponding CK treatment groups. The expression levels and functional annotations of many subcluster-4 DEGs were consistent with our recently reported findings regarding changes in firmness under the FA and CK treatments in the same postharvest berry samples(Peiet al.2023). This consistency suggests that these genes may play crucial roles in the postharvest softening process.
3.3.ldentification and analysis of AS events associated with FA treatment of grape berries
Splice variants are predominantly generated through alternative 5´ first exon (transcription start site) (TSS),alternative 3´ last exon (transcription terminal site) (TTS),alternative exon ends (AE),and skipped exon (SKIP)mechanisms. Among the identified AS events,TSS and TTS were the most prevalent types,collectively accounting for over 80% of the total AS events. They were followed by AE and SKIP,while the approximate MIR (XMIR) type was the least numerous. Overall,the FA-and CK-treated samples exhibited similar AS patterns (Fig.3-A). For analytical convenience,the AS events were categorized into five types: alternative 3´ splice site (A3SS),alternative 5´ splice site (A5SS),mutually exclusive exons (MXE),retained intron (RI),and skipped exon (SE). The number of AS events in the CKvs.FA comparison groups showed a reduction at 3,6,and 9 DAT. SE accounted for the largest proportion of alternatively spliced transcripts,followed by A3SS,MXE,and A5SS,with RI generating the smallest number of alternatively spliced transcripts (Fig.3-B).
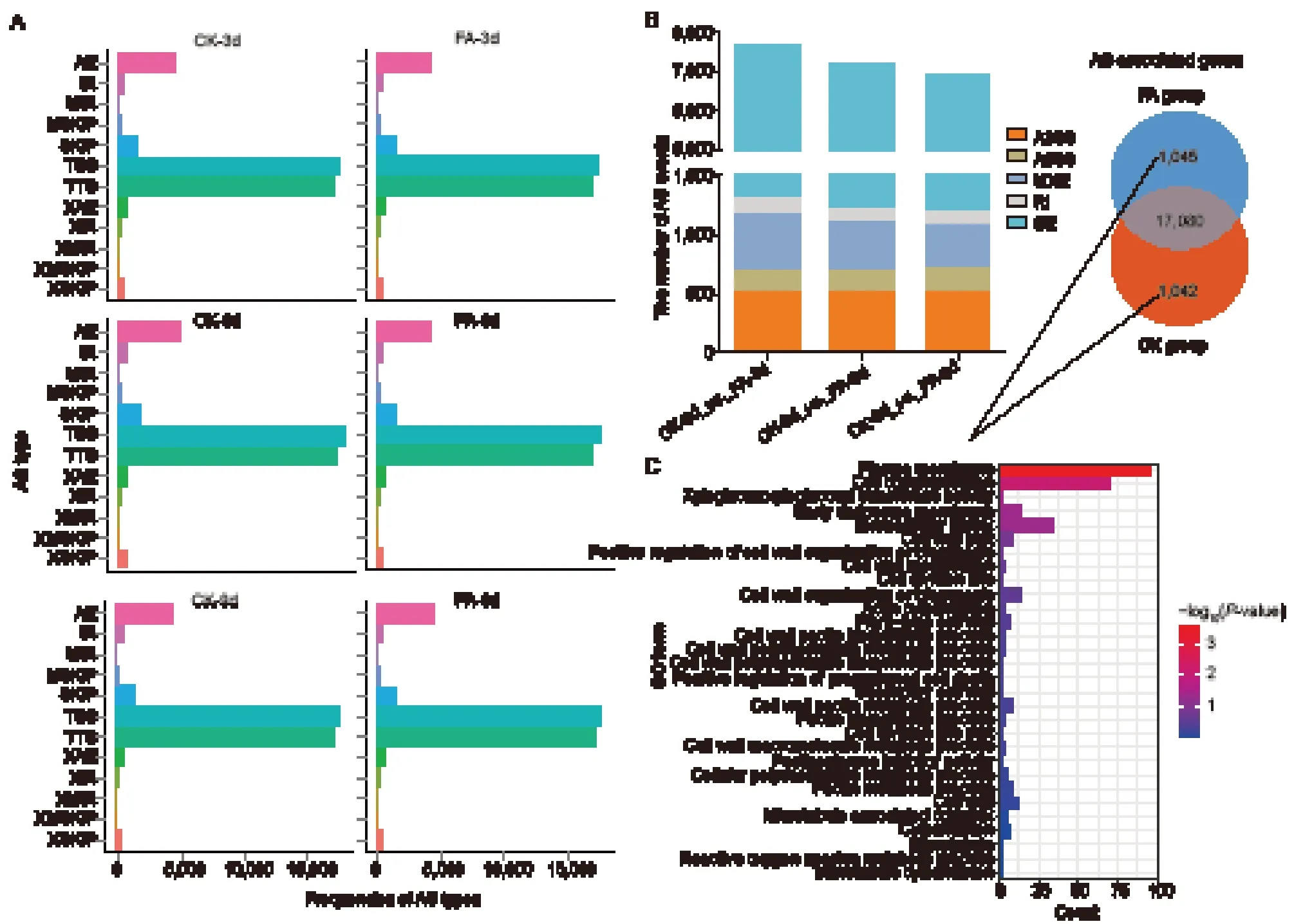
Fig. 3 Identification of alternative splicing (AS) events and functional enrichment analysis of AS-associated genes. A,frequencies of different AS types detected in the grape berries under the folic acid (FA) and CK treatments. TSS,alternative 5´ first exon(alternative transcription start site);TTS,alternative 3´ last exon (alternative transcription termination site);SKIP,skipped exon(SKIP_ON,SKIP_OFF pair);XSKIP,approximate SKIP (XSKIP_ON,XSKIP_OFF pair);MSKIP,multi-exon SKIP (MSKIP_ON,MSKIP_OFF pair);XMSKIP,approximate MSKIP (XMSKIP_ON,XMSKIP_OFF pair);IR,intron retention (IR_ON,IR_OFF pair);XIR,approximate IR (XIR_ON,XIR_OFF pair);MIR,multi-IR (MIR_ON,MIR_OFF pair);XMIR,approximate MIR (XMIR_ON,XMIR_OFF pair);AE,alternative exon ends (5´,3´,or both);XAE,approximate AE. B,number of AS events in the CK vs.FA comparison groups at 3,6,and 9 days after treatment (DAT) and AS-associated genes. A3SS,alternative 3’ splice site;A5SS,alternative 5´ splice site;MXE,mutually exclusive exons;RI,retained intron;SE,skipped exon. C,functional enrichment analysis of specific AS events in the FA and CK groups.
To identify the specific AS events responsive to FA treatment,we compared AS events between the CK and FA treatment groups. Genes undergoing AS events were designated as AS-associated genes. We identified 1,045 FA-specific AS-associated genes and 1,042 CKspecific AS-associated genes (Fig.3-B). GO enrichment analysis revealed that specific AS-associated genes were implicated in “cell communication”,“cell wall”,and various aspects of cell wall pectin metabolism,including“cell wall pectin metabolic progress”,“pectin metabolic progress”,and “pectin biosynthetic progress” (Fig.3-C).In summary,the AS-associated genes primarily played roles in regulating cell wall pectin metabolism during the postharvest quality deterioration.
3.4.AS events associated with the PECTINESTERASE gene
The expression levels of DEGs from subcluster-4 in the CK treatment were significantly higher than those of the FA treatment,which was consistent with the difference in firmness between FA and CK treatments. However,the remaining clusters did not show a significant correlation with the dynamic changes in firmness. To delve deeper into the pivotal DEGs influenced by AS events,particularly those associated with cell wall pectin metabolism,we selected eight AS-associated DEGs from subcluster-4. Of these,three were from the FA-specific AS-associated gene set and five were from the CKspecific AS-associated gene set (Fig.4-A). Notably,this group included aPECTINESTERASEgene (annotated asVvPE2,Vitvi15g00704) encoding a cell wall-degrading enzyme,from the FA-specific AS-associated gene set. Furthermore,VvPE2consistently exhibited high expression levels in the CK-treated berries and reduced expression in the FA-treated berries (Fig.4-B),suggesting thatVvPE2may play a significant role in berry cell wall degradation and postharvest softening.
The structural analysis of theVvPE2gene revealed that it contains two exons (exon_1 and exon_2) and one intron. Transcriptome analysis of the AS events further illuminated the occurrence of AS events in theVvPE2gene. Exon_2 was predicted to contain AS sites of the A3SS type (Fig.4-C). This AS event may lead to a premature termination codon,resulting in the truncation of the VvPE2 protein or producing a protein that cannot perform the typical functions. The FA-specific AS event inVvPE2could be a critical factor in its differential expression between CK-treated and FA-treated berries.
3.5.Verification of AS events occurring on the VvPE2 gene
To validate the AS events detected by RNA-seq,we conducted an RT-PCR analysis ofVvPE2gene expression using RNA samples from the FA-and CK-treated berries at 3,6,and 9 DAT. Agarose gel electrophoresis of the amplifiedVvPE2revealed two PCR fragments,a larger fragment of 1,542 base pairs (bp) and a smaller fragment of 1,317 bp,indicating the presence of two splice variants(Fig.5-A). These two splice variants were namedVvPE2.1andVvPE2.2,respectively. Sequence analysisviaClustal X multiple sequence alignment confirmed an A3SS in exon_2 at position 1,079 bp,resulting in a 225-nucleotide indel betweenVvPE2.1andVvPE2.2.This splicing event followed the ‘GT-AG’ rule (Fig.5-B).Overall,A3SS inVvPE2caused a deletion in exon_2,resulting in a shortened PE protein with a significantly different sequence from the long-formVvPE2.
Furthermore,the FA-specific alternatively splicedVvPE2(VvPE2.1andVvPE2.2) was validated by quantifying their relative abundances through qRT-PCR. The relative expression ratios between the FA-and CK-treated berries forVvPE2.1andVvPE2.2were also determined.BothVvPE2.1andVvPE2.2were detected by qRTPCR in all samples,but the expression ratios of the two transcript variants differed among the treatment samples(Fig.5-C).VvPE2.1was predominantly expressed in CKtreated berries (accounting for over 99.5% of the total),whileVvPE2.2was almost undetectable. Conversely,the abundance ofVvPE2.2increased in the FA-treated berries compared with the CK-treated berries. However,the expression ratio declined from 76.1% at 3 d to only 4.9% at 9 d,indicating a weakening effect of FA during storage (Fig.5-C). Overall,qRT-PCR demonstrated that the A3SS event on the last exon (exon_2) affected the normal expression abundance ofVvPE2,suggesting that FA treatment delayed postharvest cell wall degradation in grape berries by increasing the expression ratio of the short form ofVvPE2.

Fig. 4 Identification and analysis of the key differentially expressed genes (DEGs) affected by alternative splicing (AS) events. A,Venn diagram illustrating the distribution of shared gene numbers among the folic acid (FA)-specific AS-associated gene set,the CK-specific AS-associated gene set,and the subcluster-4 DEGs. B,heatmap displaying the expression patterns of the screened candidate genes. C,analysis of VvPE2 gene structure and prediction of the AS events.
To further analyze the functional differences between the two forms of VvPE2,we conducted ORF and conserved domain searches. The ORF ofVvPE2.1is 1,542 bp long and encodes 513 amino acids,and it is 1,317 bp long and encodes 438 amino acids forVvPE2.2according to the ORF finder. The alignment results of the deduced amino acid sequences fromVvPE2.1andVvPE2.2showed that their identity score was 85.38%. VvPE2.2 has a deletion of 75 amino acids compared with VvPE2.1,due to an alternative splicing event (Appendix D). We also searched for the conserved domains on VvPE2.1 and VvPE2.2. The domain search results indicated that VvPE2.1 is composed of complete “Pectinesterase” and “PMEI” domains (specific hit),belonging to the “Pectinesterase subfamily” and the“PMEI_like subfamily”,respectively. VvPE2.2 contains a“Probable pectinesterase/pectinesterase inhibitor” domain,belonging to the “PLN02995 super family” (superfamily hit) (Appendix D). These results indicated that the shorter protein (VvPE2.2) might not encode a typical pectinesterase enzyme due to the partial absence of the structural domain.

Fig. 5 Validation of the alternative splicing (AS) events in VvPE2 under folic acid (FA) and CK treatments by PCR assay. A,alternative splicing expression profile of VvPE2. B,alternative splicing sites in exons of VvPE2. C,expression pattern analysis of VvPE2.
In conclusion,we propose a novel regulatory mechanism for fruit softening in table grapes (Fig.6).During the postharvest storage of FA-treated berries,theVvPE2gene,which encodes a cell wall-degrading enzyme,undergoes alternative splicing giving rise to two transcripts: the full-length isoformVvPE2.1and the alternatively spliced formVvPE2.2.VvPE2.1possesses the complete structure of thePECTINESTERASEgene,and the encoded protein maintains full enzyme functionality,whereasVvPE2.2encodes an incomplete protein. The up-regulation ofVvPE2.2in FA-treated berries is expected to reduce the accumulation ofVvPE2.1,subsequently leading to reduced enzymatic degradation of pectin by PE,thus delaying berry softening.
4.Discussion
4.1.FA treatment induces gene expression changes and alternative splicing in postharvest grape berries

Fig. 6 Proposed model for the regulation of postharvest fruit softening in table grape by alternative splicing of VvPE2.VvPE2.1,full-length isoform;VvPE2.2,alternately spliced form.
Postharvest berry softening significantly impacts grape quality,and the application of FA treatment has been shown to delay this process (Peiet al.2023). This study employed RNA-seq to scrutinize differential gene expression and AS events in FA-and CK-treated berries at 3,6,and 9 DAT. The GO annotation of the DEGs revealed their involvement in “response to reactive oxygen metabolism”,“cell wall organization”,(“biological process” category) and “xyloglucan metabolic process”(“molecular function”). The FA treatment led to shifts in gene expression related to cell wall metabolism and ROS(Fig.1-C). Our prior investigations established that FA counters ROS production by mitigating the influence of VvWRKY31 on the promoter ofVvRboh(which encodes respiratory burst oxidase homolog,a key enzyme in ROS production),thus reducing oxidative stress-induced damage to membranes and postponing postharvest quality deterioration in table grapes (Peiet al.2023).Similar trends in ROS content were observed in FAtreated broccoli (Brassicaoleraceavar.Italica),with diminished accumulation of malondialdehyde (MDA) and ROS (Xuet al.2021).
The analysis of AS events indicated that FA treatment did not alter the proportions of alternative splicing types(Fig.3-B),but did impact the total number of differential AS events. The observed AS events progressively declined after treatment,with the highest number occurring on day three (CK-3dvs.FA-3d). These findings suggest that AS activity is heightened during the early stages of FA treatment,potentially contributing to the delay in softening. This contrasts with FA-treated apples,where AS activity peaked in the later stages of postharvest storage (Chenet al.2021).
The GO annotation of 1,045 FA-specific AS-associated genes and 1,042 CK-specific AS-associated genes revealed that the specific AS-associated genes in the CK group were chiefly enriched in cell wall metabolic processes,especially cell wall degradation processes (Fig.3-C). This aligns with the pivotal role of cell wall degrading enzymes in the fruit softening process (Liuet al.2022;Penget al.2022;Yanget al.2023). In summary,postharvest FA treatment influenced the expression of genes related to ROS and cell wall metabolism,potentially impacting the cell wall degradation processes in table grapes.
4.2.FA increases the expression of a short form of VvPE2
Postharvest grape berry softening involves intricate physiological and biochemical changes governed by cell wall degrading enzymes (Castellarinet al.2016).Generally,during berry softening,the cell wall undergoes metabolic reactions,including pectin demethylation by PE,the degradation of de-esterified polygalacturonic acid by PL,and the hydrolysis of α-1,4-galacturonic acid to uronic acid by PG (Penget al.2022). Pectin,the primary polymer in the middle lamella,regulates intercellular adhesion,and its disassembly is pivotal in textural changes (Paniaguaet al.2014;Aghdametal2023).Experiments involving genetic manipulation in tomatoes and strawberries have indicated that alterations inPEexpression levels significantly influence the fruit softening process (Wenet al.2013;Xueet al.2020).
This study focused on the AS events associated withPEsand,through transcriptome analysis and PCR validation,confirmed thatVvPE2undergoes AS to generate two transcript variants in FA-treated berries. The FA treatment induced an A3SS AS event,resulting in a shorterVvPE2.2transcript (with a 225-bp fragment deletion) and the longerVvPE2.1transcript (Fig.5-A and B). The FA treatment increased the ratio ofVvPE2.2toVvPE2.1transcripts in the berries,likely reducing VvPE2 activity.This reduction in the enzymatic degradation of pectin by VvPE2 would be expected to delay postharvest softening in these FA-treated grapes (Fig.6). Therefore,the AS event ofVvPE2in FA-treated grapes produces a shorter transcript to regulate fruit softening in table grapes. The short form of VvPE2 does not appear to be a typical pectinerase,so it does not have the function of promoting berry softening like VvPE2.1.
This study utilized a transcriptome analysis approach to identify alternative splicing in thePectinerase2gene associated with FA treatment of grape berries. The results shed light on a potential mechanism controlling postharvest berry softening,and further analysis could deepen our understanding of this critical process.
5.Conclusion
In the present study,we identified 2,559 DEGs from FA and CK treatment groups,including 1,045 FA-specific ASassociated genes and 1,042 CK-specific AS-associated genes. The GO annotation indicated that the specific ASassociated genes were predominantly enriched in cell wall degradation processes.Pectinesterase2(VvPE2,Vitvi15g00704),which encodes a cell wall-degrading enzyme,was predicted to undergo an A3SS event. PCR assays confirmed thatVvPE2undergoes AS to generate two transcript variants (VvPE2.1andVvPE2.2) in FAtreated berries. The FA treatment induced an A3SS AS event,resulting in a shorterVvPE2.2transcript (with a 225-bp fragment deletion) and the longerVvPE2.1transcript. This study provides deep insight into the roles that AS events play in the postharvest softening of table grapes.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (32202560 and 32302470),the Program for Innovative Research Team (in Science and Technology) in University of Henan Province,China(21IRTSTHN021),the Natural Science Foundation of Henan,China (232300421112),the Program for Science&Technology Innovation Talents in Universities of Henan Province,China (21HASTIT035),and the PhD Research Startup Foundation of Henan University of Science and Technology,China (13480068 and 13480067).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available on https://doi.org/10.1016/j.jia.2023.11.023
杂志排行
Journal of Integrative Agriculture的其它文章
- Molecular mechanisms of stress resistance in sorghum: lmplications for crop improvement strategies
- Artificial selection of the Green Revolution gene Semidwarf 1 is implicated in upland rice breeding
- Dynamics and genetic regulation of macronutrient concentrations during grain development in maize
- The NAC transcription factor LuNAC61 negatively regulates fiber development in flax (Linum usitatissimum L.)
- The underlying mechanism of variety–water–nitrogen–stubble damage interactions on yield formation in ratoon rice with low stubble height under mechanized harvesting
- Rice canopy temperature is affected by nitrogen fertilizer
