Evolution of Diagenetic Fluid of the Dawsonite-Bearing Sandstone in the Jiyang Depression, Eastern China
2024-03-12LIFulaiMAWenkuanZHANGChunandWANGKaining
LI Fulai , MA Wenkuan , ZHANG Chun and WANG Kaining
1) National Key Laboratory of Deep Oil and Gas, China University of Petroleum (East China), Qingdao 266580,China
2) Laboratory for Marine Mineral Resources, Qingdao National Laboratory for Marine Science and Technology,Qingdao 266071, China
3) Shandong Geological Exploration Institute of China Chemical Geology and Mine Bureau, Jinan 250013,China
Abstract Based on the petrology, isotope geochemistry and fluid inclusions analysis, we established the evolutionary mode of the diagenetic fluid of dawsonite-bearing sandstone in the Jiyang Depression. Dawsonite-bearing sandstone is characterized by double injection of CO2 and oil-gas in the Jiyang Depression that have experienced a relatively complex diagenetic fluid evolution process.The diagenetic sequence of secondary minerals involves secondary enlargement of quartz, kaolinite, first-stage calcite, dawsonite,second-stage calcite, ferrocalcite, dolomite and ankerite. Hydrocarbon charging in the dawsonite-bearing sandstone occurred at around 2.6 – 0 Myr. The CO2 charging event occurred during Dongying tectonism, forming the Pingfangwang CO2 gas reservoir,which provided an abundant carbon source for dawsonite precipitation. Carbon and oxygen isotopic compositions of dawsonite demonstrate that CO2 forming the dawsonite was of an inorganic origin derived from the mantle, and that water mediating the process during dawsonite precipitation was sequestered brine with a fluid temperature of 82℃. The evolutionary sequence of the diagenetic fluid in the dawsonite-bearing sandstone was: alkaline syngenetic fluids, weak alkaline fluids during organic acid formation, acidic fluids in the early stage of CO2 injection, alkaline fluids in the late stage of CO2 injection, and weak alkaline fluids during oil and gas charging. The mode indicates an increase in HCO3− because of the CO2 injection, and the loss of Ca2+ and Mg2+ due to the precipitation of carbonate minerals. Therefore, the evolutionary mode of diagenetic fluids is in good agreement with high HCO3−,low Ca2+ and low Mg2+ composition of the present formation water in the dawsonite-bearing sandstone.
Key words evolution of diagenetic fluid; dawsonite; CO2 injection and hydrocarbon charging; mineral diagenetic sequence; isotopic geochemistry; fluid inclusions
1 Introduction
Dawsonite (NaAl(OH)2CO3) is an orthorhombic carbonate mineral, which generally occurs in forms of radial,beam-like, hair-like or columnar aggregates in clastic rocks (Bakeret al., 1995; Worden, 2006; Gaoet al., 2009;Okuyama, 2014; Li and Li, 2016), pyroclastic sedimentary rocks (Zhouet al., 2014), oil shales (Smith and Milton, 1966), coals (Loughnan and Goldbery, 1972; Golabet al., 2006; Minget al., 2017), marine carbonates (Bakeret al., 1995) and soils (Hay, 1963). Natural dawsonite was firstly identified at the joints of trachyte dikes in the Montreal area, eastern North America, and abundant dawsonites were found in the volcanic ash of Lake Natron and Olduvai Valley in eastern Africa and in the stratified dolomite and oil shale of the Green River Formation in Colorado, USA in a lateral study (Harrington, 1874; Hay,1963; Smith and Milton, 1966). And the dawsonite present in sandstones was firstly found in the clastic sandstones of the Sydney Basin (Loughnan and See, 1967).Previous studies of dawsonite in sandstones in China primarily focused on the Hailaer Basin, Songliao Basin,Subei Basin, Lishui Basin and Dongying Basin (Gaoet al.,2009; Yuet al., 2014; Li and Li, 2017; Zhaoet al., 2018;Liuet al., 2020). Numerical simulation and thermodynamic experiments demonstrate that dawsonite is generally formed in alkaline fluids characterized by high CO2partial pressure and enriched Na+and Al3+(Zhanget al.,2004; Alvarez-Ayuso and Nugteren, 2005; Hellevanget al.,2005; Vanet al., 2013; Shiet al., 2019; Zhanget al., 2021;Liet al., 2022).
Due to the inevitability of CO2during the precipitation of dawsonite, it has been recognized as a typical mineral for tracing CO2migration and accumulation, and thus has specific geological significance (Worden, 2006; Liuet al.,2016, 2020). Therefore, sandstones with dawsonite occurring are generally refer to as dawsonite-bearing sandstone, which is not directly linked to the content of dawsonite (Gaoet al., 2008; Liet al., 2009). Dawsonite commonly serves as a CO2tracer mineral, which is commonly associated with CO2gas reservoirs (Worden, 2006; Liuet al., 2016; Li and Li, 2017; Liuet al., 2020). The CO2injection will affect the physical-chemical balance between underground fluids and rocks, causing large-scale CO2-fluid-rock interaction. The acidic fluids due to CO2dissolved in formation water can promote intense dissolution of silicaluminate minerals such as feldspars, and release a large amount of Na+, K+, Ca2+, Al3+, SiO2(aq)etc.,and then the secondary pores generate (Bjørlykke and Jahren, 2012; Zhouet al., 2014; Liet al., 2018; Yuanet al.,2019). Dissolution products carried by acidic fluids may eventually precipitate in the form of carbonate minerals such as calcite, dolomite, ankerite or dawsonite, blocking pore space and thus resulting in worse reservoir physical properties (Worden, 2006; Quet al., 2007; Liuet al., 2019;Zhouet al., 2020). The dawsonite-bearing sandstone of the Fourth Member of the Shahejie Formation, Jiyang Depression is developed in oil, CO2gas and water layers,indicating that the dawsonite-bearing sandstone in the Jiyang Depression has the characteristics of dual charging of oil-gas and CO2, and has undergone complex fluid evolution process, which would affect the reservoir quality, and the accumulation of oil-gas or CO2resources.This study focuses on the diagenetic fluid evolutionary mode of dawsonite-bearing sandstone in the Jiyang Depression, which can illustrate the interaction mechanism of CO2-fluid-sandstone, improve current understanding of reservoir characteristics in the study area, and provide a natural analogy for CO2capture and geological sequestration.
2 Geological Setting
The Jiyang Depression is located at the southeast of the Bohai Bay Basin, which is surrounded by the Luxi Uplift in the south, Chengning Uplift in the north and west,Tanlu Fault in the east, consisting of four sags (Huimin,Chezhen, Zhanhua, and Dongying) and nine bulges(Chengzikou, Yihezhuang, Gudao, Wudi, Binxian, Qingcheng, Guangrao,etc.) (Fig.1a). The tectonic evolution of Jiyang Depression can be divided into three stages: rifting stage (65 – 24.6 Myr), post-rift depression stage (24.6 – 5.1 Myr) and tectonic activation stage (5.1 – 0 Myr) (Wuet al.,2003; Linet al., 2005; Guoet al., 2012; Li and Li, 2016).The basement subsidence of the Jiyang Depression is episodic in the rifting stage, and the tectonic subsidence is intermittent and sudden. Both the Paleogene and Neogene are important stages for the formation of the Jiyang Depression. The Paleogene and Neogene are widely distributed and thick in the depression, and the Paleogene can be divided into Kongdian Formation, Shahejie Formation and Dongying Formation from bottom to top in a stratigraphic sequence. Mesozoic magmatic rocks and Cenozoic magmatic rocks are developed in the Jiyang Depression. The former is igneous rock formation in orogenic period, and the latter is continental rift volcanic rock formation. Cenozoic volcanic activity includes three periods:Paleogene, Neogene and Quaternary, and the magmatic rocks are predominantly distributed in the Huimin Sag and the western of the Dongying Sag (Wang, 2005; Guo,2006; Li and Li, 2017; Duet al., 2021).
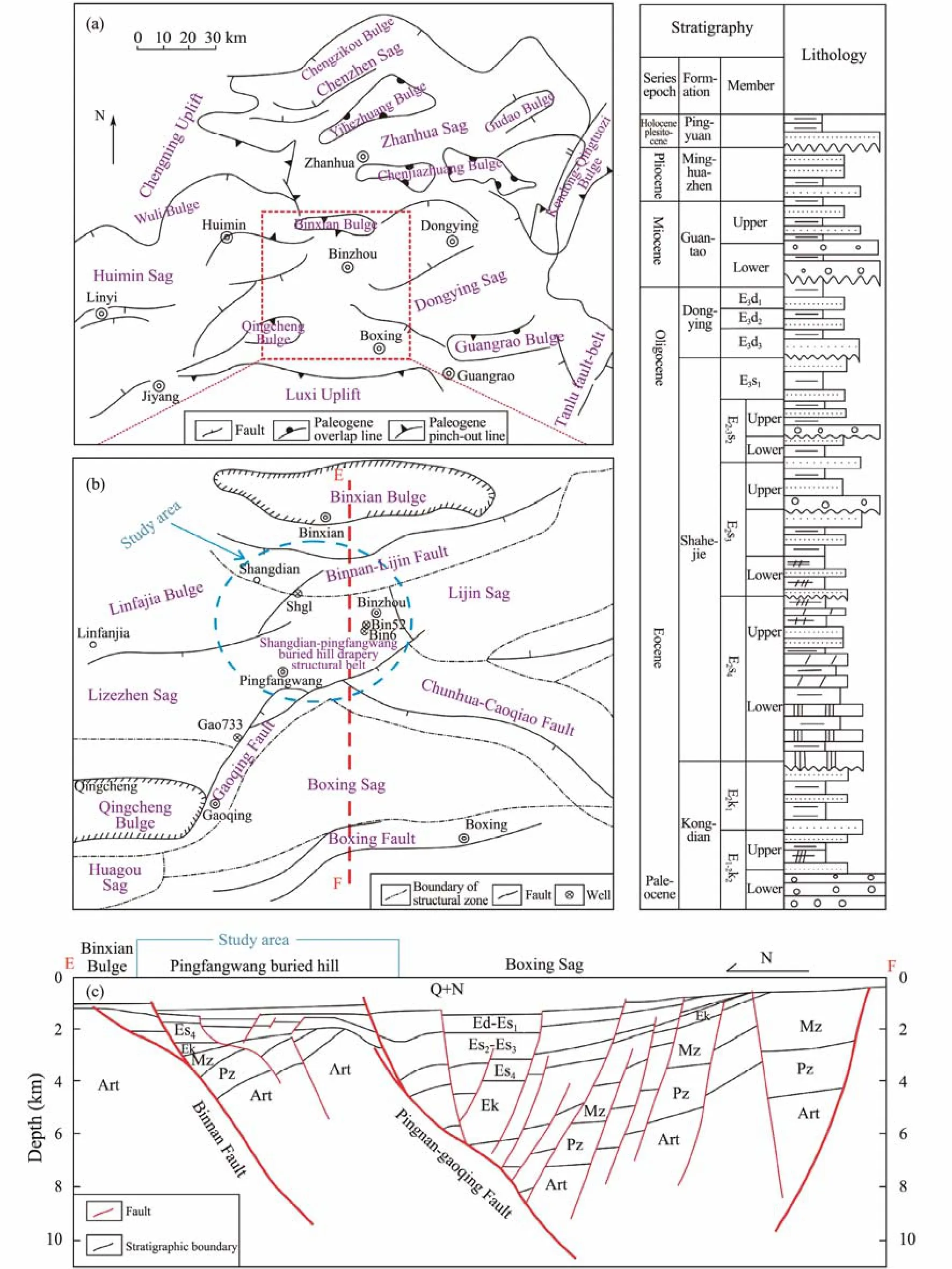
Fig.1 (a), location map of the study area showing the tectonic units of the Jiyang Depression; (b), distribution of sub-tectonic units within the study area with the locations of sampled wells and the location of cross-section EF; (c), N-S cross-section (EF) of the study area showing the various tectonic-structural zones and key stratigraphic intervals.
The study area is located at the central area of the Jiyang Depression and the west of the Dongying Sag, in the south of Binnan-Lijin Fault Belt and Binxian Uplift, in the east of Lizezhen Subsag and Linfanjia Structure, in the west of Lijin Sag, in the north of Gaoqing-Pingnan Fault Belt and Boxing Sag (Fig.1b). Buried hill structures are developed in the lower part of the Cenozoic in the study area. Kongdian and Shahejie Formations directly overlie buried hill structures, forming buried hill drapery structural belt (Fig.1c).
The study area is located at the uplifting plate of the Gaoqing-Pingnan Fault Zone, which continued to rise during the early Paleogene. In the late sedimentary period of the Fourth Member of the Shahejie Formation, reefbeach facies carbonate rocks were deposited in the Pingfangwang buried hill area at the tidal flat of platform margin in the Dongying Basin, forming the north-south axial buried hill drapery structure. Since the early deposition period of the Third Member of Shahejie Formation,the Pingfangwang area located on the hanging wall of the fault zone had been rising continuously, resulting in thinner stratum from the lower sub-member of the Third Member of Shahejie Formation to the Dongying Formation in the study area than the corresponding stratum in the footwall of Gaoqing-Pingnan Fault Zone (Cheng,2005; Wang, 2005; Guo, 2006). Reef carbonate rocks are formed in the upper part of the Fourth Member of Shahejie Formation in the Pingfangwang area, and various pore types are developed due to the erosion. Siltstone and silt-fine sandstone are dominant in the middle of the Fourth Member of Shahejie Formation, which can be good oil and gas reservoirs. Grey mudstone is developed in the Third and Second Member of Shahejie Formation,which is stable in the Pingfangwang area and have a breakthrough pressure of 0.7 – 3.1 MPa. It can be a good cap for the Fourth Member reservoir. Under the Pingfangwang CO2gas reservoir, the oil reservoir is developed with a gas-oil interface at about 1510 m. The reservoir is mainly composed of siltstone in the middle of the Fourth Member of Shahejie Formation (Guo, 2006: Wang,2005; Tanget al., 2022). Dawsonites are precipitated in the cores from the wells including Bin16, Bin52, Bin77,Bin214, Gao733 and Shg1 in the study area.
3 Samples and Methods
In this study, samples were collected from the dawsonite-bearing sandstones of the Fourth Member of the Shahejie Formation and the Kongdian Formation in the Jiyang Depression.
The dawsonite-bearing sandstone slices were prepared and stained with alizarine red and potassium ferricyanide.The framework clastic composition and characteristics of authigenic minerals were characterized under standard polarizing microscope. And the characteristics of dawsonite and the contact relationship between dawsonite and other authigenic minerals were observed under the scanning electron microscope (SEM). In addition, the cathodoluminescent sheets were polished and observed using CL8200 MK5-2 cathodoluminescence microscopy to identify different generations of carbonate minerals in the dawsonite-bearing sandstone.
The thin sections of inclusions were prepared to observe fluid inclusion characteristics of dawsonite-bearing sandstone. Microthermometry was carried out under the LEICA microscope equipped with THMS-600 cooling and heating stage. The measurement range was from−120℃ to 300℃ with an error ≤ 0.5℃.
The carbon and oxygen isotope testing of the dawsonite-bearing sandstone samples was then conducted using MAT-252 mass spectrometer to determine the carbon and oxygen isotope composition of dawsonite, and the reference standard for carbonate used in the analysis was the GBW04417 calcite standard. The content of dawsonite of those samples for whole-rock carbon and oxygen isotope testing was more than 8%, accounting for more than 85%of the total content of carbonate minerals. The carbon and oxygen isotopic composition measured by phosphoric acid method can be considered as that of dawsonite approximately.
The present formation water data from the common sandstone and dawsonite-bearing sandstone of the study area in the Jiyang Depression and typical areas was collected. Compared with the present formation water from the common sandstone of the study area in the Jiyang Depression and from dawsonite-bearing sandstone of typical areas (Wuerxun Sag and Songliao Basin), the present formation water from dawsonite-bearing sandstone in the Jiyang Depression was analyzed.
4 Results
4.1 Petrology
Based on the framework clastic composition of dawsonite-bearing sandstone samples from the wells including Bin16, Bin52, Bin77, Shg1 and Gao733, the lithology of the dawsonite-bearing sandstone in the Jiyang Depression is mainly arkose and lithic arkose, with a small amount of feldspathic litharenite. Quartz is mainly monocrystalline(polycrystalline in some cases), xenomorphicgranular,sub-angular, and deformation, fragmentation and secondary enlargement are observed in some quartz particles.Feldspars are mainly alkaline feldspar, followed by plagioclase. The occurrence of feldspar is dominated by tabular and columnar, and the barbor-shaped crystal exists due to the strong dissolution of feldspar. The proportion of feldspar is relatively high, which indicates the relatively low mineral maturity of the dawsonite-bearing sandstone in the Jiyang Depression. The rock fragments are minor (a volume fraction of about 10%) and dominated by metamorphic and sedimentary rock fragments.
The total content of carbonate minerals in Pingfangwang area ranges from 2.2% to 39.8%, with an average of 14.5%, showing a decreasing trend. The total content of carbonate minerals is relatively high at 1440 – 1500 m,generally over 10%, while the total content of carbonate minerals is relatively constant, mainly in a range of 8% –18% at 1500 – 1600 m. The porosity ranges from 7.9% to 32.9%, with an average value of 23.0%, showing a slight increase trend, which has a good negative correlation with the total content of carbonate minerals in the Pingfangwang area. The porosity is about 20% at 1440 – 1500 m,while the porosity is concentrated between 20% and 30%generally and relatively constant at 1500 – 1600 m. The permeability ranges from 0.5 – 708 mD with an average of 104.4 mD. The permeability is relatively higher in the depths of 1470, 1500, 1510 – 1555 and 1565 – 1590 m, and shows a negative correlation with the content of carbonate minerals.
Authigenic minerals in dawsonite-bearing sandstone mainly consist of dawsonite, the secondary enlargement of quartz, kaolinite, calcite, ferrocalcite, dolomite and ankerite. The content of dawsonite reaches up to 20%,and dawsonite occurs in the intergranular pores of clastic particles and replaces clastic particles in some cases. Its single columnar crystal can be observed under the scanning electron microscope (SEM). Dawsonite aggregates appear radial, beam-like, hair-like or pinnate, which consist of multiple connected or crossed single crystals (Figs.2a and b). There are two main occurrence types of dawsonite: one occurs as cement filling in the intergranular pores of clastic particles, and the other occurs as a metasomatic product of clastic particles. The growth morphology of dawsonite filling the intergranular pores is often restricted by the pore morphology, and it may replace detrital particles around the pores. As the product of the metasomatism of feldspars and quartz, dawsonite usually occurs as columnar or platelike aggregates. Clastic quartz grains are partially metasomatized, while clastic feldspar grains metasomatized more strongly are replaced partially or completely, a part of whom are harbor-shaped or only the residual crystals are left when the feldspar grains are metasomatized intensely (Fig.2c).

Fig.2 Micrographs of the authigenic minerals in the dawsonite-bearing sandstones. (a), the dawsonite aggregates consist of multiple connected or crossed single crystals (G733, 1844.4 m, SEM); (b), radial dawsonite (Bin52, 1498.05 m, crossed nicols); (c), the residual crystal of feldspar (Bin52, 1500.10 m, crossed nicols); (d), the dawsonite replaced the first-stage calcite (Bin16, 1631.11m, crossed nicols); (e), the second-stage calcite replaced the dawsonite (Bin52, 1498.36 m, crossed nicols); (f), the two stage of calcite (Bin16, 1631.24 m, CL); (g), the dolomite replaced the ferrocalcite (Bin52, 1474.50 m,crossed nicols); (h), the iron-dolomitization of dolomite (Bin16, 1614.00 m, crossed nicols); (i), the contact relationship of kaolinite and secondary enlargement of quartz (Bin16, 1620.50 m, crossed nicols). Daw, dawsonite; F, feldspar; I-Cal, the first-stage calcite; II-Cal, the second-stage calcite; Fe-Cal, ferrocalcite; Dol, dolomite; Ank, ankerite; Q, quartz; Qse, secondary enlargement of quartz; Kao, kaolinite.
4.2 Hydrocarbon Inclusions
A large number of hydrocarbon inclusions are developed in the dawsonite-bearing sandstone in the Jiyang Depression, which are mainly classified as primary hydrocarbon inclusions in carbonate cement with a few secondary hydrocarbon inclusions in structural fractures and dissolution pores. The hydrocarbon fluids could be captured to form the hydrocarbon inclusions during carbonate precipitation, which are commonly isolated. In addition, the secondary inclusions could form at the dissolution pores of carbonate cement when the carbonate cementation again (Figs.3a – c). The secondary inclusions are usually irregular and restricted by the morphology of dissolution pores. The secondary hydrocarbon inclusions are present in a linear alignment also can be observed in quartz healing cracks (Fig.3d).
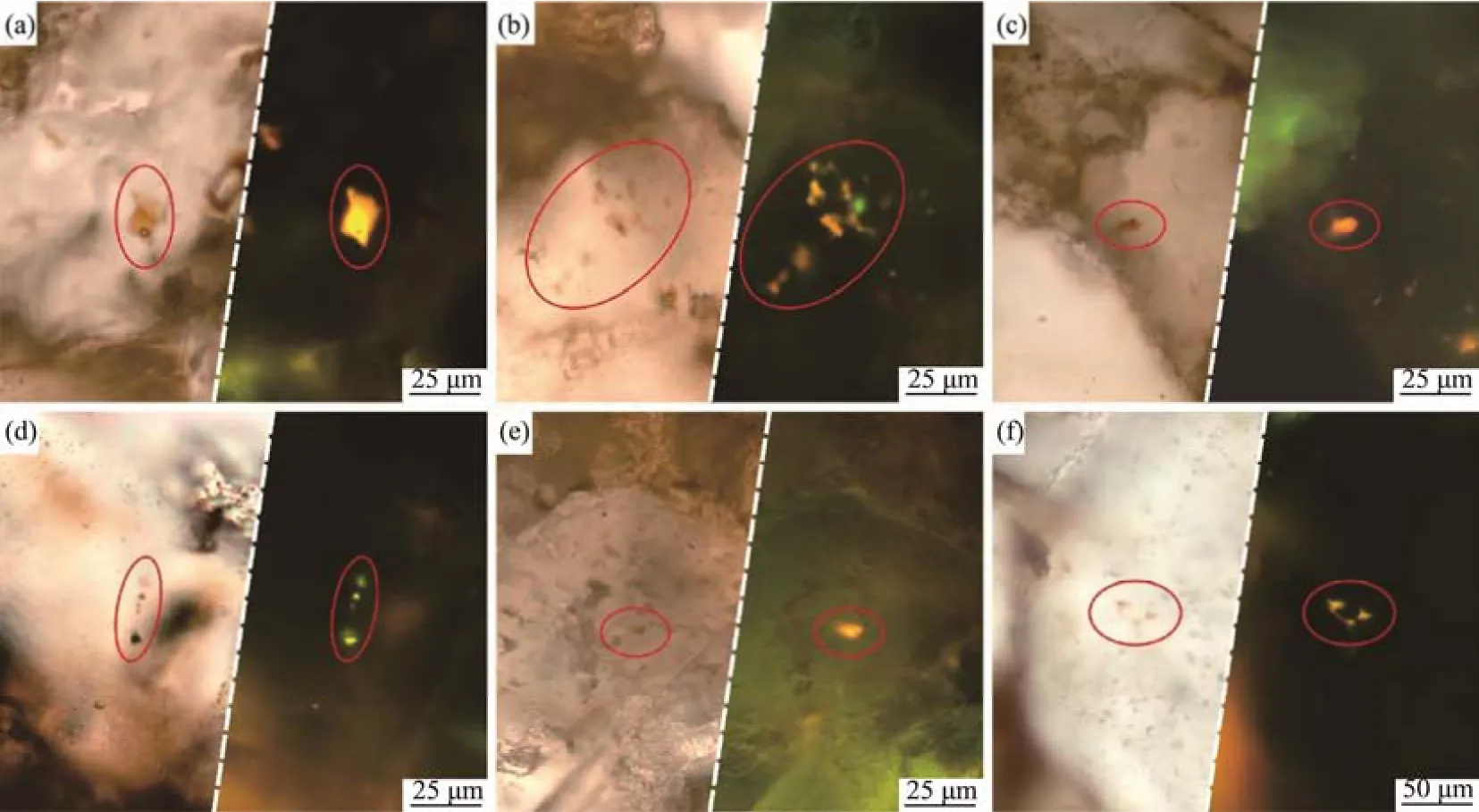
Fig.3 Micrographs of hydrocarbon inclusions under the transmitted light (left) and fluorescence light (right). (a), the isolated hydrocarbon inclusion with light yellow fluorescence in the carbonate cement (Bin52, 1500.07 m); (b), the hydrocarbon inclusion with dark yellow fluorescence in the carbonate cement (Bin52, 1471.39 m); (c), the isolated hydrocarbon inclusion with dark yellow fluorescence in the carbonate cement (Bin52, 1471.39 m); (d), the hydrocarbon inclusions arranged in a linear alignment in the quartz healing cracks (Bin16, 1531.11m); (e), the hydrocarbon inclusion with dark yellow fluorescence in the dissolution pores of feldspar (Bin52, 1471.39 m); (f), the hydrocarbon inclusion with yellow fluorescence in the quartz healing cracks (Bin16, 1531.24 m).
The liquid phase of hydrocarbon is dilute brown to brown under the single polarization light, and the fluorescence color is dark yellow to light yellow. The black boundary between the gas phase and liquid phase can be observed, and the black bubbles do not glow under fluorescence (Figs.3a – c). Isolated hydrocarbon inclusions with dark yellow fluorescence are observed in the dissolution pores of feldspar, which are also observed in the quartz healing cracks. According to the fluorescence color of the hydrocarbon inclusions, the maturity information of the hydrocarbon can be inferred. The hydrocarbon inclusion petrography indicates that the maturity of the hydrocarbon inclusions in studied dawsonite-bearing sandstone is relatively low, and only one stage of the hydrocarbon inclusions is charged. The homogenization temperature of the hydrocarbon inclusions is about 80 –100℃, and the homogenization temperature of the homochromous saline inclusions is 90 – 110℃.
4.3 Carbon and Oxygen Isotopic Compositions of Dawsonite
The carbon and oxygen isotopic compositions of dawsonite in the Jiyang Depression are provided in Table 1.The δ13C and δ18OPDBvalues of the whole rock are−2.49‰ – +3.50‰ and −13.85‰ – −10.82‰, respectively.The δ13C and δ18OPDBvalues ofin-situdawsonite are−2.18‰ – −0.16‰ and −14.96‰ – −12.59‰, respectively.The results of whole-rock carbon and oxygen isotope test are in good agreement with those ofin-situdawsonite.The δ13C values of the dawsonite minerals (whole rock andin-situdawsonite) range from −2.49‰ to +3.50‰,with an average of −1.17‰, and most of the samples have the δ13C values less than 0‰. The δ18OPDBvalues range from −14.73‰ to −10.82‰, with an average of −12.63‰.The calculated δ18OSMOWvalues range from 16.64‰ to 19.76‰, with an average value of 17.55‰ using the δ18OPDBand δ18OSMOWconversion formula:

Table 1 The carbon and oxygen isotope compositions of dawsonite in the Jiyang Depression
The carbon and oxygen isotope compositions of dawsonite in the Jiyang Depression and other areas are shown in the Fig.4. The δ13CPDBand δ18OSMOWvalues of dawsonite in the Tamtsag Basin and Wuerxun Depression are in the range of −1‰ to −6‰ and 5‰ to 10‰, respectively(Liuet al., 2006; Gaoet al., 2007; Donget al., 2011). In the Sydney Basin, Denison Trough, upper Hunter Valley area, Gunnedah Basin, Qan’an Oilfield, Gudian area and Jiyang Depression, the dawsonites have the relatively heavy carbon and oxygen isotope compositions, and its δ13CPDBvalues range from −2.2‰ to +2.4‰, while the δ18OSMOWvalues range from 11‰ to 20‰ (Bakeret al.,1995; Golabet al., 2006; Li, 2009; Yu, 2015).
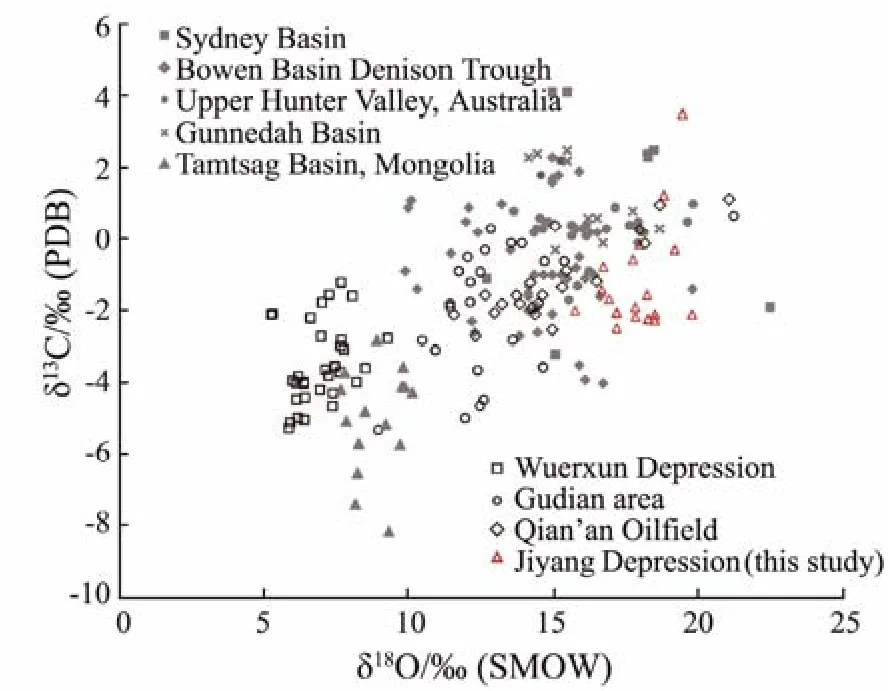
Fig.4 Characteristics of the carbon and oxygen isotope compositions of dawsonite.
4.4 Present Formation Water
Compared with the present formation water from dawsonite-bearing sandstone of Wuerxun Sag and Songliao Basin and the common sandstone (non-dawsonite) of the Jiyang Depression, the present formation water from dawsonite-bearing sandstone of the Jiyang Depression was statistically analyzed (Table 2) (Gaoet al., 2007; Li,2009; Yu, 2015). The total dissolved solids (TDS) of the present formation water from the dawsonite-bearing sandstone in the Jiyang Depression is 9.30 – 82.31 g L−1,with an average of 49.33 g L−1, which is lower than that of the common sandstone of the Fourth Member (84.44 g L−1)and Third Member (76.04 g L−1) of the Shahejie Formation, similar to that of the Second Member of Shahejie Formation (49.34 g L−1) that in the same depth with dawso-nite-bearing sandstone, and higher than that of the First Member of Shahejie Formation (35.95 g L−1). It indicates that the TDS of present formation water in the study area is mainly controlled by the burial depth and increases with depth (Fig.5).
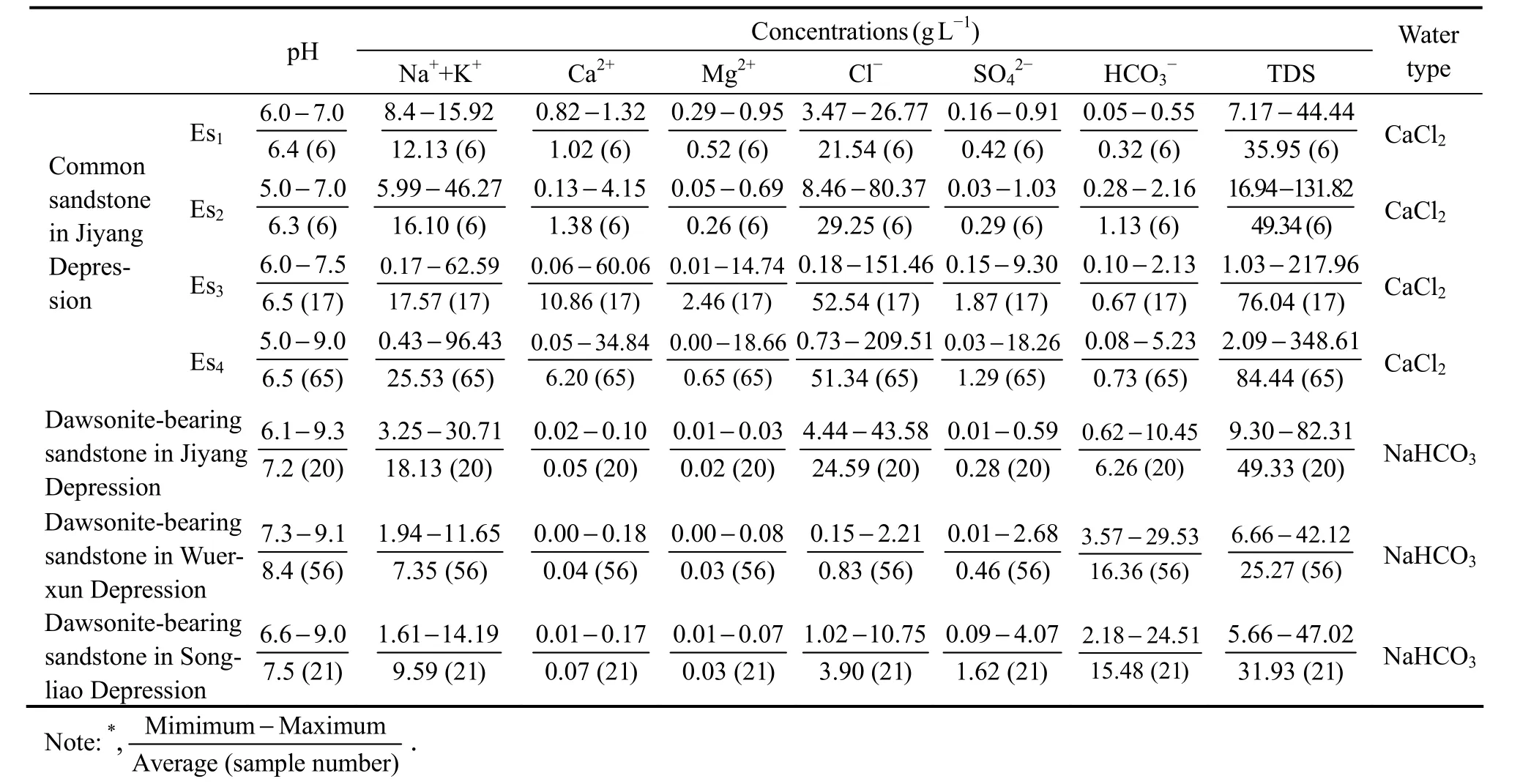
Table 2 Characteristics of present formation water from dawsonite-bearing sandstones and common sandstones*
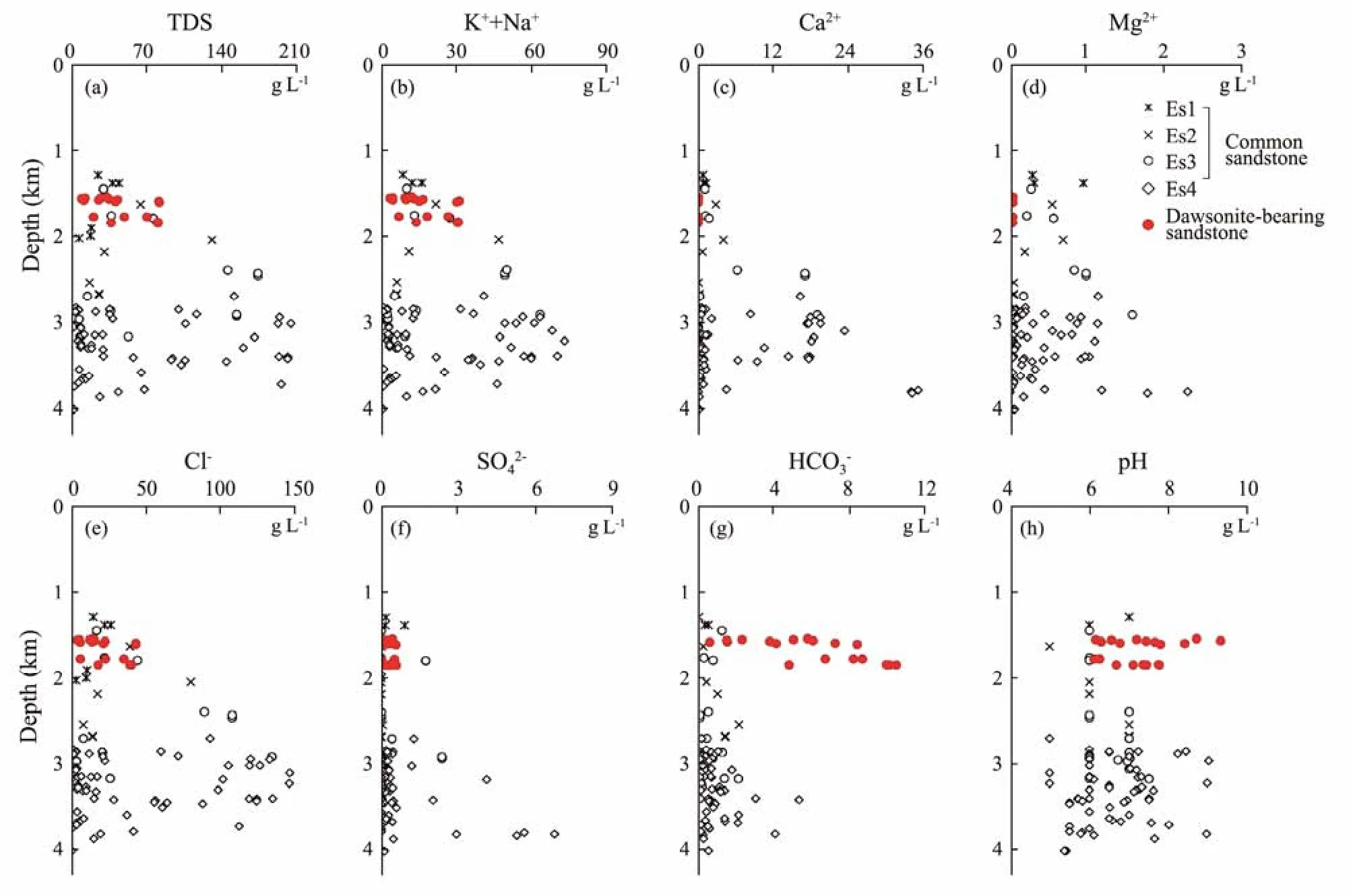
Fig.5 Characteristics of present formation water from dawsonite-bearing sandstones and common sandstones in the Jiyang Depression.
The cationic composition of present formation water from dawsonite-bearing sandstone in the Jiyang Depression is (Na++ K+) > Ca2+> Mg2+, which is similar to that of common sandstones in the Jiyang Depression. The contents of Na+and K+of present formation water from dawsonite-bearing sandstone (18.13 g L−1) are lower than that of the common sandstone (25.53 g L−1) of the Fourth Member, similar to that of the Second and Third Member(17.57 g L−1and 16.10 g L−1, respectively), higher than that of the First Member of Shahejie Formation (12.13 g L−1). Therefore, the content of Na+and K+in the present formation water (from dawsonite-bearing and common sandstone) in the Jiyang Depression is mainly controlled by the burial depth (Fig.5). The Ca2+and Mg2+concentrations of present formation water increase with burial depth, but both the concentrations of present formation water from dawsonite-bearing sandstone (Ca2+concentration is 0.05 g L−1, and Mg2+concentration is 0.02 g L−1) are significantly lower than that of common sandstone (Ca2+and Mg2+concentration of the Shahejie Formation is 6.40 g L−1and 0.94 g L−1) in the Jiyang Depression (Fig.5).
In the Jiyang Depression, the anionic composition of present formation water from dawsonite-bearing sandstone is Cl−> HCO3−> SO42−, while that of common sandstone is Cl−> HCO3−> SO42−or Cl−> SO42−> HCO3−. The Cl−and SO42−contents of present formation water is controlled by the burial depth, and their contents in the dawsonite-bearing sandstone is lower than that of the Fourth and Third Member of the Shahejie formation and similar to that of the Second Member of the Shahejie Formation at the same depth with the dawsonite-bearing sandstone.The HCO3−content of present formation water in the dawsonite-bearing sandstone (0.62 – 10.45 g L−1, with an average of 6.26 g L−1) is higher than that of common sandstone (that of the Fourth, Third, Second, First Member of the Shahejie formation is 0.73, 0.67, 1.13 and 0.32 g L−1, respectively) in the Jiyang Depression.
In the Jiyang Depression, the water type of the present formation water of the dawsonite-bearing sandstone belongs to the NaHCO3type, while the water type of the common sandstone is mainly the CaCl2type, with Na2SO4and NaHCO3types in some cases. The pH value of present formation water from common sandstone is about 6.4,showing weak acidity. The pH value of the dawsonitebearing sandstone is 6.1 – 9.3, with an average value of 7.2, showing weak alkalinity, consistent with the alkaline fluid environment with high CO2partial pressure forming dawsonite.
5 Discussion
5.1 Diagenetic Sequence
The formation sequence of authigenic minerals can be determined based on the contact relationship among minerals under microscope.
Both the replacement of dawsonite by calcite and the replacement of calcite by dawsonite can be found in studied dawsonite-bearing sandstones. Considering the two observed generations of calcite cementation under the cathodoluminescence, therefore, the replacement of the first-stage calcite by dawsonite, and the replacement of dawsonite by second-stage calcite can be determined(Figs.2d – f). That is to say, the precipitation of dawsonite occurs between the two generations calcite cementation.Ferrocalcite may replace the calcite, and the ferrocalcite is replaced by dolomite (Fig.2g). The dolomite shows iron-dolomitization (Fig.2h), indicating the formation sequence is calcite, ferrocalcite, dolomite and ankerite.
Kaolinite and secondary enlargement of quartz occur before the cementation of calcite and dawsonite (Fig.2i).In acidic fluid environments, feldspars ((Na,K)AlSi3O8)can react with H+in formation fluids, providing material basis (e.g., Al and Si) and spatial conditions (i.e., feldspar dissolution pores) for the formation of the kaolinite(Al4(Si4O10)(OH)8). Furthermore, the dissolution of feldspars can also provide a material basis for the secondary enlargement of quartz (SiO2). Therefore, the dissolution of feldspars, the secondary enlargement of quartz and the formation of kaolinite can be considered as the product of the action of the same acidic formation fluids, and they are formed quasi-simultaneously. According to the contact relationship among the three processes observed under the microscope (Fig.2i), the sequence of the three processes of dawsonite-bearing sandstone in the Jiyang Depression is the dissolution of feldspars, secondary enlargement of quartz, and the formation of kaolinite.
In conclusion, the sequence of authigenic minerals in the dawsonite-bearing sandstone is the secondary enlargement of quartz, kaolinite, first-stage calcite, dawsonite,second-stage calcite, ferrocalcite, dolomite and ankerite.
According to the tectonic evolution characteristics of the study area and the fluid environment in the stage of authigenic mineral precipitation, the diagenetic sequence can be divided into three authigenic mineral assemblages:the assemblage of secondary enlargement of quartz and kaolinite, the assemblage of first-stage calcite, dawsonite and second-stage calcite, and the assemblage of ferrocalcite, dolomite and ankerite.
5.2 Characteristic of Fluid Charging
5.2.1 Hydrocarbon charging
The timing of hydrocarbon charging into the dawsonite-bearing sandstone is discussed based on petrographic observation and microscopic temperature measurement.Previous studies have been carried out on denudation thickness, paleo-heat flow value and geothermal gradient in the study area (Bao, 2009; Zhanget al., 2015; Liu,2018), the denudation thickness at the end stage of the Dongying Formation in the Pingfangwang area is about 320 m (Bao, 2009; Zhanget al., 2015). The paleo-heat flow and geothermal gradient were 88 mW m−2and 48℃km−1at 50 Myr, 83 mW m−2and 41℃ km−1at 38 Myr, 75 mW m−2and 38℃ km−1at 24 Myr, 74 mW m−2and 37℃km−1at 14 Myr, respectively, while the paleo-heat flow value and geothermal gradient at present are 71 mW m−2and 36℃ km−1, respectively (Bao, 2009; Liu, 2018). The burial and thermal evolution history of Well Bin16 and Well Bin52 are simulated using PetroMod, and the microscopic temperature measurement results of inclusions are projected into their burial and thermal evolution history maps to estimate the hydrocarbon charging time(Figs.6a – b).
In comprehensive consideration of the homogenization temperature of homochromous saline inclusions (90 –100℃ and 90 – 110℃, respectively) and the burial and thermal evolution history of Well Bin16 and Bin52, the hydrocarbon charging periods are 2.57 – 0.25 Ma and 2.69– 0 Myr. In summary, the hydrocarbon charging period is from the late sedimentary period of the Minghuazhen Formation to the present (Figs.6a – b).
A comparison of source rock biomarkers, geochemical characteristics of crude oil, logging oil and gas, oil test results and HMIE (Hydrocarbon Effective Migration Index, HMIE = (50% fluorescence + oil stain + oil spot + oil immersion + oil-bearing)/sand body thickness) shows that the source of Fourth Member of Shahejie Reservoir in the Pingfangwang area was the mixed oil and gas from the Fourth and Third Member of the Shahejie Formation in the Boxing Sag, taking the Pingfangwang Fault as the migration channel (Fig.6c) (Jia, 2019). Therefore, the hydrocarbon charging in the study area has similar characteristics to that in the Boxing Sag. According to the previous study of fluid inclusion in the Dongying Basin(Liet al., 2008; Caiet al., 2009; Li, 2014; Menget al.,2014), there was only one stage of oil and gas charging in the Boxing Sag and the southern gentle slope belt. The charging time is from the sedimentary period of the Minghuazhen Formation to present (5 – 0 Myr) approximately. The hydrocarbon charging time of the Fourth Member of Shahejie Formation in the Pingfangwang area is approximately 2.6 – 0 Myr, relatively lagging that of the Boxing sag, and may represent the process of hydrocarbon migration.

Fig.6 (a), the curve of burial and thermal evolution history of Well Bin16; (b), the curve of burial and thermal evolution history of Well Bin52; (c), profile of N-S oil and gas migration in Pingfangwang area (from Jia, 2019).
5.2.2 CO2injection
The Pingfangwang gas cap reservoir developed in the buried hill drape structure, and the lithology of the reservoir is organic reef carbonate rocks of the Fourth and First Member of the Shahejie Formation. The gas reservoir of the First Member of the Shahejie Formation is dominated by methane gas, while the Fourth is dominated by CO2, with the average content about 70%. The δ13CCO2value of CO2in the Pingfangwang gas reservoir is −7.5‰to −4.3‰ which is consistent with the carbon isotope composition of the inorganic CO2, and consistent with that of the mantle-derived CO2(Daiet al., 1995; Cheng,2005; Guo, 2006). It indicates that the mixing of the mantle-derived Helium when the3He/4He is over the3He/4He in air with the value of 1.4 × 10−6, so the Pingfangwang gas reservoir is the mixing of the mantle-de- rived Helium,with the3He/4He of 3.6 × 10−6to 3.9 × 10−6(Cheng, 2005;Guo, 2006). The carbon isotopes and Helium isotopes of the Pingfangwang gas reservoir indicate that the CO2in the reservoir was derived from magmatic- volcanic activity.
According to the spatial relationship between gas reservoir and magmatic intrusion, the CO2gas reservoir in the Jiyang Depression can be divided into three types:volcanic channel-gas reservoir type, magmatic invasion-gas reservoir type and magmatic invasion-fault-gas reservoir type. The CO2gas reservoirs of volcanic channel-gas reservoir type and magma intrusion-gas reservoir type have the similar accumulation characteristics: CO2generated during magma crystallization degassing was charged into the reservoirs in contact with volcanic channel-gas reservoir or magma intrusion and accumulated.The magmatic intrusion-fault-gas reservoir type of CO2gas reservoir does not directly contact with the magmatic intrusion but communicates with it through the fault tectonic zone, and the CO2generated by the degassing of magma crystallization migrates to the trap through the tectonic fault zone and accumulates to form the gas reservoirs. The magmatic intrusive-fault-gas reservoir type of CO2gas reservoir is generally developed along the fault zone, and the shallow reservoir is generally enriched in methane, helium and nitrogen, while the deep reservoir is generally enriched in CO2. The Pingfangwang gas reservoir is a magmatic intrusion-fault-gas reservoir type,with gas reservoirs in the Fourth and the First Member of the Shahejie Formation. The deep Fourth Member is rich in CO2, while the shallow First Member is rich in methane (Cheng, 2005; Wang, 2005; Guo, 2006). The magma intrusion is found in the Well Bingu1 in the study area,and the lithology of the intrusion is diorite porphyrite,orthophyre and lamprophyre, respectively. The upper stratum above the intrusion exists obvious thinning phenomenon, reflecting the influence of the intruding magma.Add that into the consideration with southern fracture cutting to the Guantao Formation, therefore, the time of intrusion was the deposition sedimentation period of late Shahejie Formation to Guantao Formation presumably,which is probably resulted from the volcano-magmatic activity during the Dongying tectonism.
The accumulation mode of the Pingfangwang CO2gas reservoir is depicted in Fig.7. The carbonate reservoir of the Fourth Member of Shahejie Formation and the mudstone caprock of the Second and Third Member of Shahejie Formation had been prepared in the period of the First Member Shahejie Formation in the study area. A large amount of CO2was released due to the reduction of temperature and pressure in the process of intrusion of Dongying tectonism, and the CO2accumulated in the upper reservoir of the Fourth Member of the Shahejie Formation with the migration along the fault zone. After the deposition of the Guantao Formation, the magma activity basically attenuated, and a large amount of CO2was released unceasingly because of the cooling and crystallization of magma, which continuously charged into the reservoir of the Fourth Member of the Shahejie Formation through the fault zone, providing a rich carbon source for the deposition of dawsonite.
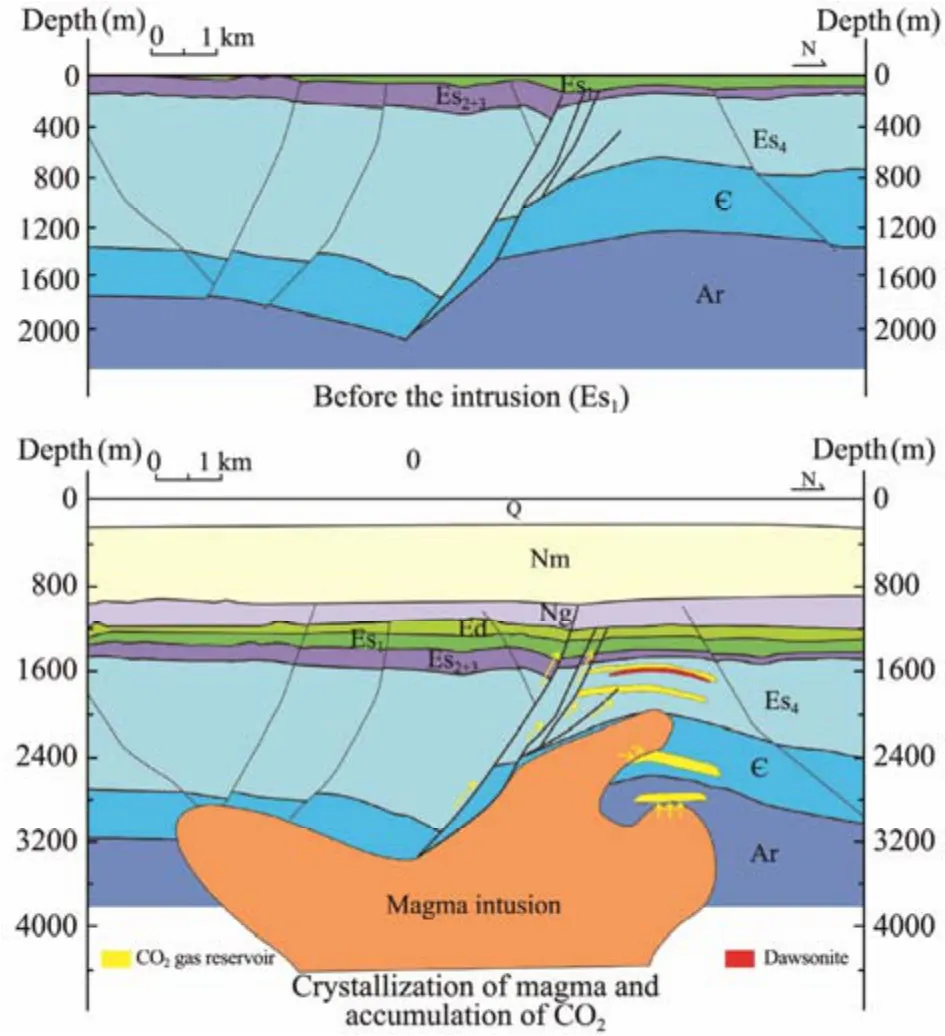
Fig.7 Schematic profile of the Pingfangwang CO2 gas reservoir formation process.
5.3 Paleo-Fluid Characteristics During the Formation of Dawsonite
5.3.1 Carbon source
Daiet al. (1995) summarized the δ13C values of various organic and inorganic carbon-containing materials.The δ13C values of inorganic carbon-containing materials range from −11.0‰ to +6.0‰, while the δ13C values of organic carbon-containing materials mainly range from−35.04‰ to −19.38‰. The δ13C values of dawsonite in the Jiyang Depression range from −2.49‰ to +3.5‰,which is consistent with the carbon isotope composition of inorganic carbon-containing materials, indicating that the carbon forming dawsonite was inorganic. Previous studies on carbon isotopes of dawsonite from the Wuerxun Sag, Songliao Basin, Qan’an Oilfield and Gudian area show the characteristics of inorganic carbon, with δ13C values ranging from −5.25‰ to −1.21‰, −2.7‰ to+0.38‰ and −5.3‰ to +0.67‰, respectively (Liuet al.,2006; Gaoet al., 2007; Li, 2009). The δ13C values of various carbon-bearing materials were collated to draw the δ13C comparison plot of different carbon-bearing materials (Daiet al., 1995). Putting the δ13C values of dawsonite in the Wuerxun Sag in the Hailar Basin, Qan’an oilfield and Gudian area in the Songliao Basin as well as the dawsonite tested this study into the classification plot, and these dawsonites show characteristics of δ13C values of inorganic carbon (Fig.8).

Fig.8 δ13C of carbonaceous material, dawsonite, various genetic CO2 and the CO2 forming the dawsonites (Dai et al., 1995).
Those researchers mainly raised the following ideas about the sources of CO2: 1) Ohmoto and Rye (1979)believed that CO2in deep underground mainly came from the intense tectonic activity of the crust, which resulted in the intensification of magmatic and faulting activities,and its δ13CPDBvalue was −8.0‰ to −4‰. 2) Worden(2006) suggested that atmospheric CO2is mainly enriched in areas with active groundwater systems and can be charged into the ground with atmospheric water, and its δ13CPDBvalue is around −7‰. 3) Tissot and Welte(1984) concluded that organic CO2could be produced by fermentation, microbial oxidation and sulfate reduction,which is mainly derived from organic matter in sedimentary rocks (mostly mud shale), with low δ13CPDBvalues ranging from −25‰ to −20‰. Based on the widely distributed Tertiary batholites, dikes and extrusive rocks in the BGS (Bowen-Gunnedah-Sydney) basin system and the magmatic CO2occurring in coal seams in the Bowen Basin and Sydney Basin, Bakeret al. (1995) determined that the carbon source of dawsonite was mantle-derived magmatism. The research on dawsonite from Permian coal measures in the upper Hunter Valley of the Sydney Basin reinforced Baker’s view (Golabet al., 2006).
The δ13CCO2value of CO2in equilibrium with dawsonite in BGS Basin System were calculated by Bakeret al.(1995), who regarded the calcite-CO2fractionation coefficient as equivalent to the dawsonite-CO2fractionation coefficient at the same temperature, and adopted the calcite-CO2fractionation equation of Ohmoto and Rye (1979)(Eq. (1)). Ohmoto and Goldhaber (1997) then further improved the calcite-CO2fractionation equation of Eq. (2).
In this paper, the method of Bakeret al. (1995) was adopted to regard the fractionation coefficient of calcite-CO2as equivalent to the fractionation coefficient of dawsonite-CO2at the same temperature. The δ13CCO2value of CO2in equilibrium with dawsonite in the Jiyang Depression was calculated by using the fractionation Eq. (3):
In the above equation, δ13Ccalciteis the δ13C value of calcite, δ13Cdawsoniteis the δ13C value of dawsonite, δ13CCO2is the δ13C value of CO2in equilibrium with the calcite or dawsonite, and T is formation temperature. The calculated results showed that the δ13CCO2values of equilibrium CO2during the formation of dawsonite in the Jiyang Depression ranged from −7.18‰ to −1.61‰, with an average of−5.64‰.
Daiet al. (1995) summarized the δ13CCO2values of various types of organic CO2and inorganic CO2based on the carbon isotope discrimination criteria of many researchers: the δ13CCO2values of inorganic CO2are less than −10‰ and mainly distributed between −30‰ and−10‰, while the δ13CCO2values of organic CO2are larger than −8‰ and mainly distributed between −8‰ and +3‰(Fig.8). The δ13CCO2values of inorganic CO2of carbonate metamorphic origin are in the range of −3‰ – +3‰, and the δ13CCO2values of inorganic mantle-derived CO2are in the range of −8‰ – −4‰. Therefore, the δ13CCO2values of the CO2forming the dawsonite in the Jiyang Depression range from −7.18‰ to −1.61‰, which is completely consistent with inorganic CO2, and most is consistent with the mantle-derived CO2, indicating that the CO2forming the dawsonite in the Jiyang Depression mainly came from magma-volcanic activity, and a little carbonate metamorphic CO2may have been mixed (Fig.8).
The carbon isotope composition of the CO2forming the dawsonite in Qian’an Oilfield with the δ13CCO2values ranging from −8.35‰ to −4.54‰ is similar to that of the Jiyang Depression, which is basically consistent with the characteristics of mantle-derived CO2, indicating that the CO2was derived from magmatic-volcanic activity. The CO2forming the dawsonite in Wuerxun Sag and Gudian area have a relatively lighter carbon isotope composition,with δ13CCO2values ranging from −11.82‰ to −5.11‰and −11.72‰ to −6.90‰, respectively, which are located in the boundary section of inorganic and organic CO2. It is suggested that the CO2may be a mixture of inorganic origin (magmatic-volcanic activity and mantle-derived origin) and organic origin (hydrocarbon charging). There are Cenozoic magmatic intrusions in the south of the dawsonite development area in the Pingfangwang area.Therefore, it is speculated that the CO2forming the dawsonite in the Jiyang Depression came from the corresponding magmatic activities in the Cenozoic.
5.3.2 Water medium
Based on the calcite-H2O fractionation equation presented by O’Neilet al. (1969) (Eq. (4)), the δ18OH2Ovalue of the water medium forming the dawsonite in the Jiyang Depression was calculated in this paper, adopting the dawsonite-H2O fractionation Eq. (5):
In the above equation, αcalcite-CO2is the fractionation coefficient of calcite-CO2, αdawsonite-CO2is the fractionation coefficient of dawsonite-H2O, δ18Ocalciteis the δ18O value of calcite, δ18Odawsoniteis the δ18O value of dawsonite,δ18OH2Ois the δ18O value of water medium during the formation of dawsonite or calcite.Tis formation temperature.
The δ18OH2Oof the water medium during the formation of dawsonite in the Jiyang Depression range from−3.76‰ to +1.84‰, with an average value of −1.16‰.The δ18OH2Ovalues of meteoric water are less than −2‰,while the δ18OH2Ovalues of sequestrated underground brines and underground hydrothermal are in the range of−2‰ – +6‰ and +6‰ – +10‰, respectively (Li and Li,2016). The δ18O values of the water medium during the formation of dawsonite in the Jiyang Depression basically conform to the oxygen isotope composition of the sequestrated underground brines, indicating that the water medium during the formation of dawsonite was the sequestrated underground brine.
The oxygen isotope (δ18O) of ancient lake water during the sedimentation period of the Fourth Member of the Shahejie Formation in the Dongying Basin was about−4.8‰ (Yuanet al., 2015; Maet al., 2016), while the oxygen isotope (δ18OH2O) of the water medium during the formation of dawsonite in the Jiyang Depression was heavier than that of the ancient lake water during the deposition process. Considering the tectonic evolution characteristics of the study area, it is speculated that the formation fluids of the Fourth Member of the Shahejie Formation may have been mixed with underground magmatic-hydrothermal fluids during the diagenetic fluid evolution process, resulting in the relatively heavier oxygen isotope composition during the dawsonite precipitation stage. The CO2in the Pingfangwang gas reservoir may be charged correspondingly with magmatic hydrothermal fluids, which was the product of Cenozoic magmatic intrusion in the Pingfangwang area. The study area is located at Shangdian-Pingfangwang buried hill drapery structural belt, and the Third and Second Member of the Shahejie Formation are developed gray mudstone, which is the good cap rocks of the Fourth Member of the Shahejie Formation. The existence of CO2gas cap reservoir of the Fourth Member of the Shahejie Formation indicates the good sealing property of mudstone cap rocks of the Third and Second Member of the Shahejie Formation.The Fourth Member of the Shahejie Formation reservoir received magmatic hydrothermal fluids and CO2from deep migration, which was locked in the Pingfangwang anticline, so the fluid system was relatively closed.
5.3.3 Temperature of paleo-fluid
The temperature of the paleo-fluid during the precipitate period of the carbonate cement can be estimated bythe oxygen isotopic composition of the carbonate cement.Craig (1961) proposed a calculation equation for paleo-fluid temperature during the precipitate period of carbonate minerals (Eq. (6)), and Fonteset al. (1993)proposed a new calculation Eq. (7), which was used to calculate approximately the temperature of the paleo-fluid during the precipitate period of dawsonite by the oxygen isotopic composition of the dawsonite in this paper:
In the equation,δrepresents the difference between the δ18O value of CO2released by dawsonite and the δ18O value of CO2in isotopic equilibrium with aqueous medium, andTprepresents the temperature of paleo-fluid when dawsonite was precipitated.
In the equation, δ18Odawsonite-CO2represents the δ18O value of CO2released by dawsonite, and δ18OH2O-CO2represents the δ18O value of CO2in equilibrium with the aqueous medium.
The equation of δ18Odawsonite-CO2and δ18OH2O-CO2is shown below:
In the equation, α18Odawsonite-CO2is the fractionation coefficient of dawsonite-CO2at 25℃, δ18Odawsoniteis the δ18OSMOWvalue of the dawsonite mineral, α18OH2O-CO2is the fractionation coefficient of H2O-CO2at 25℃, δ18OH2Ois the δ18OSMOWvalue of aqueous medium.
The fractionation coefficient of calcite-CO2(α18Ocalcite-CO2)by the phosphoric acid method is 1.01025 at 25℃ (Kim and O’Neil, 1997), which is also used as the fractionation coefficient of dawsonite-CO2in this study. The fractionation coefficient of H2O-CO2(α18OH2O-CO2) is 1.04120 (Liet al., 1997), and the δ18OH2Ovalue is the average value of δ18OH2Oof the water medium calculated above (−1.16‰)during the formation of dawsonite. The paleo-fluid temperature during the precipitation of dawsonite in the Jiyang Depression is 69.30 – 96.82℃, with the average paleo-fluid temperature of 82℃.
The temperature of paleo-fluid during the deposition of dawsonite in the Pingfangwang area mainly concentrate in 70 – 85℃. According to the thermal evolution characteristics of the Pingfangwang area (Fig.6), the corresponding geological history period of temperature is 6.0 – 3.0 Ma, which is the main precipitation period of dawsonite.
5.4 Origin of Present Formation Water from Dawsonite-Bearing Sandstone
Compared with the formation water in common sandstones, the formation water in the dawsonite-bearing sandstone in the Jiyang Depression is characterized by high HCO3−, low Ca2+and low Mg2+, and its pH value is larger than 7, showing weak alkaline, which is different from the weak acidity of the formation water from common sandstone (Table 2). The high HCO3-content of formation water from dawsonite-bearing sandstone is related to CO2gas dissolved in water in gas cap reservoir of Pingfangwang in the Fourth Member of the Shahejie Formation, while the low content of Ca2+and Mg2+may be ascribed to CO32−and HCO3−reaction in formation water. In detail, because of the precipitation of carbonate minerals such as calcite, dolomite and ankerite, the depletion of Ca2+and Mg2+in the formation fluids resulted in the low Ca2+and Mg2+contents in the formation water from dawsonite-bearing sandstone.
Compared with the formation water from the typical area, the formation water from the dawsonite-bearing sandstone in the Jiyang Depression shows similar characteristics of low Ca2+and Mg2+concentrations, but also include some differences (Table 2). The present formation water from the dawsonite-bearing sandstone in the Jiyang Depression also has a high content of HCO3−, but its content is lower than that of Cl−. The present formation water from the dawsonite-bearing sandstone in typical areas not only shows a high content of HCO3−, but its content is significantly higher than that of Cl−. The present formation water from the Wuerxun Sag is alkaline and NaHCO3type, and the Cl−content of the present formation water from the dawsonite-bearing sandstone and common sandstone is 0.83 g L−1and 0.66 g L−1, respectively (Gaoet al.,2007). The present formation water from the Songliao Basin is weakly alkaline, and the water type is NaHCO3type. And the Cl−content of the present formation water from the dawsonite-bearing sandstone and common sandstone is 3.9 g L−1and 5.8 g L−1, respectively (Li, 2009; Yu,2015). Therefore, the difference of Cl−content in present formation water from the dawsonite-bearing sandstone between the Jiyang Depression and the typical area is determined by the geochemical characteristics of regional formation water. On account of the charging of CO2, the formation water changed from CaCl2type to NaHCO3type and from weak acid to weak alkaline in the Jiyang Depression. However, the formation water was weakly alkaline, and the water type was NaHCO3type before CO2charging in the typical area of dawsonite sandstone.And the CO2charging caused the increase of HCO3−concentrations but did not cause the change of the acidity and basicity and the water type (Gaoet al., 2007; Li, 2009;Quet al., 2010; Yu, 2015).
5.5 Evolutionary Mode of Diagenetic Fluid
The dissolution of clastic mineral particles and the precipitation and dissolution of secondary minerals directly correspond to diagenetic environment and diagenetic fluid properties. Therefore, the diagenetic environment and the fluid properties can be speculated through the research on the precipitation and dissolution of clastic mineral particles and secondary minerals (Li, 2009;Bankoleet al., 2015; Hu, 2016; Caoet al., 2017).
The maturation of organic matter in source rocks is accompanied by the generation of organic acids and hydrocarbons which can be used as weak reducing agents,and the organic acids dissolve in the formation fluids,forming acidic fluids. In addition, the dissolution of CO2into the formation also forms the acidic fluids. Therefore,the injection of CO2and hydrocarbon can change the physicochemical properties of diagenetic fluids and control the evolution of reservoir diagenesis (Surdamet al.,1989; Li, 2009; Li and Li, 2017). The area of dawsonite-bearing sandstone in the Jiyang Depression has the characteristics of double charging of CO2and hydrocarbon and have the complex diagenetic fluid evolution. In this paper, according to the petrology, fluid inclusion and regional geological evolution, the diagenetic sequence,the stage of hydrocarbon charging and the accumulation characteristics of the Pingfangwang CO2gas reservoir were determined. Ultimately, the diagenetic fluid evolutionary mode of dawsonite-bearing sandstone in the Jiyang Depression was established (Fig.9).
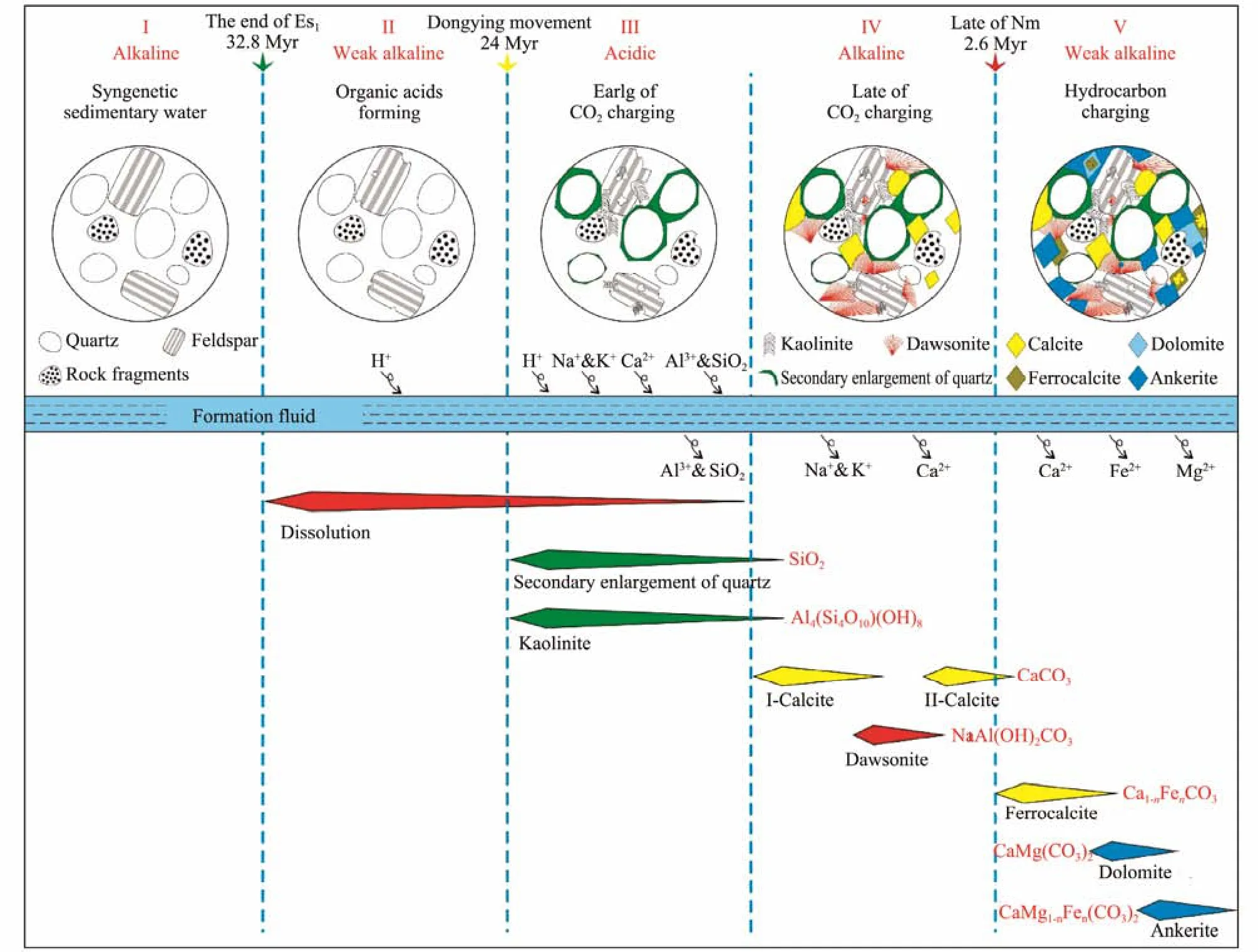
Fig.9 Diagram of diagenetic fluid evolution mode of dawsonite-bearing sandstone in Jiyang Depression.
1) The alkaline syngenetic fluid environment
The syngenetic sedimentary water at the sedimentary period of the Shahejie Formation in the Dongying Sag showed a certain evolution in geochemical composition but a high salinity alkaline fluid environment as a whole(Zeng, 2000; Zhanget al., 2008; Menget al., 2018). In the late sedimentary period of the Fourth Member of the Shahejie Formation, the climate was drought and heat,and the evaporation of lake water was large. The reefshoal carbonate rocks were deposited in Pingfangwang area on the platform at the edge of the Dongying Basin.The syngenetic sedimentary water was alkaline brine, rich in Ca2+, Mg2+, K+, Na+and Cl−.
2) The weak alkaline fluid environment during the formation of organic acids
With the continuous deposition of the strata, the burial depth of the Fourth Member of the Shahejie Formation increased continually, and the mechanical compaction resulted in the continuous decrease of the porosity of the formation, the tight arrangement of clastic particles, and the consolidation of rocks. The formation temperature of 60 – 90℃ was the stage of mass formation of organic acids (Tayloret al., 2010; Yuanet al., 2013). According to the simulation results of thermal evolution history in the Pingfangwang area, the formation temperature of the Fourth Member of the Shahejie Formation was larger than 65℃ at the beginning of deposition of the Dongying Formation, and the organic acids generated by the thermal evolution of organic matter were integrated into the formation fluids, resulting in a decrease of alkalinity of formation fluids.
3) The acidic fluid environment in the early stage of CO2injection
The Dongying tectonism resulted in uplift and denudation in the Dingying Basin, and the temperature of the Fourth Member of the Shahejie Formation decreased. The magmatic activity occurred in the Pingfangwang area,and a large amount of CO2was released from magma due to the decrease of temperature and pressure in the process of intrusion. CO2was injected into the Pingfangwang uplift along the Pingfangwang Fault and accumulated in the upper part of the Fourth Member of the Shahejie Formation. In the gas reservoir, CO2dissolved in formation fluids to form unstable carbonic acid, which decomposed into HCO3−and CO32−and released a large amount of H+,and the formation fluids became acidic. Unstable minerals dissolved in large quantities and produced a lot of secondary pores. The dissolution of carbonate minerals released a large amount of Ca2+and Mg2+, and the dissolution of feldspar ((Na,K)AlSi3O8) released a large amount of Na+and Al3+. Feldspar or other aluminum-rich minerals reacted with CO2to form kaolinite in an acidic fluid environment, and with the continuous dissolution of feldspar, SiO2was supersaturated and precipitated, which generally occurred in the form of secondary enlargement of quartz (SiO2) (Peltonenet al., 2009; Hyodoet al.,2014). The reaction equation is:
4) The alkaline fluid environment in the late stage of CO2injection
The dissolution of unstable minerals resulted in the depletion of H+in the formation fluids, the increasing of the formation fluid salinity, and the change of fluid properties from acidic to alkaline gradually. Because the main CO2gas reservoir lithology was biological reef limestone, CO2charging would lead to the dissolution of carbonate mineral, resulting in the increasing of Ca2+content of formation fluids. And these Ca2+would precipitate as calcite(first-stage calcite) when the fluid properties into alkaline.After the cessation of magmatic activity, magma cooled and crystallized, and CO2was generated by continuous degassing. Therefore, CO2charging was a continuous process. With the continuous charging of CO2, the partial pressure of CO2in the gas reservoir increased continuously. When the formation fluids meet the alkaline fluid environment required for the deposition of dawsonite with the high partial pressure of CO2, high concentration of Na+and high concentration of Al3+, Na+and Al3+in the formation fluids will combined with HCO3−to form dawsonite (NaAl(OH)2CO3):
With the continuous precipitation of dawsonite, the Al3+and HCO3−were consumed in large quantities in the formation fluids, and the CO2generated by magma degassed gradually decreased with time evolution, resulting in the decrease of CO2partial pressure in the formation fluids, which cannot meet the fluid condition required for the deposition of dawsonite, and calcite (second-stage calcite) was precipitated again.
5) The weak alkaline fluid environment during the hydrocarbon charging
The development of a large number of primary hydrocarbon inclusions in carbonate minerals indicated that there were abundant hydrocarbons in the formation fluids during this period. Hydrocarbon charging brought a lot of organic acids and hydrocarbons, which would neutralize the alkaline fluid environment and decrease the pH.Meanwhile, part of Fe3+in the fluids was activated by metal ions and was transformed into Fe2+under the reduction of hydrocarbons (Wigleyet al., 2012). And the ferrocalcite began to precipitate in the study area. And part of the calcite (CaCO3) would undergo ion replacement and become ferrocalcite (Ca1-nFenCO3). With the continuous precipitation of ferrocalcite, Ca2+and Fe2+were consumed in formation fluids, the ratio of Mg2+and Ca2+increased, and dolomite (CaMg(CO3)2) began to precipitate. As the burial deepened and the formation temperature increased, the organic matter in the source rocks of the Fourth Member of the Shahejie Formation maturated and began to generate hydrocarbons. These hydrocarbons, as reducing agents, reduced part of Fe3+to Fe2+in the formation fluids, and the ankerite (CaMg1-nFen(CO3)2) began to precipitate, and part of the dolomite was iron-dolomitization.
The characteristics of present formation water in the Jiyang Depression show that the present formation water from common sandstones (without dawsonite) is weakly acidic and mainly CaCl2type, while the present formation water from dawsonite-bearing sandstone is alkaline and NaHCO3type, and the contents of Ca2+and Mg2+are significantly lower than that of common sandstone. A large number of carbonate minerals, such as calcite, dolomite,ferrocalcite and ankerite, were developed in the distribution area of the dawsonite-bearing sandstone. The precipitation of these carbonate minerals consumed a large amount of Ca2+and Mg2+in the formation fluids, leading to a significantly low content of Ca2+and Mg2+in the present formation water from dawsonite-bearing sandstone, which was significantly different from common sandstone. Therefore, the diagenetic fluid evolutionary mode was in good agreement with the present formation water characteristics from dawsonite-bearing sandstone.
The diagenetic fluid evolutionary mode of dawsonitebearing sandstone including five individual stages in the Jiyang Depression has been established, revealing the interaction process of CO2-H2O and sandstone, which provides support for the development of oil-gas and CO2.More importantly, the evolutionary mode provides a natural analogy for CO2capture and storage, indicating the potential of artificial CO2geological storage.
6 Conclusions
Based on the analysis of petrology, fluid inclusions,isotopic geochemistry and hydrogeochemistry of dawsonite-bearing sandstone in the Jiyang Depression, the following conclusions can be drawn:
1) The lithology of dawsonite-bearing sandstone in the Jiyang Depression is mainly arkose and lithic arkose, and the diagenetic sequence is: secondary enlargement of quartz, kaolinite, first-stage calcite, dawsonite, second-stage calcite, ferrocalcite, dolomite, and ankerite.
2) The hydrocarbon charging is determined in the dawsonite-bearing sandstone in the Jiyang Depression,with the time at around 2.6 – 0 Myr. The source of hydrocarbon is the mixed oil and gas from the Third and Fourth Member of the Shahejie Formation in Boxing Sag.
3) The δ13CCO2of the CO2forming dawsonite is −7.18‰ – −1.61‰, which is the mantle-derived inorganic origin and had the same source as the CO2in the Pingfangwang gas reservoir. The δ18O of the water medium during the formation of dawsonite is −3.76‰ – +1.84‰, which suggests sequestered underground brine. The paleo-fluid temperature in the period of the formation of dawsonite ranges from 69.30℃ to 96.82℃, with an average of 82℃.
4) The evolution of diagenetic fluid of dawsonite-bearing sandstone in the Jiyang Depression can be divided into five stages: the first stage is the alkaline syngenetic fluid; the second stage is the weak alkaline fluids in the period of organic acids formation; the third stage is the acidic fluids in the early stage of CO2injection; the fourth stage is the alkaline fluids in the late stage of CO2injection; the fifth stage is the weak alkaline fluids in the stage of oil and gas charging. The evolutionary mode of diagenetic fluid is in good agreement with the high HCO3−,low Ca2+and low Mg2+nature of present formation water from dawsonite-bearing sandstone in the Jiyang Depression.
Acknowledgement
The authors thank the editors and anonymous reviewer for their careful check and helpful comments and modification of the manuscript. This study was supported by the National Natural Science Foundation of China (Nos.42072130, 41872152).
杂志排行
Journal of Ocean University of China的其它文章
- Using Natural Radionuclides to Trace Sources of Suspended Particles in the Lower Reaches of the Yellow River
- Eutrophication of Jiangsu Coastal Water and Its Role in the Formation of Green Tide
- Evaluation of the Shallow Gas Hydrate Production Based on the Radial Drilling-Heat Injection-Back Fill Method
- Microstructure Characterization of Bubbles in Gassy Soil Based on the Fractal Theory
- Morphological and Sulfur-Isotopic Characteristics of Pyrites in the Deep Sediments from Xisha Trough, South China Sea
- Deformation Characteristics of Hydrate-Bearing Sediments
