Eutrophication of Jiangsu Coastal Water and Its Role in the Formation of Green Tide
2024-03-12XIAOMingyanSONGWeinaZHANGHaiboSHIXiaoyongandSURongguo
XIAO Mingyan, SONG Weina, ZHANG Haibo, SHI Xiaoyong, , and SU Rongguo,
1) Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education, Ocean University of China,Qingdao 266100, China
2) National Marine Environmental Monitoring Center, Dalian 116000, China
3) National Marine Hazard Mitigation Service, Beijing 100194, China
Abstract Since 2007, the large-scale green tide caused by Ulva prolifera (U. prolifera) have occurred as a recurrent phenomenon in the southern Yellow Sea of China. Field surveys and satellite remote sensing showed that the small scattered patches of green tide algae were first observed along the Porphyra agriculture area of the Subei Shoal in late April. In this study, we attempted to identify the role of eutrophication in the origin of the green tide in the Subei Shoal and its adjacent area. Subei Shoal and its adjacent area are characterized by rich nutrients, especially NO3−-N, NH4+-N, PO43−-P, and other bioavailable components (such as urea-N and amino acids). In the spring of 2017, the average concentrations of NO3−-N were 19.01 ± 11.01 μmol L−1, accounting for 86.68% of the dissolved inorganic nitrogen (DIN). In addition, the average concentration of NH4+-N was 2.51 ± 1.60 μmol L−1. PO43−-P had an average concentration of 0.14 ± 0.13 μmol L−1. The average concentrations of urea-N and total hydrolyzed amino acids (THAA) were 1.73 ±1.36 μmol L−1 and 1.33 ± 0.80 μmol L−1, respectively. Rich nutritive substances play a key role in the rapid production of U. prolifera and make the Jiangsu coastal water an incubator for green tide.
Key words green tide; nutrients; algae; Ulva prolifera; eutrophication; Subei Shoal
1 Introduction
Blooms of green macroalgae occur worldwide (Huet al.,2014; Yu and Liu, 2016) and are commonly associated with coastal eutrophication caused by the inputs of nutrients, such as nitrogen (N) and phosphorus (P), resulting from rapid human population growth, excessive agricultural fertilization, intensive mariculture, and other anthropogenic perturbations (Liuet al., 2012; Yuet al.,2018; Liet al., 2021). Since 2007, green tide withUlva prolifera(U. prolifera) as the dominant species have occurred every summer in the Yellow Sea (Xiaoet al.,2021). Large-scale green tide can cause a series of deleterious impacts on the local ecosystem, including hindering the growth of benthic seagrasses by increasing light attenuation, altering benthic fauna, disturbing the carbon and nitrogen cycles, and causing hypoxia resulting from the degradation of blooming algae (Sellneret al., 2003).
Some researchers have studied the occurrence and development process of green tide in the South Yellow Sea(SYS) from multiple perspectives, among them, eutrophication and biological factors were the focus (Songet al.,2014; Chenet al., 2020; Liet al., 2021; Shaet al., 2021).Seawater eutrophication is considered a key factor for the initiation and development of green tide (Berman and Chava, 1999; Yinet al., 2008; Wanget al., 2019; Wurtsbaughet al., 2019). Field studies have indicated that inorganic nutrients, especially dissolved inorganic nitrogen(DIN), are the key factors controlling the scale and output ofU. proliferagreen tide (Zhanget al., 2020b). Laboratory experiments have shown thatU. proliferacould simultaneously take up and utilize inorganic and organic nitrogen compositions, and sources rich in DIN facilitate the fast growth ofU. prolifera(Liet al., 2019). With the rapid development of industry and aquaculture, the use of chemical synthetic fertilizers has increased significantly,and a large amount of wastewater containing nitrogen,phosphorus and other nutrients is discharged into the SYS(Geet al., 2017; Chenet al., 2020; Zhaoet al., 2021). In addition, the expansion of the Yangtze River dilute water,and the upwelling along the northern coast of Jiangsu transports nutrients into the SYS (Cuiet al., 2012). Excess nutrients favor the outbreak of green tide (Liet al.,2017).U. proliferaoften occurs as the dominant algal species in green tide; it is opportunist and can quickly adapt to changes in temperature and salinity (Liet al.,2021).U. proliferaalso has strong growth and cell regeneration ability in eutrophic water environments (Duanet al.,2012). Its spores can be preserved for a long time or in a dormant state and can easily reproduce in different seasons in estuaries, bays and other environments to form green tide disasters (Fenget al., 2012; Wuet al., 2018).These biological characteristics are important factors makingU. proliferathe dominant green tide species in the SYS (Zhaoet al., 2018). Other environmental factors,such as temperature, irradiance, salinity, field current and monsoons, can also affect the growth, scale, floating paths,and distribution area of green tide in the SYS (Largoet al.,2004; Zhanget al., 2010; Keesinget al., 2011; Liuet al.,2013).
The Subei Shoal is one of the largestPorphyracultivation bases, and the aquaculture area is expanded from the Rudong tidal flat (32˚12´–32˚36´N) to north of Lianyungang (34˚N).Porphyraaquaculture rafts are confirmed as nurseries for macroalgal blooms. The macroalgae attached to the aquaculture rafts and the propagules of algae are scraped off and arbitrarily deposited into the sea by farmers. Propagules, which are widely distributed in the Jiangsu coastal area, may play important roles during macroalgal recruitment and the succession of green tide(Hoffmann and Santelices, 1991; Linet al., 2021). Previous researchers on the green tide in the Yellow Sea have mainly focused on the effects of environmental factors on the scale, floating paths, and distribution area of green tide in the SYS, such as bioavailable nutrients (Shiet al.,2015; Zhanget al., 2017; Zhaoet al., 2021), temperature(Kimet al., 2011; Xiaoet al., 2016; Jinet al., 2018), irradiance (Leeet al., 2011), salinity, field current, monsoons, and reproductive system (Sonet al., 2015; Wanget al., 2015; Cao and Han, 2020). However, what are the characteristics of the nutrient structure and distribution in the Subei Shoal and its adjacent area waters? Why can the Subei Shoal and its adjacent areas be green tide incubators? The answers to these questions are not entirely clear,and further research is needed. In the present study, we aimed to 1) investigate the nutrient structure and distribution in the Subei Shoal and its adjacent area in April, May and June from 2017 to 2018; 2) characterize the development process of green tide in this area and 3) analyze the reasons that make the Subei Shoal and its adjacent area waters a green tide incubator. This study will provide theoretical support for the scientific prevention and control of green tide in the SYS.
2 Materials and Methods
2.1 Study Area
The study area is located in the Subei Shoal and its adjacent area (Fig.1). The Subei Shoal in the nearshore water of the SYS starts from the Yangtze River Delta and continues up to the Sheyang River, covering an area of approximately 18000 km2(Zhanget al., 2019b). The water depth in this area is less than 10 m at high tide, and the hydrographic conditions generally feature strong tidal forces and permanent water turbidity (Zhanget al., 2019a;Huoet al., 2020). Besides, the Subei Shoal is the major aquaculture area of the seaweedPorphyra yezoensisin China (Zhanget al., 2020b). The Jiangsu coastal region is densely populated, and there is developed industry and intensive agriculture. There is a large amount of coastal runoff around the Subei Shoal, and more than 20 billion m3of water enters the sea in the Subei Shoal and its adjacent area (Jiangsu Province Water Resources Bulletin,2017). Eutrophication has become one of the most serious aquatic environmental problems (Liet al., 2017).
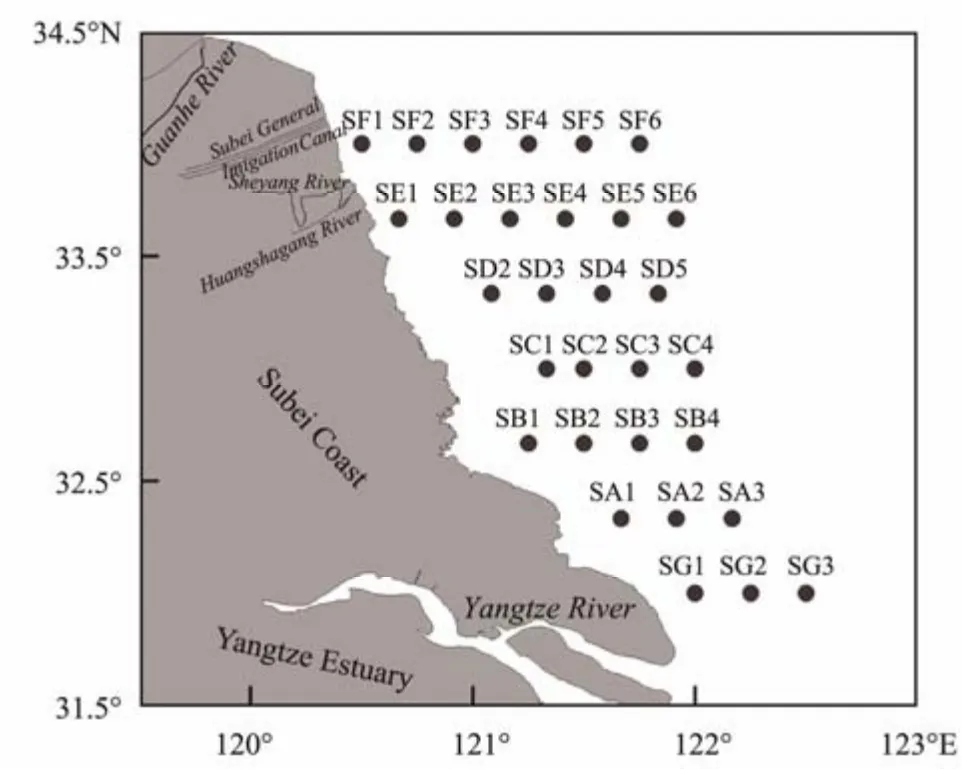
Fig.1 Map of the study area with sampling stations.
2.2 Sampling and Analysis
The field investigation was conducted in the Subei Shoal (31.5˚–34.5˚N, 120˚–123˚E) during April (in spring,the early outbreak period ofU. proliferagreen tide), May(in spring, the increasing period ofU. proliferagreen tide)and June (in summer, the period ofU. proliferalarge outbreaks) from 2017 to 2018. Seawater samples were collected with Niskin bottles and filtered through pre-combusted (450℃ for 6 h) GF/F (Whatman, 0.7 μm) filters.The filtrates were collected in polyethylene bottles at−20℃ for laboratory nutrient analysis. Nutrient concentration was measured by a continuous flow autoanalyzer system (AAIII, HR). The concentrations of NOx−-N(NO3−-N and NO2−-N) were determined using the cadmium-copper reduction method and standard pink azo dye method, respectively (Strickland and Parsons, 1972).The concentration of NH4+-N was determined by the indophenol blue method (Koroleff, 1970), and that of PO43−-P was determined using the molybdate blue method(Murphy and Riley, 1962). DIN was calculated as the sum of the concentrations of NO3−-N, NO2−-N, and NH4+-N.The detection limits of the NO3−-N, NO2−-N, NH4+-N,and PO43−-P were 0.06, 0.02, 0.09 and 0.03 μmol L−1, respectively. Urea-N was determined using the diacetyl monoxime-semicarbazide hydrochloride method with a relative standard deviation of 4% (Mulvenna and Savidge,1992). The total hydrolyzed amino acids (THAA) were measured by high-performance liquid chromatography using phenyl isothiocyanate derivatization after the samples were hydrolyzed with 6 mol L−1HCl at 110℃ for 24 h (Cohen and Strydom, 1988). Seawater temperature and salinity were measured in situ by a CTD multiparameter probe (Seabird 911, USA).
The data of coverage area of floating green tide patches during 2015 – 2018 was obtained from the National Marine Hazard Mitigation Service and the North China Sea Marine Forecasting Center of State Oceanic Administration.
2.3 Statistics Method
Pearson correlation matrix (CM) was determined to analyze the relationship between different nutrients and salinity in the Subei Shoal and its adjacent area. The statistical analysis was performed with the software Origin 2018 (Origin Lab).
3 Results and Discussion
3.1 Analysis on the Development State of U. prolifera
In the period from 2015 to 2018, the floatingU. proliferapatches formed and initially scaled in the area south of 34.5˚N with an initial coverage area of 100 km2; subsequently, the biomass rapidly accumulated from 34.5˚N to 35.5˚N, and the coverage area expanded, forming a large-scaleU. proliferagreen tide coverage area; then in the area north of 35.5˚N, the scale ofU. proliferagreen tide basically reached stability and did not increase (Fig.2).In late spring, especially in late April, sporadicU. proliferaappeared around the laver raft frame (approximately 32˚N – 33˚N). The seaweed breeding raft frame was cleaned by coastal seaweed farmers in Jiangsu. In May, the initial algae attached to the raft, with a total amount of 10000 tons of green tide algae, continued to enter the sea water, and this process was an important source of algae in the initial green tide. In the initial stage of green tide(the area south of 34.5˚N), the algal bodies ofU. proliferawere dark green with high contents of chlorophyll (1.1 –1.4 mg g−1), nitrogen and phosphorus (N > 40 mg g−1,P>0.6 mg g−1, dry weight) and were mainly distributed in scattered patches (< 100 cm2) or strips (< 3 m2) (Fig.3 left)(Zhanget al., 2013). This small form distribution is more conducive to the absorption and utilization of nutrients in sea water to achieve rapid growth and reproduction(Zhanget al., 2013). In this area, the average relative growth rate (RGR, the daily growth rate of the area covered byU. proliferagreen tide) of the green tide could reach 30%, showing an exponential increase overall. Although the total biomass and scale of the algae were at low-scale levels, approximately 17% of the green tide scale developed at this stage (Zhanget al., 2020a). The N and P contents ofU. proliferain the Subei Shoal and its adjacent area were greater than 40 g kg−1and 0.8 g kg−1,respectively (Ding, 2014). There are two indexes used to evaluate the nutrient content inUlvaorEnteromorpha,the subsistence quotas of each nutrient: XQ, the minimum tissue nutrient concentration needed to sustain growth and the critical concentration of each nutrient, and XC, the minimum tissue concentration of a nutrient needed to support maximum growth rates. This means that when the nutrient content inUlvaorEnteromorphawas lower than XQ, the growth ofUlvaorEnteromorphawas limited by nutrient scarcity; however, when the nutrient content was between XQand XC,UlvaorEnteromorphacould keep growing at a slower rate until the subsistence quota was reached. When the nutrient content was higher than XC,UlvaorEnteromorphacould escape grazing control and,in a short time, built up high biomasses (Pedersen and Borum, 1996). ForU. prolifera, its NQ, NC, PQand PCwere 8.13, 24.50, 0.27 and 0.81 g kg−1, respectively(Villares and Carballeira, 2010). The growth ofU. proliferain the Subei Shoal and its adjacent area was not limited by nutrients. When the N content in algae > 20 g kg−1,the algae had more nutrients available than they needed,and the algae could store nutrients (Xiuet al., 2019). The abundant nutrients stored byU. proliferacould provide nutritional support for subsequent growth. When green tide drifted to the coastal waters of the southern Shandong Peninsula, the nutrient contents ofU. proliferadecreased to less than 20 g kg−1for N and less than 0.3 g kg−1for P(Ding, 2014). Zhanget al. (2020a) reported that the consumption of N and P nutrients by green tide in the Subei Shoal and its adjacent area accounted for 50% of the total supply of nutrients in the whole green tide development period.
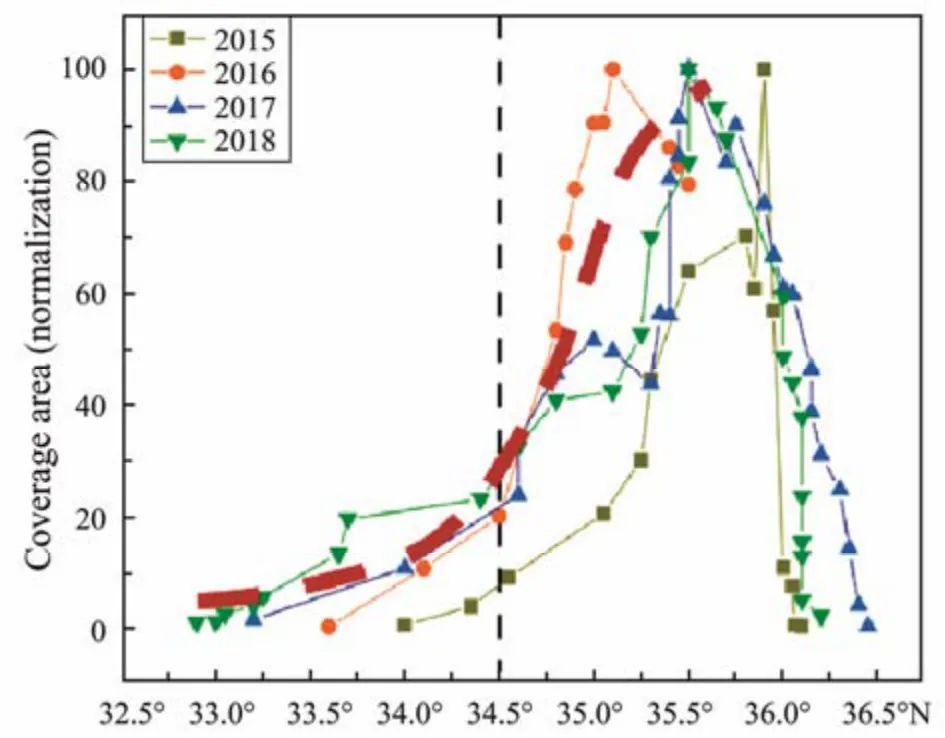
Fig.2 The coverage area of green tide varied with latitude(the red thick line is the increasing trend curve of the green tide coverage area).

Fig.3 Initial states of green tide (left) (Zhang et al., 2020c) and U. prolifera (right) (Zhang, 2020).
3.2 Distribution, Structure and Source of Nutrients
In the Subei Shoal and its adjacent area, nutrient concentrations showed temporal variations, being higher in spring than in summer. The DIN and PO43−-P concentrations in April 2017 and 2018 were in the range of 9.56 –58.79 μmol L−1and 0.87 – 55.23 μmol L−1and 0.02 – 0.58 μmol L−1and 0.02 – 0.79 μmol L−1, respectively (Table 1).The mean concentrations of DIN decreased from 21.93 ±11.79 μmol L−1in April to 14.69 ± 7.53 μmol L−1in June 2017 and 21.08 ± 13.13μmol L−1in April to 14.46 ± 6.99 μmol L−1in June 2018. Similarly, the mean concentrations of PO43--P changed from 0.14 ± 0.13μmol L−1in April to 0.17 ± 0.16 μmol L−1in June 2017 and 0.28 ± 0.19 μmol L−1in April to 0.24 ± 0.20 μmol L−1in June 2018. The spatial distributions of DIN and PO43−-P had an obvious decreasing trend from inshore to offshore areas. Higher DIN and PO43−-P concentrations (DIN > 30 μmol L−1, PO43−-P >0.30 μmol L−1) were mainly concentrated in coastal areas,especially in the northwestern area (near the estuary of the Sheyang River and Guanhe River) (Fig.4).
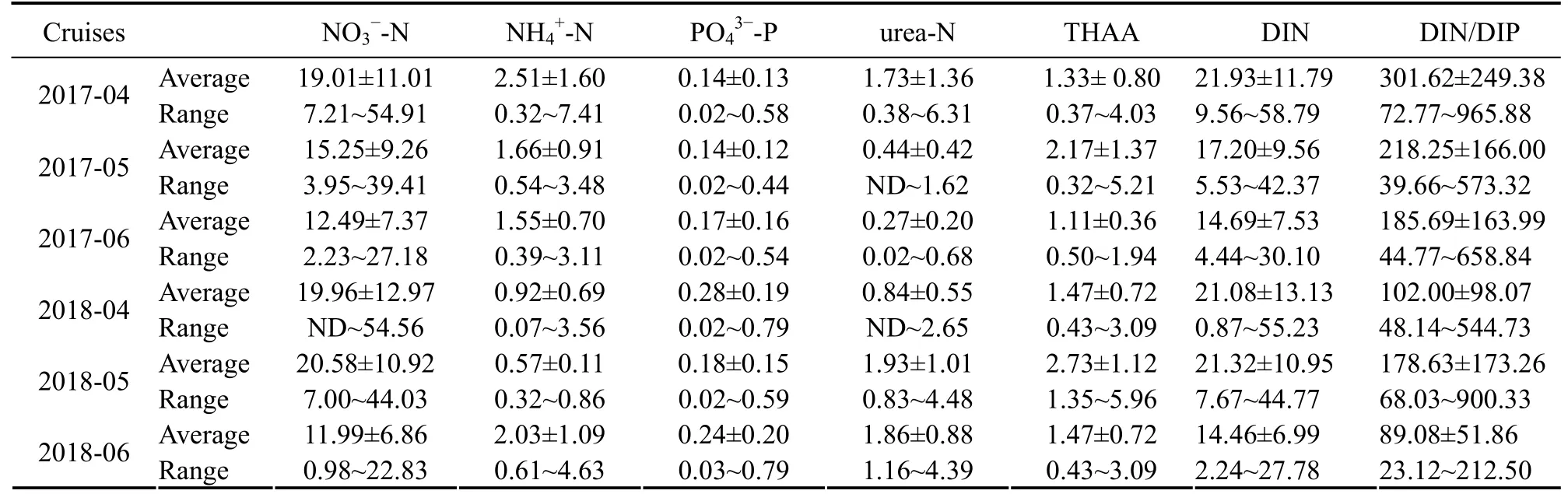
Table 1 Nutrient components (μmol L−1) in surface water during green tide in 2017 – 2018 in the Subei Shoal and its adjacent area

Fig.4 A Horizontal distribution of nutrients (μmol L−1) in 2017 – 2018 in the Subei Shoal and its adjacent area.
In May and June, high-value areas of DIN and PO43−-P extended southward along the coastline, and their concentrations decreased significantly. From April to June,the ratio of inorganic nitrogen to phosphorus (DIN/DIP)was in the range of 23.12 – 965.88, showing a gradually decreasing trend.
The concentration of urea-N recorded from April to June was in the range of ND – 6.31 μmol L−1(Table 1).High urea-N concentrations primarily occurred in the southern portion of the survey area and then occurred in the southeastern portion of the survey area (Fig.4). From April to June, the concentration of THAA was in the range of 0.32 – 5.96 μmol L−1(Table 1). The high-value areas of THAA (> 2 μmol L−1) were concentrated in the aquaculture areas and the northern Yangtze River Estuary.
In comparison, the nutrient concentrations in spring in the north part of green tide area (north of 35.5˚N) were much lower. The DIN and PO43−-P concentrations were in the range of 0.55 – 1.62 μmol L−1and 0.01 – 0.02 μmol L−1in April 2017 and their mean concentrations were 0.88 ±0.34 and 0.02 ± 0.00 μmol L−1, respectively.
A large number of rivers around the Subei Shoal could carry nutrients into the coastal area (Weiet al., 2015).The correlations of nutrients with salinity (S) from April to June of 2017 and 2018 were analyzed (Fig.5). The DIN,PO43−-P and urea-N all showed a linear dependence relation withS(r= −0.73, −0.70, and −0.62,P< 0.05), which suggested that terrestrial input was the main source of these nutrients and determined the distribution, level and structure of nutrients in the Subei Shoal and its adjacent waters. The amounts of DIN input to the Subei Shoal by the Sheyang River, Huangshagang River and North Jiangsu Irrigation Canal were approximately 7217 t, 2317 tons and 1734 t, respectively, and the amounts of TP input were approximately 1338 t, 682 t and 310 t, respectively(The Bulletin of China Marine Ecological Environment Status, 2017). The high nutrient discharge from rivers should be the main contributor to the eutrophication of the Subei Shoal and its adjacent waters (Maet al., 2010;Liuet al., 2013; Liet al., 2015). There were no linear correlations between THAA and salinity(Fig.5). The THAA in coastal waters was greatly affected by biological activities, and its occurrence reflected a balance between production and assimilation (Liet al., 2015; Zhanget al., 2021). Potential sources of amino acids include phytoplankton exudates, zooplankton feeding and the decomposition of particulate organic matter such as algae,while the main loss is generally ascribed to heterotrophic bacteria (Jørgensen, 1987).

Fig.5 Correlations of nutrients with salinity.
3.3 Relationship Between the Nutrient Composition Characteristics and the Green Tide Outbreak
Nitrogen and phosphorus are the basic elements for the growth of organisms, and their available components are important for sustaining micro/macroalgal growth and primary productivity (Gilbert, 2017).
In the 2017-04 and 2018-04 cruises, the average concentrations of NO3−-N in the Subei Shoal and its adjacent area were 19.01 ± 11.01 μmol L−1and 19.96 ± 12.97 μmol L−1, accounting for 86.68% and 94.69% of the DIN(Fig.6), respectively. NH4+-N had average concentrations of 2.51 ± 1.60 μmol L−1and 0.92 ± 0.69 μmol L−1, respectively. In the 2017-05 and 2018-05 cruises, NO3−-N was also considered to be the main component of DIN, with average concentrations of 15.25 ± 9.26 μmol L−1and 20.58± 10.92 μmol L−1, accounting for 88.66% and 96.52% of the DIN, respectively. NH4+-N had average concentrations of 1.66 ± 0.91 μmol L−1and 0.57 ± 0.11 μmol L−1. In the 2017-06 and 2018-06 cruises, compared with the above four cruises, NO3−-N had an obvious decreasingtrend, with average concentrations of 12.49 ± 7.37 μmol L−1and 11.99 ± 6.86 μmol L−1, respectively. Studies exploring the influence of nitrogen sources on the growth ofU. proliferashowed that in a medium with only NO3−-N added, the weight ofU. proliferaincreased, and this state could continue for a long time (Li, 2011). Wuet al. (2010)designed a mesocosm experiment and showed thatU.proliferahad a fast absorption rate of nitrate, and in the early stage of the experiment, the average absorption rate of NO3−-N reached 0.54 μmol g−1h−1. The fluxes of nitrate absorbed byU. proliferaincreased with increasing nitrate concentrations (Wanget al., 2019). Rich nutrients (NO3−-N and PO43−-P) not only promoted the rapid growth ofU.proliferabut also promoted the rapid propagation ofU.prolifera(Maet al., 2010). The abundant NO3−-N in the investigated area may play an important role in the initial stage of green tide.
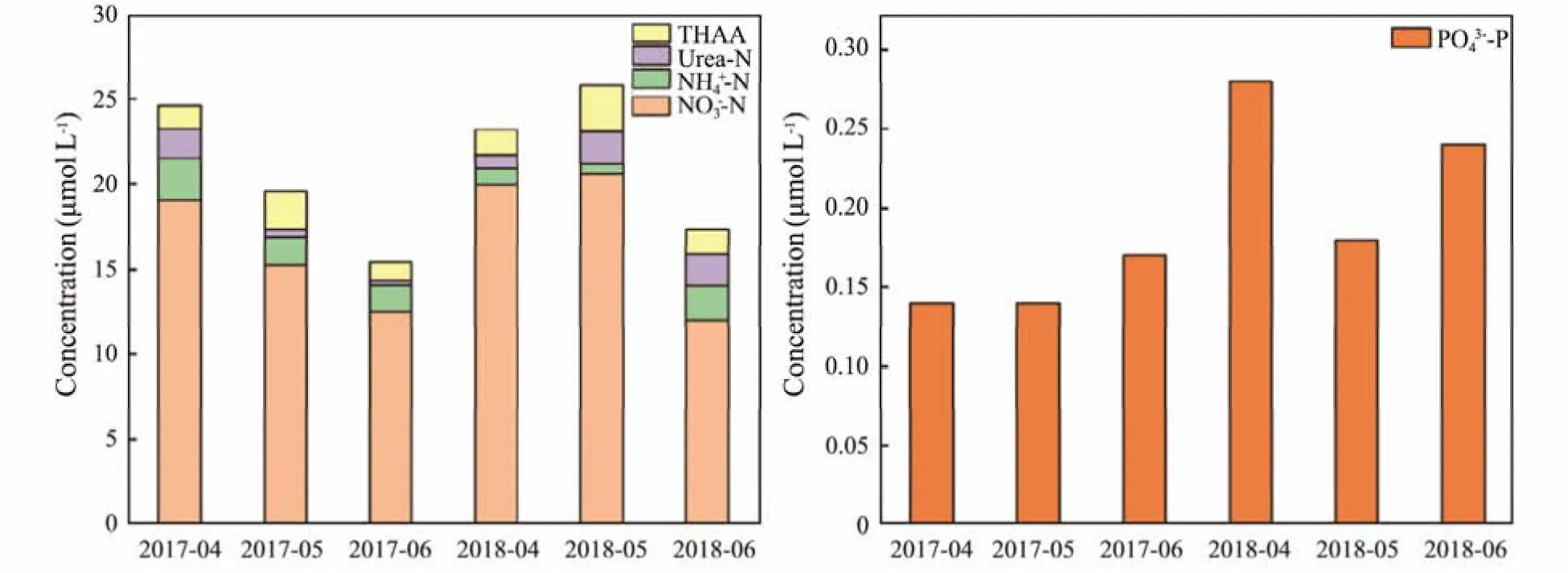
Fig.6 Characteristics of nutrient compositions in surface water during green tide in 2017 – 2018 in the Subei Shoal and its adjacent area.
NH4+-N could be absorbed and utilized as nutrients byU. prolifera. According to previous research reports, algae in the growth process can absorb and utilize NH4+-N.There was a significant linear correlation betweenU. proliferaand the concentration of NH4+-N (Tianet al., 2010),and the maximum absorption rate of algae increased with increasing NH4+-N concentration in a certain range.Pedersen (1994) found thatUlva, which grew in the field,could absorb NH4+-N 20 times as much as it could than other nitrogen components in the first 15 min of exposure to indoor high concentrations of NH4+-N. Li (2011) reported that in a medium of NH4+-N,U. proliferagrew rapidly at the early stage of the experiment. A moderate amount of NH4+-N in the Subei Shoal and its adjacent area waters was favorable to the initiation of green tide.
In the 2017-04 and 2018-04 cruises, the average concentrations of PO43−-P were 0.14 ± 0.13 μmol L−1and 0.28± 0.19 μmol L−1, respectively. In the other four cruises, the average concentrations of PO43−-P were in the range of 0.14 ± 0.12 μmol L−1and 0.24 ± 0.20 μmol L−1. Phosphorus is an important limiting factor for phytoplankton growth,and the level of PO43−-P concentration in seawater can significantly change the physiological and ecological characteristics of marine plants and even affect cell metabolic activities and genetic signal transduction (Thingstadet al.,2005; Rodriguezet al., 2021). Algae did not grow and propagate rapidly in a low-concentration PO43−-P environment (Linyu and Lin, 2000). Li (2015) found that PO43−-P addition improved the growth state ofU. prolifera, and the maximum absorption rate was up to 0.075 μmol g−1h−1(Wuet al., 2010).
In the 2017-04 and 2018-04 cruises, the average concentrations of urea-N were 1.73 ± 1.36 μmol L−1and 0.84 ±0.55 μmol L−1, respectively. The average concentrations of THAA were 1.33 ± 0.80 μmol L−1and 1.47 ± 0.72 μmol L−1,respectively. In the other four cruises, the average concentration ranges of urea-N and THAA were 0.27 ± 0.20 μmol L−1to 1.93 ± 1.0 μmol L−1and 1.11 ± 0.36 μmol L−1to 2.73 ± 1.12 μmol L−1. Dissolved organic nitrogen (DON),especially small molecules such as urea-N and amino acids, is an important N source for phytoplankton (Palenik and Morel, 1990; Bronk and Gilbert, 1993; Fan and Gilbert, 2005). Laboratory culture experiments have found that algae have a high affinity for urea-N (Huet al.,2010). Urea-N is often the optimum nitrogen source for algal growth.Although the concentration of urea-N in the marine system was not high, the urea-N had a fast turnaround time and affected phytoplankton growth and reproduction in coastal waters (Solomonet al., 2010). Some algae can directly use amino acids as the only nitrogen source for their growth, and amino acid transporters of the cell plasma membrane were found in their gene chromosome (Palenik and Morel, 1990; John and Flynn,1999; Anderssonet al., 2006; Huet al., 2012). Li (2011)found thatU. proliferashowed the best growth state in yeast extract, which had a variety of nutrients, such as protein, amino acids, B vitamins, minerals, nucleic acids,organic acids and sugars.
The Subei Shoal and its adjacent area are characterized by rich nutrients, especially NO3−-N, NH4+-N, PO43−-P,and other bioavailable components (such as urea-N and amino acids). This area is also rich in vitamins (Songet al.,2019) and trace elements (Fe3+, Zn2+, Mn2+and so on)(Guet al., 2017; Zhenget al., 2017; Zhanget al., 2019a).Rich nutritive substances play a key role in spore germination and rapid production ofU. prolifera(Luoet al.,2012) and make the Subei Shoal and its adjacent area waters an incubator for green tide.
4 Conclusions
The Subei Shoal and its adjacent waters were considered green tide-forming regions. In this area, the RGR ofU. proliferareached 30%, and nearly 50% of nutrients for the green tide were provided. Rich nutritive substances made the Subei Shoal and its adjacent waters an incubator for green tide. Reducing terrestrial pollutant emissions from the Jiangsu coastal region could be an efficient measure to purify nutrients and alleviate coastal eutrophication, which would effectively inhibit the germination and growth of green tide algae.
Acknowledgements
This work was supported by the Joint Fund between NSFC and Shandong Province (No. U1906210), and the China National Key Research and Development Program(No. 2016YFC1402101). And thanks are given to the cooperation fund project of China and Association of Southeast Asian Nations (ASEAN) and Ministry of Natural Resources of the People’s Republic of China.
杂志排行
Journal of Ocean University of China的其它文章
- Using Natural Radionuclides to Trace Sources of Suspended Particles in the Lower Reaches of the Yellow River
- Evaluation of the Shallow Gas Hydrate Production Based on the Radial Drilling-Heat Injection-Back Fill Method
- Microstructure Characterization of Bubbles in Gassy Soil Based on the Fractal Theory
- Morphological and Sulfur-Isotopic Characteristics of Pyrites in the Deep Sediments from Xisha Trough, South China Sea
- Deformation Characteristics of Hydrate-Bearing Sediments
- Genetic Analysis of Structural Styles in the Makran Accretionary Wedge – Insight from Physical Simulations
