New Antibacterial Dihydropyrones Induced by Co-Culture of Penicillium crustosum PRB-2and Penicillium citrinum HDN11-186
2024-03-12YUGuihongZHOULuningWUGuangweiandLIDehai
YU Guihong , ZHOU Luning WU Guangwei, and LI Dehai
1) Shandong Province Key Laboratory of Applied Mycology, and Qingdao International Center on Microbes Utilizing Biogas, School of Life Sciences, Qingdao Agricultural University, Qingdao 266109, China
2) School of Medicine and Pharmacy, and Sanya Oceanographic Institution, Ocean University of China,Qingdao 266003, China
3) College of Chemical Engineering, Nanjing Forestry University, Nanjing 210037, China
4) Laboratory for Marine Drugs and Bioproducts, Laoshan Laboratory, Qingdao 266237, China
Abstract Two new dihydropyrones, rhytismatones C (1) and D (2), and a known compound, penicillenol A1 (3), were isolated from the co-culture broth of the deep-sea-derived fungus Penicillium crustosum PRB-2 and Suaeda salsa-derived endophytic fungus Penicillium citrinum HDN11-186. Their structures were elucidated through comprehensive analysis of nuclear magnetic resonance (NMR)spectra and mass spectra. The absolute configurations of new compounds were determined by calculating the electronic circular dichroism (ECD) spectrum. UPLC-MS data showed that compounds 1 – 3 could only be detected in the media of co-culture, suggesting new biosynthetic pathways were activated in the co-cultured fungi. Compound 1 showed obvious antibacterial activities against Proteus sp. MMBC-1002 and Bacillus subtilis MMBC-1004 with minimum inhibitory concentration (MIC) both at 25 μmol L−1.
Key words co-culture; Penicillium crustosum; Penicillium citrinum; dihydropyrones; antibacterial activity
1 Introduction
Fungi biosynthesize a variety of secondary metabolites that help them to survive in their habitats, and these metabolites have been proved to be an important source for the novel drug. Studies indicate that fungi possess abundant biosynthetic gene clusters. For example, at least 30 biosynthetic gene clusters have been detected in manyAspergillusspp. (Knowleset al., 2022). However, a great deal of gene clusters in fungi are silent under pure culture, largely because their strong biosynthetic potential was maintained through communication and interaction with neighbors in complex natural environment (Boddy, 2000; Quet al., 2019).Thus, the co-culture strategy, which simulates the relationship of different fungi in their habitats, is an effective way to activate the silent biosynthetic pathways (Moody, 2014).Moreover, the natural products induced by co-culture strategy tend to exhibit excellent bioactivities, such as antimicrobial, anti-tumor and anti-fouling activities (Watanabeet al.,1982; Cho and Kim, 2012; Liet al., 2014; Knowleset al.,2019).
In our previous work, many novel bioactive compounds have been identified fromPenicillium crustosumPRB-2, a deep-sea fungus which showed great biosynthesis potential(Wuet al., 2012, 2022; Yuet al., 2019, 2022). AntiSMASH analysis of the genome sequence of PRB-2 revealed the presence of at least 38 biosynthetic gene clusters, however,the metabolites encoded by most of these clusters were unknown (Fig.1). To tap the biosynthetic potential and search for more bioactive secondary metabolites, PRB-2 was further co-cultured with many other strains derived from diverse habitats. During this process, we found that co-culturing of PRB-2 andPenicillium citrinumHDN11-186, an endophytic fungus derived fromSuaeda salsa, resulted in the production of new metabolites as indicated by the HPLC profiles. Further study of the co-cultivation system led to the discovery of two new dihydropyrones, rhytismatones C(1) and D (2), and a known compound penicillenol A1(3).Here, the details of isolation, structure elucidation, and bioactivity evaluation were reported.
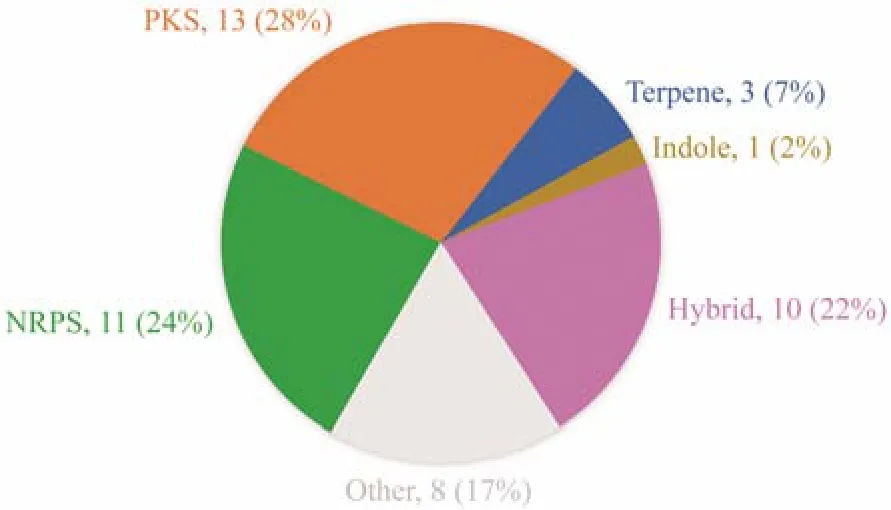
Fig.1 Types, quantity and corresponding proportion of gene clusters in PRB-2.
2 Materials and Methods
2.1 General Experimental Procedures
UPLC-MS spectra were measured on Waters ACQUITYSQ UPLC-mass spectrometer. HPLC was applied using an YMC ODS column (10 mm × 250 mm, 5 µm). NMR data were measured on an Agilent DD2 spectrometer, using TMS as internal standard (500 MHz). ECD data were obtained on a JASCO J-715 spectrometer. UV spectra and optical rotations were recorded on Waters 2487 and JASCO P-1020,respectively. HRESIMS data were acquired by a Thermo Scientific LTQ-Orbitrap XL spectrometer and ESIMS data were tested on a Waters Micromass Q-TOF spectrometer.
2.2 Fungal Material
The strainHDN11-186 was isolated from the leave ofSuaeda salsacollected from Dongying, Shandong, China,and was identified asPenicillium citrinumby analyzing ITS andβ-tubulin gene sequences (accession number: OM757 912). The corresponding information ofP. crustosumPRB-2 has been reported in our early research (Wuet al., 2012).Those fungal materials were deposited in our laboratory.
2.3 Fermentation and Extraction
PRB-2 and HDN11-186were co-cultured in 500 mL flasks with 150 mL medium, consisting of glycerinum (20.0 g L−1),peptone (2.0 g L−1), yeast extract (2.0 g L−1), and seawater.After the cultivation at 28℃, with shaking at 180 r min−1for 9 days, the whole broth (20.0 L) was collected and filtered through cheesecloth. Then, the wet mycelia and the supernatant were extracted with MeOH and EtOAc, respectively, to obtain a crude extract, which was about 27 g(Yuet al., 2018).
2.4 Purification
The crude extract was fractionated into six fractions (fractions 1 – 6) by a C-18 ODS column using gradient elution with MeOH-H2O (5% – 100%) as the solvent. Based on the UPLC-MS data, fractions 2 (eluted with 20:80 MeOHH2O) and 5 (eluted with 80:20 MeOH-H2O) were selected for further research. Fraction 2 was separated by HPLC(43:57 MeOH-H2O, 3 mL min−1) to furnish 1 (15.0 mg, tR21.0 min) and furnish 2 (30.0 mg, tR17.5 min). Fraction 5 was purified on HPLC (80:20 MeOH-H2O, 3 mL min−1) to furnish 3 (15.0 mg, tR20.0 min).
Rhytismatone C (1): pale yellow oil; [α]20D−46.8 (c0.1, CHCl3); UV (MeOH)λmax(logε): 235 (3.96), 268(3.99) nm;1H and13C NMR data, see Table 1; HRESIMSm/z269.1025 [M-H]−(calcd. for C13H17O6, 269.1031).

Table 1 1H (500 MHz) and 13C (125 MHz) NMR data of compounds 1 and 2 (DMSO-d6, δ ppm)
Rhytismatone D (2): pale yellow oil; [α]20D−66.7 (c0.1, CHCl3); UV (MeOH)λmax(logε): 235 (3.95), 267(3.99) nm;1H and13C NMR data, see Table 1; HRESIMSm/z255.0878 [M-H]−(calcd. for C12H15O6, 255.0874).
2.5 ECD Calculations
Merck molecular force field was adopted for conformational search. The conformer with a distribution above 5.0%was re-optimized and calculated at B3LYP/6-31+G (d) level with PCM model for MeOH. ECD spectrum was obtained using SpecDis3 with the sigma/gamma setting as 0.4 eV and UV-shift setting as 5 nm for better comparison with the experimental data (Yuet al., 2016).
2.6 Assay of Cytotoxicity Inhibitory Activity and Antimicrobial Activities
The cytotoxicities were measured by methylthiazoletrazolium (MTT) or Sulforhodamine B (SRB) method, and the antimicrobial activities were tested in the 96 wells plate, as previously reported (Yuet al., 2018).
3 Results and Discussion
The two strains PRB-2 andHDN11-186were co-cultured for 9 days at 28℃, with shaking at 180 r min−1. Guided by UPLC-MS analysis, the total extract (27.0 g) was purifiedviacolumn chromatography and semi-preparative HPLC to obtain the newly generated compounds 1 − 3 (Fig.2).
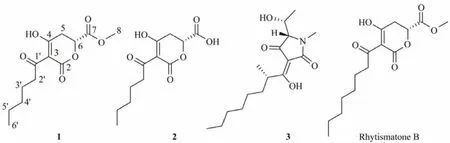
Fig.2 Structures of compounds 1 – 3 and similar compound rhytismatone B.
Rhytismatone C (1) was determined as C13H18O6based on the HREIMS signal atm/z269.1025 [M-H]−. The1H and13C NMR data (Table 1) suggested the existence of five nonprotonated sp2carbons (δC193.2, 192.6, 171.6, 170.1,97.0), one oxygenated methine (δC75.7), five methylenes,and two methyls including one oxygenated (δC51.7). The COSY correlation between H-2' and H-3', and the HMBC correlations from H3-6' to C-4'/C-5', from H2-3' to C-1'/C-4'/C-5', from H2-2' to C-1'/C-4'/C-3 suggested the presence of an unsaturated aliphatic chain (C-3, C-1' – C-6') (Fig.3). The HMBC signals from H3-8 to C-7 and from H2-5 to C-4/C-6/C-7 suggested the presence of a fragment containing methyl ester group (C-4 – C-8) (Fig.3). Based on chemical shifts of C-6 (δC75.7) and the left carbonyl (δC171.6), we speculated that there was an ester fragment which link to C-6. At last, a trisubstituted-dihydropyrone-ring was deduced by comprehensive analysis of chemical shifts of C-2(δC171.6), C-3 (δC97.0) and C-4 (δC192.6), as well as the molecular weight (Fig.2; Table 1). These chemical shifts were consistent with that of rhytismatone B (C-2 (δC174.1),C-3 (δC99.6) and C-4 (δC197.6) in CD3OD), a compound with different length of unsaturated aliphatic chain (compound 1: C6; rhytismatone B: C8) (Fig.2) (McMullinet al.,2017). The result of the chiral HPLC analysis indicated that compound 1 was a pure substance rather than an enantiomeric mixture, as evidenced by the presence of a major peak.Thus, the absolute stereochemistry of 1 was further determined by comparing the calculated ECD spectrum with the experimental one (Fig.4) (Yuet al., 2016). The ECD spectrum were calculated using the fragment 1a as the truncated model structure of 1 (Fig.4). A conformer with a Boltzmann distribution 97.2% was obtained and calculated. As the calculated ECD spectrum ofR-1a was identical to the experimental result of 1, the absolute stereochemistry of 1 was determined asR(Fig.4).

Fig.3 Key COSY and HMBC correlations of 1 and 2.
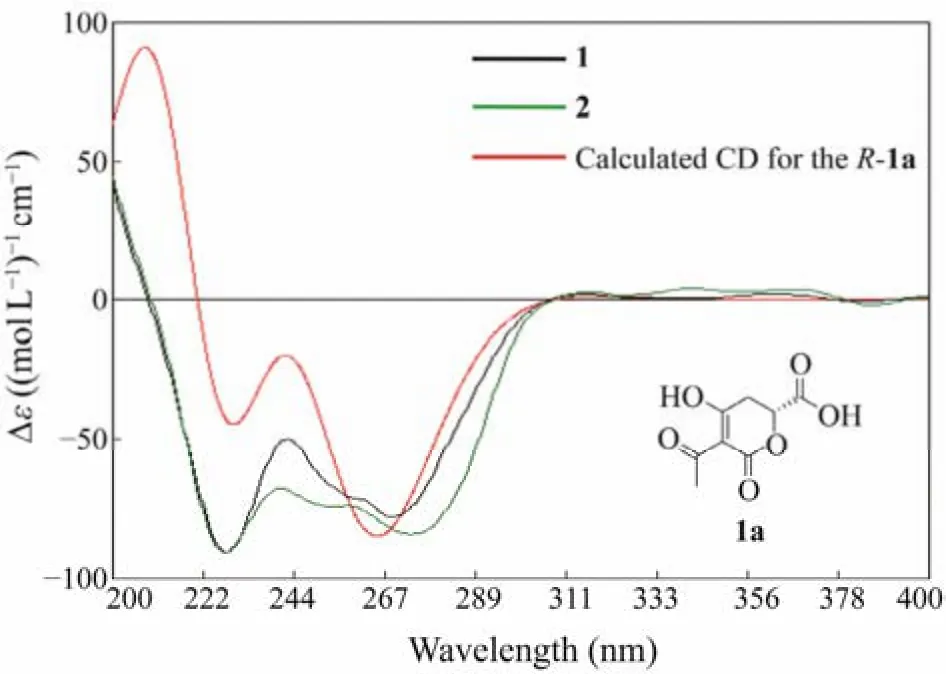
Fig.4 Experimental ECD spectra of compounds 1 and 2 and the calculated spectrum for R-1a.
Rhytismatone D (2) was determined as C12H16O6based on HREIMS signal atm/z255.0878 [M-H]−. The1H and13C NMR data (Table 1) of 2 suggested the existence of five nonprotonated sp2carbons (δC193.4, 193.3, 172.4, 171.3,96.3), one oxygenated methine (δC75.9), five methylenes,one methyl, which were very similar to the result of compound 1. Compared with 1, the resonances atδH3.61 (s)andδC51.7 were disappeared in 2, suggested that it was not esterified at C-7 (Fig.2). The analysis of COSY and HMBC spectra further confirmed our speculation (Fig.3). The presence of a main peak in the chiral HPLC analysis of compound 2 indicates that it is a pure substance instead of a mixture of enantiomers, too. Furthermore, the absolute stereochemistry of compound 2 was determined asR, based on the highly similarity of its ECD spectrum to that of compound 1.
Rhytismatones C (1) and D (2) were identified as new compounds with a dihydropyrone core structure. Natural products with the same core structure have been reported in other organisms and most of them showed remarkable antimicrobial activities. For example, alternaric acid fromAlternaria solanishowed inhibitory activities againstAbsidia glaucaandMyrothecium verrucaria; rhytismatones A and B fromRhytismataceaesp. showed inhibitory activities againstSaccharomyces cerevisiae; and podoblastins A − C fromPodophyllum peltatumshowed inhibitory activities againstPyricularia oryzae.Compound 3 was determined as penicillenol A1based on the coincident1H NMR data and the close optical rotation, which has been isolated from aPenicilliumsp. and reported as a potent cytotoxic substance against HL-60 with the IC50value of 0.76 µmol L−1(Sengokuet al., 2010).
The cytotoxicities of 1 and 2 were measured using twelve cell lines (including HL-60, SH-SY5Y, Hela, L-02,etc.)by MTT or SRB method. However, both of them showed no activity at 30 µmol L−1. Inspired by the antimicrobial activities of other dihydropyrone derivatives, the antimicrobial activities of 1 and 2 were measured using eight kinds of microorganisms including one kind of fungus,CandidaalbicansMMBC-2001, and seven kinds of bacteria,includingEscherichia coliMMBC-1001,Proteussp. MMBC-1002,B. subtilisMMBC-1004,Mycobacterium phleiMMBC-1005,Bacillus cereusMMBC-1007, Methicillinresistance coagulase negativeStaphylococci(MRCNS)MMBC-1009, andAcinetobacter baumanniiMMBC-1012.Compound 1 displayed obvious activities againstProteussp. MMBC-1002 andB. subtilisMMBC-1004 with the MIC values both at 25.0 µmol L−1(Table 2). Ciprofloxacin,the positive control, showed inhibitory effect on two bacteria with the MIC values of 0.2 µmol L−1and 13.0 nmol L−1.
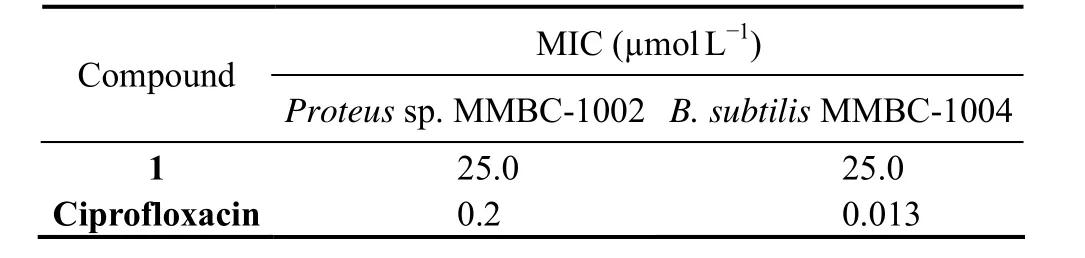
Table 2 Antibacterial activities of compound 1
The UPLC-MS profiles revealed that compounds 1 − 3 were not produced in mono-culture of these two fungi under the same condition used for co-culture. This indicated that the gene clusters synthesizing them were activated by the interaction of two fungi in co-culture condition(Fig.5).
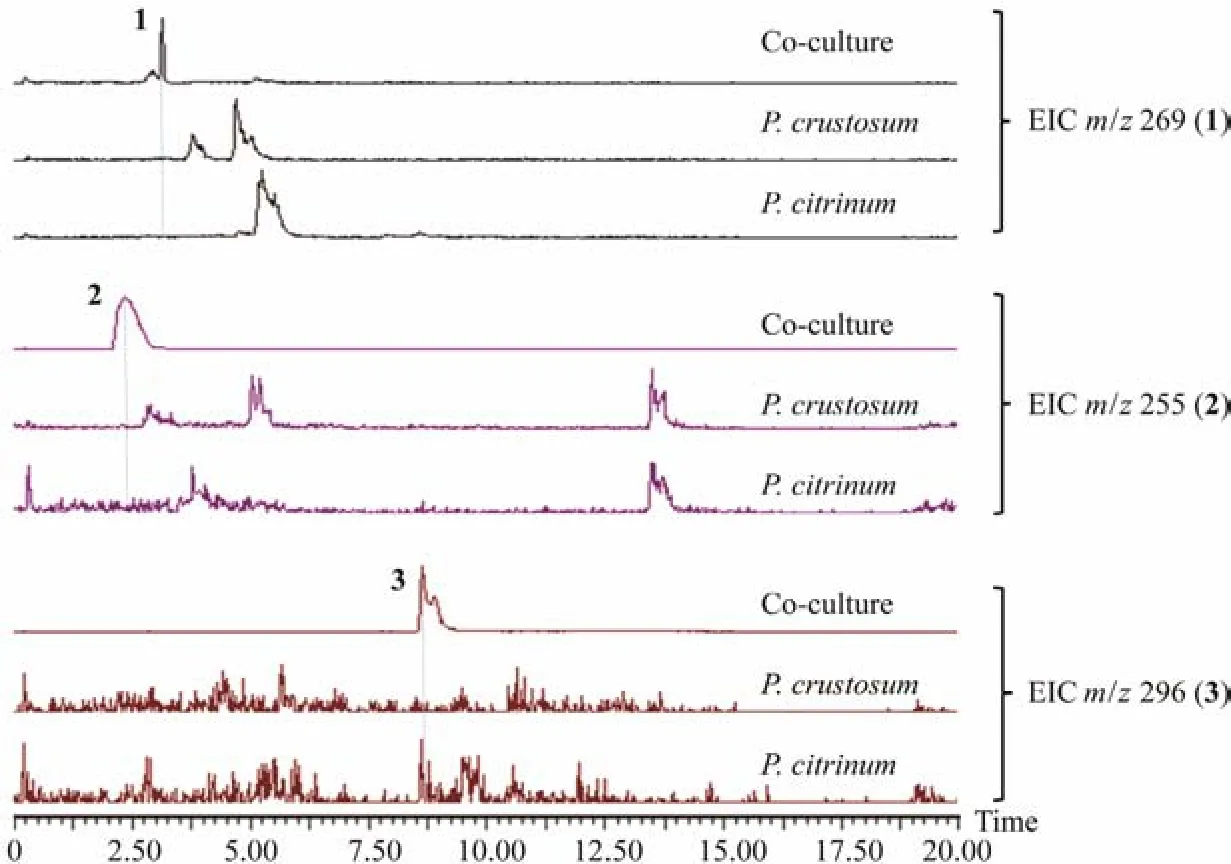
Fig.5 UPLC-MS analysis of the 9th day fermentation broth extracts from co-culture of P. crustosum PRB-2 and P. citrinum HDN11-186, as well as culturing them alone (concentration: 5 mg mL−1, extracted negative ion peak ([M-H]−) of compounds 1 – 3).
4 Conclusions
Chemical investigation of the co-culture extract of the fungi PRB-2 and LD-11 led to the discovery of two new dihydropyrones, rhytismatones C (1) and D (2), as well as a known compound, penicillenol A1(3). The absolute configurations of 1 and 2 were elucidated by the calculation of ECD spectrum. Compound 1 showed obvious antibacterial effects onProteussp. MMBC-1002 andB. subtilisMMBC-1004 with the MIC values at 25 µmol L−1. All three compounds were only produced in co-culture condition, suggested that co-culture strategy was an effective method to activate the silent biosynthetic pathways.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 41806167), the High-Level Talents Research Fund of Qingdao Agricultural University(No. 665/1120034), the NSFC-Shandong Joint Fund (No.U1906212), the Major Project of the 14th Five-Year Plan(No. 2022QNLM030003-1), the Natural Science Foundation of Shandong Province (No. ZR2021ZD28), the Hainan Provincial Joint Project of Sanya Yazhou Bay Science and Technology City (No. 2021CXLH0012), and the Youth Innovation Plan of Shandong Province (No. 2019KJM004).
杂志排行
Journal of Ocean University of China的其它文章
- Using Natural Radionuclides to Trace Sources of Suspended Particles in the Lower Reaches of the Yellow River
- Eutrophication of Jiangsu Coastal Water and Its Role in the Formation of Green Tide
- Evaluation of the Shallow Gas Hydrate Production Based on the Radial Drilling-Heat Injection-Back Fill Method
- Microstructure Characterization of Bubbles in Gassy Soil Based on the Fractal Theory
- Morphological and Sulfur-Isotopic Characteristics of Pyrites in the Deep Sediments from Xisha Trough, South China Sea
- Deformation Characteristics of Hydrate-Bearing Sediments
