Expression and Characterization of a Novel λ-Carrageenase Cgl150A_Wa from Wenyingzhuangia aestuarii
2024-03-12SUNYuhaoCAOSiqiZHANGYuyingXUEChanghuXIAOHangandCHANGYaoguang
SUN Yuhao , CAO Siqi ZHANG Yuying XUE Changhu , XIAO Hang,and CHANG Yaoguang ,
1) College of Food Science and Engineering, Ocean University of China, Qingdao 266003,China
2) Marine Life Research Center, Laoshan National Laboratory, Qingdao 266237, China
3) Department of Food Science, University of Massachusetts Amherst, MA 01003, United States
Abstract λ-Carrageenan is a highly sulfated polysaccharide alternating of 1,4-O-α-D-galactopyranose-2,6-sulfate (D2S,6S) and 1,3-O-β-D-galactopyranose-2-sulfate (G2S). λ-Carrageenases are desirable tools for λ-carrageenan degradation. Based on the genome mining, a novel λ-carrageenase Cgl150A_Wa was cloned from the bacterium Wenyingzhuangia aestuarii and expressed in Escherichia coli. Cgl150A_Wa was an endo-acting enzyme and exhibited its maximum activity at 30℃ and pH 8.0. By employing a glycomics strategy that combined ultra-performance liquid chromatography-mass spectrometry analysis and glycoinformatics, Cgl150A_Wa was proven to degrade λ-carrageenan octaose and hexaose, and the major hydrolysis product of Cgl150A_Wa was λ-carrageenan tetrose.In addition to the typical λ-carrageenan motifs, the active center of Cgl150A_Wa might tolerate desulfated λ-carrageenan motifs.Cgl150A_Wa is a potential biotechnological tool for preparing λ-carrageenan oligosaccharides and structural investigation.
Key words carrageenan; λ-carrageenase; LC-MS; oligosaccharide; GH150
1 Introduction
Carrageenan, a class of natural polysaccharides in the cell walls of red algae, mainly consists of 1,3-O-β-D-galactopyranose (G) and 1,4-O-α-D-galactopyranose residues (D)or 1,4-O-3,6-anhydroα-D-galactopyranose residues (DA)repeating disaccharide units (Zhuet al., 2018). According to the difference of the position and number of sulfate groups(S) on galactose as well as the amount of DA, the carrageenan disaccharide units can be divided into different types(Sedayuet al., 2019).λ-Carrageenan, which is primarily generated from the tetrasporic phase ofGigartinceae, consists of D2S,6S-G2S and contains the largest content of sulfate groups (Chauhanet al., 2016; Guoet al., 2022). Due to the absence of DA,λ-carrageenan is more hydrophilic thanκ-andι-carrageenan, although its aqueous solution is very viscous. Consequently,λ-carrageenan is utilized as thickening and stabilizer agents in food, cosmetics and pharmaceutical industries (Ghanbarzadehet al., 2018). More importantly,λ-carrageenan, especially low molecular weightλ-carrageenan andλ-carrageenan oligosaccharides possess versatile biological activities, including anti-tumor, anticoagulant and immunomodulation activities (Zhouet al.,2004; Zhouet al., 2006; Groultet al., 2019; Guoet al.,2022), which have generated increasing interest and showed promising applications in the food and medical fields.
By the virtues of the specificity and efficiency, enzymes are considered as desirable tools for polysaccharides degradation and oligosaccharides preparation. To the best of our knowledge, few studies have reported the cloning and characterization ofλ-carrageenases. Ohta and Hatada (2006)isolated and purified the firstλ-carrageenase CglA fromPseudoalteromonassp.CL19 and they clarified the gene sequence of CglA in 2006. Guibetet al. (2007) heterologously expressed anotherλ-carrageenase CglA, investigated its hydrolysis pattern and comfirmed that the enzyme contains at least 8 subsides; moreover, created a new category forλ-carrageenase. Recently,λ-carrageenase OUC-CglA was successfully expressed, which showed cold-adaption in the degradation process (Luet al., 2022). Even though threeλ-carrageenases have been successfully cloned, there are no available commercial enzymes for degradingλ-carrageenan yet.
In order to enrich theλ-carrageenase species, recently, we isolated and screened a marine bacterium namedWenyingzhuangia aestuarii, and revealed a putativeλ-carrageenase gene sequence (GenBank accession no. OP730521) in the genome of this strain by using BLASTP algorithm against the Swiss-Prot database. In this study, the sequence was cloned and heterologously expressed inEscherichia coliBL21(DE3), and a newλ-carrageenase Cgl150A_Wa was produced. The biochemical characteristics of the enzyme were investigated and its hydrolysis process and products were determined by a glycomics strategy.
2 Material and Methods
2.1 Bioinformatics Analysis
dbCAN (Yinet al., 2012) and SignalP 4.1 (Petersenet al.,2011) were used to predict the domain of Cgl150A_Wa.The sequence similarity was examined by using BLASTP(Altschulet al., 1997). The isoelectric point and theoretical molecular weight (Mw) of Cgl150A_Wa were estimated by ExPASy (Artimoet al., 2012). Evolutionary relationship between Cgl150A_Wa and all the enzymes of the GH150 family in CAZy was analyzed by ClustalX2 and MEGA6 (Thompsonet al., 1997). The ClustalX2 was used to performed the multiple sequence alignment, and the phylogenetic tree was constructed by using MEGA6 based on neighbor-joining algorithm.
2.2 Plasmid Construction and Protein Purification
W. aestuariiwas collected and the genomic DNA was extracted as previously described (Zhanget al., 2019). The geneCgl150A_Wawithout signal peptide sequence was amplified by polymerase chain reaction (PCR), and a GST tag was added to the N-terminus of the encoded sequence to improve protein solubility. The forward primer was 5’-GACACGGATCCCAAAAGGTAGATACAAAATCAGC TTTG-3’ and the reverse primer was 5’-GACACCTCGA GTTATTTAAGTGGTTTGCTCAACTCAACATC-3’. The PCR amplification products and PGEX-4T-1 vector were digested with the restriction enzymesBamHI/XhoI, and the target fragment was inserted into the vector. The recombinant vector was transformed into BL21 (DE3) competent cells. The recombinant strains were cultivated in LB medium containing 60 ng mL−1ampicillin with a shaking at 170 r min−1and 37℃. Isopropylβ-D-thiogalactoside was added to induce Cgl150A_Wa expression with a concentration of 0.1 mmol L−1at 17℃ for 12 h. Ultimately, the cells were harvested and resuspended in 20 mmol L−1PBS (pH 8.0), disrupted by sonication, and centrifuged to obtain the crude Cgl150A_Wa solution.
The purification procedures were performed on the AKTA Prime System (GE Healthcare, Sweden). The target proteins were primarily purified by GSTrap columns (GE Healthcare, Sweden) and then with HiPrep SP Fast Flow columns (GE Healthcare, Sweden). After removing GST tag by using thrombin, Cgl150A_Wa was further purified by Superdex 75 Increase 10/300GL column (Cytiva, American), then analyzed by SDS-PAGE. The gel consisted of a stacking gel (5% polyacrylamide) and a separating gel (10%polyacrylamide). The purified enzyme concentration was examined by the Bicinchoninic Acid (BCA) Protein Assay Kit (Beyotime Biotechnology, China).
2.3 Enzyme Activity Assay
To determine theλ-carrageenase activity, 100 μL enzyme solution was incubated with 250 μL 2 mg mL−1λ-carrageenan (Shanghai Yuanye Bio-Technology, China) and 275 μL PBS at 30℃ for 10 min. The para-hydroxybenzoic acid hydrazide (pHBH) assay (Lever, 1972) was used to measure the amount of the reducing sugar. Activities of Cgl150A_Wa againstκ-carrageenan,ι-carrageenan or furcelleran were comparatively measured. One unit of Cgl150A_Wa activity(1 U) was defined as the amount of Cgl150A_Wa in hydrolyzing substrate to produce 1 nmol reducing sugar (equivalent to D-galactose) per minute.
2.4 Biochemical Characterization
The solution containing the enzyme and substrate was incubated at different temperatures from 20℃ to 60℃ to estimate the effect of temperature on Cgl150A_Wa activity.The thermal stability was detected by placing Cgl150A_Wa at temperatures ranging from 4℃ to 50℃ for 24 h,and enzyme solutions were taken at different time intervals for enzyme activity assay.
Cgl150A_Wa was mixed withλ-carrageenan solution at pH 4 to 10, then the enzyme activity was measured to determine the effect of pH on enzyme activity. To estimate the pH stability of Cgl150A_Wa, the enzyme was mixed in various buffers with pH 4 to 10 and incubated at 4℃for 1 h before the residual activity assay.
Cgl150A_Wa was incubated withλ-carrageenan solution at a NaCl final concentrations of 0 to 0.5 mol L−1for 10 min, respectively, to study the impact of Na+. In order to evaluate the effects of metal ions and chemical reagents on the enzyme activity, CuSO4, CaCl2, KCl, MgCl2, HgCl2,EDTA (Sinopharm Chemical Reagent Co., Ltd., China),SDS andβ-Mercaptoethanol (Sigma-Aldrich, Germany)were supplemented respectively to the reaction solution at final concentrations of 1 mmol L−1and 5 mmol L−1for 10 min.
2.5 Hydrolysis Pattern Analysis
To elucidate the hydrolysis pattern of Cgl150A_Wa, 150 U Cgl150A_Wa was incubated with 10 mL 2 mg mL−1λcarrageenan solution at 30℃ for 24 h. High-performance liquid chromatography (HPLC) and a refractive index detector (RID) were used to analyze the samples that were collected at various time intervals. TSK-GEL SuperAW-4000 column (Tosoh Corporation, Japan) and elution buffer 0.2 mol L−1NaCl with a flow rate of 0.2 mL min−1were employed to estimate the global profile of the hydrolysis products.
The final products of Cgl150A_Wa were prepared by incubating 1500 U enzyme and 10 mLλ-carrageenan solution whose concentration was 10 mg mL−1at 30℃ for 12 h.Thereafter, another 1500 U enzyme was added to ensure the substrates were hydrolyzed completely. After inactivation,the solution was lyophilized. The lyophilizes were dissolved in 20 mg mL−1, and centrifuged. The supernatant was further purified by AKTA Prime Plus (GE Healthcare, Sweden) and HiLoad 26/60 Superdex 30 PG (GE Healthcare,Sweden) with 5 mmol L−1ammonium formate at a flow rate of 2.6 mL min−1served as the mobile phase. The separated components were identified by liquid chromatographymass spectrometry (LC-MS). Single components with the samem/zwere combined for1H NMR detection.
The hydrolysis processing of Cgl150A_Wa was investigated by adding 3, 6, 9, 12 and 15 U Cgl150A_Wa with 1 mgλ-carrageenan to aλ-carrageenan final concentration of 1 mg mL−1, respectively, and incubated at 30℃ for 24 h.The supernatant was analyzed by ultra-performance size exclusion chromatography combined with high-resolution mass spectrometry (UPSEC-HRMS). The system was equipped with an ultra-performance liquid chromatography(UPLC) unit (Dionex Ultimate 3000, Thermo Fisher Scientific, San Jose, CA) connected to Thermo Scientific QExactive Orbitrap mass spectrometer (Thermo Fisher Scientific, San Jose, CA) and an Acquity BEH 125 SEC column (4.6 mm × 150 mm, Waters, Milford, MA). The mobile phase was composed of 20% (v/v) methanol with 10 mmol L−1ammonium acetate, and the flow rate was set as 0.2 mL min−1(Zhanget al., 2020). Mass spectrometer parameters were set as follows: mass rangem/z150 – 2000;negative ionization mode; S-lens RF level 50 V; spray voltage 2000 V; sheath gas pressure 40 psi; capillary temperature 300℃.
The generated oligosaccharides were identified by a glycoinformatics protocol. The raw data of mass spectrometry was deconvolved using DeconTools (Liet al., 2012).The outputs were processed by GlycoResoft to analyze the composition of oligosaccharides and generate the semiquantitative information (Maxwellet al., 2012).
2.6 Statistical Analysis
All experiments were performed at least three times. All the data were expressed as average ± SD. SPSS Statistics 19.0 (SPSS Inc., Chicago, IL, USA) was utilized to perform Tukey’s post-hoc test [analysis of variance (ANOVA)]. TheP-value under 0.05 was considered statistically significant.
3 Results and Discussion
3.1 Bioinformatics Analysis
The geneCgl150A_Waencodes a protein consisting of 950 amino acids. Cgl150A_Wa was predicted to contain a putative signal peptide (residues 1-35) and a GH150 family structural domain (residues 41-947). After removing the signal peptide, the molecular weight of Cgl150A_Wa was calculated as 104 kDa and the isoelectric point was supposed to be 9.24. The phylogenetic tree consisting of all GH150 family proteins recorded in the CAZy database is shown in Fig.1. Cgl150A_Wa is in a separated branch in distance of the previously reportedλ-carrageenases.BLASTP showed that Cgl150A_Wa sequence only shared 47.31%, 46.98% and 53.11% similarity with the characterizedλ-carrageenase CL19, CglA and OUC-CglA isolated fromPseudoalteromonas sp., indicating the novelty of Cgl150A_Wa.

Fig.1 Phylogenetic tree of Cgl150A_Wa (highlighted by pentacle), previously characterized λ-carrageenases (highlighted by triangle) and other GH150 proteins.
3.2 Cloning and Expression of Cgl150A_Wa
After purification by using the GSTrap and HiPrep SP Fast Flow columns, the recombinant Cgl150A_Wa presented a single band of 110 kDa in SDS-PAGE analysis (Fig.2),which was in a good agreement with the predicted value.The purified Cgl150A_Wa was active onλ-carrageenan with the activity of 236 U mg−1, while it was incapable of degradingκ-andι-carrageenan and furcellaran. The results above indicated that Cgl150A_Wa was a novelλ-carrageenase.

Fig.2 SDS-PAGE analysis of purified Cgl150A_Wa. Line 1, purified Cgl150A_Wa.
3.3 Biochemical Characteristics of Cgl150A_Wa
The optimum reaction temperature and pH of Cgl150A_Wa were 30℃ (Fig.3A) and pH 8 (Fig.3B). More than 90%activity could be retained at 4℃ for 24 h; however, the activity decreased significantly when Cgl150A_Wa was maintained at 20℃ and above (Fig.3C). As for pH stability,Cgl150A_Wa retained more than 80% activity between pH 5.5 to 8.5, and exhibited the highest activity at pH 8 (Fig.3D). NaCl could significantly promote the activity of Cgl-150A_Wa (Fig.3E), which was increased about 3.5 times except for 0.5 M NaCl solution. The notable effect of NaCl on Cgl150A_Wa indicated that Cgl150A_Wa activity was extremely enhanced by the salts and the maximum activity was reached at the presence of 0.1 mol L−1NaCl.

Fig.3 Biochemical characterization of Cgl150A_Wa. (A), effect of temperature on enzyme activity; (B), effect of pH value on enzyme activity; (C), thermal stability; (D), pH stability; (E), effect of NaCl concentration on enzyme activity.
The influence of organic reagents and metal ions on Cgl-150A_Wa are shown in Table 1. 1 mmol L−1Cu2+, Hg2+and SDS inhibited Cgl150A_Wa activity remarkably, while 5 mmol L−1Mg2+and EDTA could promote the activity of the enzyme. Ca2+, K+, andβ-Mercaptoethanol did not have significant effect on the Cgl150A_Wa activity.

Table 1 Effects of metal ions and organic reagents on Cgl150A_Wa activity
3.4 Hydrolysis Pattern of Cgl150A_Wa
To estimate the acting type of Cgl150A_Wa, the enzyme reaction was monitored by utilizing HPSEC-RID (Fig.4,the complete diagram was shown in Fig.5). A significant late evaluation was observed after 10 min of reaction. It confirmed that Cgl150A_Wa was an endo-acting enzyme,which was consistent with CglA and OUC-CglA. Until now,all theλ-carrageenases that have been identified were endotypeλ-carrageenases.

Fig.4 HPSEC-RID analysis of the hydrolysis products of Cgl150A_Wa on λ-carrageenan at different reaction times.

Fig.5 Complete diagram of HPSEC-RID analysis.
To study the enzymatic reaction process, Cgl150A_Wa with different dosages (3 – 15 U) was incubated withλ-carrageenan for 24 h, and the hydrolyzed products were analyzed by a glycomics workflow. [M-2H]2−withm/zvalue of 289.9855 and 571.9735, [M-3H]3−withm/zvalue of 568.9717 and 756.9638 were observed, which correspond respectively to theλ-carrageenan biose (D2S,6S-G2S)1,tetraose (D2S,6S-G2S)2, hexaose (D2S,6S-G2S)3and octaose (D2S,6S-G2S)4. Peak areas of the extraction ion chromatograms of each component were integrated, and the results were illustrated in Fig.6A. The putativeλ-carrageenan tetraose (D2S,6S-G2S)2was the predominant component in all samples. To further identify the component, the putative tetraose was isolated from the end products for1H-NMR identification and the chemical shifts were assigned according to the previous studies (Guibetet al., 2006;2007). Signals of δ5.51 and δ5.49 were attributed to the anomeric protons inα-configuration at the reducing end (α-G2Sr H1) and the non-reducing end (D2S,6Snr H1) (Fig.7).The internal protons (α-D2S,6S H1 andβ-D2S,6S H1) were assigned at δ5.56 and δ5.57. Combining the mass spectrometry and NMR data, the main product was confirmed to be (D2S,6S-G2S)2.
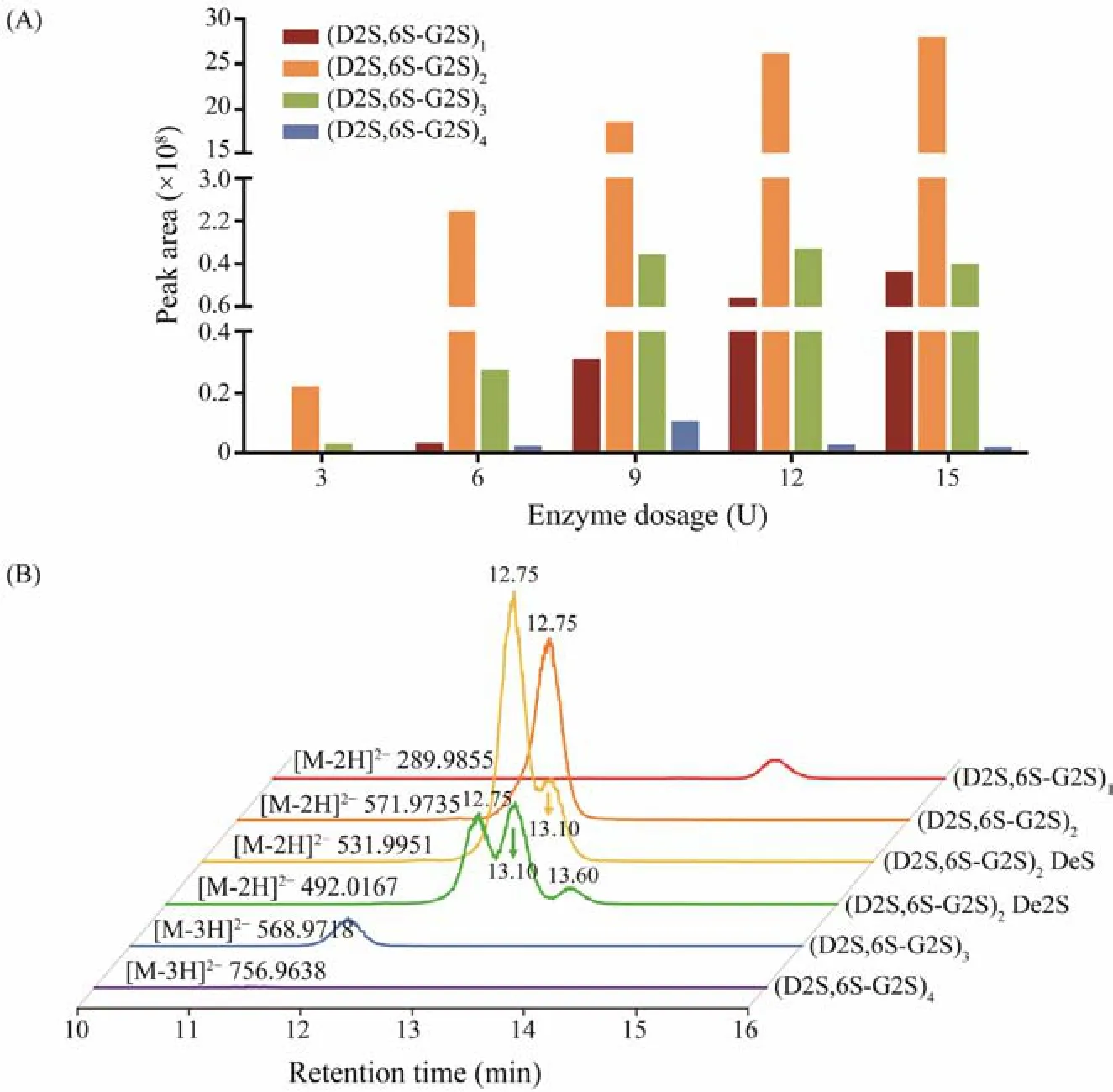
Fig.6 (A) Peak areas of extraction ion chromatograms of each oligosaccharide prepared by incubating λ-carrageenan with different dosages of Cgl150A_Wa; (B) Extraction ion chromatograms of the products hydrolyzed by 15 U Cgl150A_Wa.
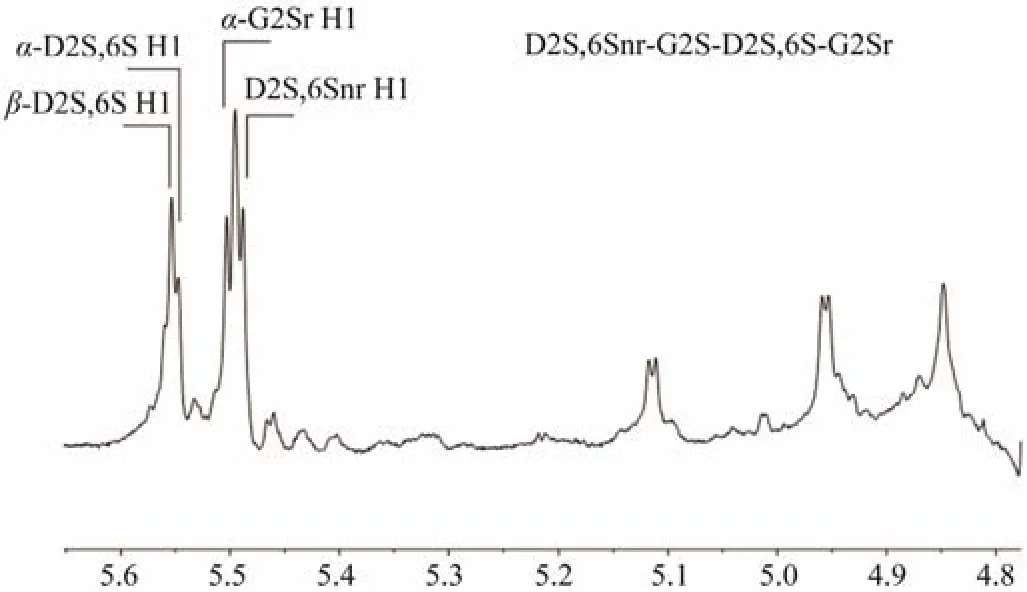
Fig.7 1H-NMR spectrum of purified λ-carrageenan tetraose D2S,6Snr-G2S-D2S,6S-G2Sr. r, reducing end; nr, non-reducing end.
With the increasing dosages of Cgl150A_Wa, the amounts of (D2S,6S-G2S)2increased sharply, coupling with a slow production of (D2S,6S-G2S)1. While the amounts of(D2S,6S-G2S)3and (D2S,6S-G2S)4increased firstly and then decreased. This indicated that Cgl150A_Wa could degradeλ-carrageenan hexaose and octaose, but the biose and tetraose could not be degraded by Cgl150A_Wa. CglA fromPseudoalteromonas carrageenovorawas capable of degrading octaose and hexaose, and generated tetrose as the main product (Guibetet al., 2007), which was consistent with Cgl150A_Wa. The results proved that Cgl150A_Wa consists of at least eight subsites. Considering that tetraose could not be degraded by Cgl150A_Wa, we presumed that the number of the active subsites is more than four, while the exact number needs to be further elucidated. As the products of OUC-CglA in the hydrolysis process were much more complex than those of Cgl150A_Wa and CglA, the biose, tetrose, hexaose, octaose and decaose were all accumulated as the degradation time increased (Luet al., 2022),causing great obstacle to clarify the degradation pattern and the number of subsites.
Intriguingly, in addition to the typicalλ-carrageenan oligosaccharides,λ-carrageenan tetrasaccharide desulfation products (D2S,6S-G2S)2DeS (m/z531.9951, [M-2H]2−,(D2S,6S-G2S)2lost one sulfate group) and (D2S,6S-G2S)2De2S (m/z492.0167, [M-2H]2−, (D2S,6S-G2S)2lost two sulfate groups, or (D2S,6S-G2S)2DeS lost one sulfate group)were also observed in the 15 U Cgl150A_Wa hydrolysis products (Fig.6B). (D2S,6S-G2S)2DeS showed two peaks at retention time of 13.10 min and 12.75 min. Desulfation of highly sulfated polysaccharides in high-energy magnetic fields of the mass spectrum is common and is an inevitable phenomenon (Anastyuket al., 2015). The peak of(D2S,6S-G2S)2DeS at 12.75 min exhibited the same retention time with (D2S,6S-G2S)2. Therefore, the ion ofm/z531.9951 at 12.75 min was supposed to be the (D2S,6S-G2S)2desulfated product in the MS detection process. Meanwhile, the peak of (D2S,6S-G2S)2DeS at 13.10 min was later than that of (D2S,6S-G2S)2, which was in accordance with the separation principle of SEC. So, it should be(D2S,6S-G2S)2DeS in the hydrolysis products. Similarly,the peak of (D2S,6S-G2S)2De2S at 13.60 min was speculated to be a product obtained from the enzyme degradation. It indicated that Cgl150A_Wa can hydrolyze desulfatedλ-carrageenan and generate corresponding desulfated oligosaccharides.
According to the above results, it was speculated that Cgl150A_Wa might be able to accommodate less sulfate groups carrageenan motifs in addition to the typicalλ-carrageenan motifs. Sinceλ-carrageenan contains more sulfate groups thanκ- andι-carrageenan,etc.,λ-carrageenase was considered to express a larger cavity than the other carrageenases to accommodate additional sulfate groups. Theoretically, the desulfated substrate possesses less steric hindrance and is not preferably accommodated into the active sites. This is the first report on the sequence ofλ-carrageenase in hydrolyzing desulfatedλ-carrageenan moieties. The study provided novel understanding of the potential degradation pattern of the GH150 family. Further identification of desulfatedλ-carrageenan oligosaccharides will lead to a better understanding of the subsite specificity of Cgl150A_Wa, which is worthy of additional investigation.
4 Conclusions
In conclusion, a novelλ-carrageenase Cgl150A_Wa was successfully cloned and well characterized. The enzyme shared the highest 53.11% identity with the characterizedλ-carrageenase, and exhibited its maximum activity at 30℃and pH 8.0. It hydrolyzedλ-carrageenan in an endo-acting manner, and was capable of degrading octaose and hexaose,and producedλ-carrageenan tetraose as the main product.Furthermore, in addition to recognizing the typicalλ-carrageenan structure, the active sites of Cgl150A_Wa might also accommodate desulfatedλ-carrageenan motifs. The characterized biochemical properties and special hydrolysis pattern suggested that Cgl150A_Wa is a promising tool to facilitate full degradation ofλ-carrageenan and oligosaccharides preparation.
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (No. 202012020), and the National Key R&D Program of China (No. 2018YFC 0311203).
杂志排行
Journal of Ocean University of China的其它文章
- Using Natural Radionuclides to Trace Sources of Suspended Particles in the Lower Reaches of the Yellow River
- Eutrophication of Jiangsu Coastal Water and Its Role in the Formation of Green Tide
- Evaluation of the Shallow Gas Hydrate Production Based on the Radial Drilling-Heat Injection-Back Fill Method
- Microstructure Characterization of Bubbles in Gassy Soil Based on the Fractal Theory
- Morphological and Sulfur-Isotopic Characteristics of Pyrites in the Deep Sediments from Xisha Trough, South China Sea
- Deformation Characteristics of Hydrate-Bearing Sediments
