Thin Layer Identification of Jiedu Shengxue Granules and Determination of Notoginsenoside R1 Content
2024-03-07ZhenyingFUBingQINGYinghongHUANGXianyiSHIMeiyanQIUXianPENGJiangcunWEIFengzhenLIWenZHONG
Zhenying FU, Bing QING, Yinghong HUANG, Xianyi SHI, Meiyan QIU, Xian PENG, Jiangcun WEI, Fengzhen LI, Wen ZHONG
Guangxi International Zhuang Medical Hospital, Nanning 530201, China
Abstract [Objectives] To establish the quality standard of hospital preparation Jiedu Shengxue granules. [Methods] Scleromitrion diffusum and Prunella vulgaris in Jiedu Shengxue granules were qualitatively identified by thin layer chromatography (TLC). A high performance liquid chromatography (HPLC) was established to determine the content of notoginsenoside R1 in the granule. [Results] The traditional Chinese medicinal materials in Jiedu Shengxue granules could be identified by TLC, and the characteristic spots were stable and clear. Notoginsenoside R1 had a good linear relationship in the range of 10.45-104.5 μg/mL, with an average recovery of 98.52% and RSD=2.36%. [Conclusions] TLC and HPLC, as the quality control methods of Jiedu Shengxue granules, have high accuracy and good repeatability, which lays a foundation for the quality control of this mixture.
Key words Jiedu Shengxue granules, Thin layer identification, Notoginsenoside R1, Quality standard
1 Introduction
The prescription of Jiedu Shengxue granules (Zhuang name: Ywlweddoeg) is derived from Zhuang folk prescription, and was declared and approved in 2010. It is an ethnic medicine preparation of Guangxi International Zhuang Medical Hospital. The prescription is processed with 13 traditional Chinese medicines, such asScleromitriondiffusum,Scutellariabarbata,Panaxnotoginseng,Solanumnigrum,PortulacaoleraceaandPrunellavulgaris, with the effects of clearing heat and removing toxicity, dispersing blood stasis, invigorating qi and blood,etc.It is used for acute leukemia and chronic leukemia caused by heat toxin, stasis toxin, and deficiency due to disease, combined with chemotherapy drugs or alone.
There are no research data of content determination of notoginsenoside R1, the signature ingredient of Jiedu Shengxue granules, and the original quality standard of the preparation is low due to large color rendering error and poor stability and reproducibility of thin layer chromatography (TLC). Therefore, it is necessary to improve its quality standards. Improving the quality standards of Jiedu Shengxue granules is conducive to the stable control of product quality and the safety and effectiveness of clinical medication. Moreover, it also provides quality assurance for further expanding the usable range of this preparation, and offers a new drug choice for the treatment of leukemia in Guangxi, thus accelerating the development of Guangxi Zhuang and Yao medicinal materials and promoting the growth of special Zhuang and Yao ethnic medicine preparations.
2 Materials and methods
2.1 InstrumentsIntelligent dark box three-purpose ultraviolet analyzer (ZF-7ND, Shanghai Jiapeng Technology Co., Ltd.); One over ten-thousand analytical balance [ME155DU, Mettler Toledo International Trade (Shanghai) Co., Ltd.]; Electro-thermostatic blast oven (PHG-9206A, Shanghai Yiheng Scientific Instrument Co., Ltd.); Electro-thermostatic water bath (HWS-24, Shanghai Yiheng Scientific Instrument Co., Ltd.); Ultrasonic cleaners (JP-100S, Shenzhen Jiemeng Cleaning Equipment Co., Ltd.); Thin layer chromatography expansion cylinder (double-cylinder, Shanghai Yiheng Scientific Instrument Co., Ltd.).
2.2 Main reagents and samplesThe main reagents used in the test included ethyl acetate, petroleum ether (60-90 ℃), methanol, trichloromethane, glacial acetic acid, 5% vanillin sulfuric acid solution, anhydrous ethanol, cyclohexane and 10% sulfuric acid ethanol solution, all of which were analytically pure.S.diffusumreference (121183-201605) was derived from National Institutes for Food and Drug Control. The plates for TLC were silica gel G pre-coated plate (20191228, Qingdao Haiyang Chemical Co., Ltd.), Silica gel GF254pre-coated plate, and high efficiency silica gel G pre-coated plate (20150316, Qingdao Haiyang Chemical Co., Ltd.). Jiedu Shengxue granules (batch No.: 200701, 200702, 200703) were provided by Zhuang and Yao Medicine Preparation Center of Guangxi International Zhuang Medical Hospital.
2.3 TLC identification of S. diffusum
2.3.1Preparation of test solution and reference solution. Accurately 5.0 g (sample 1) and 2.0 g (sample 2) of Jiedu Shengxue granules were added with anhydrous ethanol separately, treated by ultrasonic sound (250 W, 37 kHz) for 30 min, and filtered. After drying, the residue was dissolved with 1 mL of methanol and used as the test solution. Reference solution was prepared with 0.5 g ofS.diffusumreference by the same method. According to the prescription, the herbs other thanS.diffusumwere weighed, and the negative particles withoutS.diffusumwere prepared according to the preparation technology. Afterwards, the negative reference solution was prepared by the same method.
2.3.2TLC identification. Accurately 5.0 g (sample 1) and 2.0 g (sample 2) of Jiedu Shengxue granules were dissolved with 25 mL water by ultrasonic sound, and extracted with equal volume of ethyl acetate for 3 times. The extracts were merged and concentrated to 1 mL, and used as the test solution. Precisely 1.0 g ofS.diffusumreference were added with 30 mL of petroleum ether (60-90 ℃), treated by ultrasonic sound for 30 min, and filtered. After drained of petroleum ether, the filter residue was added with 30 mL of methanol, treated by ultrasonic sound for 30 min, and filtered. After drying, the residue was dissolved with 1 mL of methanol and used as the reference solution.
Accurately 10 μL of each of the above solutions were absorbed and dotted on the same silica gel GF254pre-coated plate. With trichlomethane-methanol-ice acetic acid (12:3:0.5) as the developing agent, the samples were unfolded, taken out, dried, sprayed with 5% vanilline sulfuric acid solution, and finally heated at 105 ℃ until the spots showed clear color.
Fig.1A showed that two samples showed spots of the same color at points a and b of reference drug, but the two spots were not completely separated. The developing agent would be adjusted in the subsequent tests in order to separate the two spots and determine the identification method ofS.diffusum.
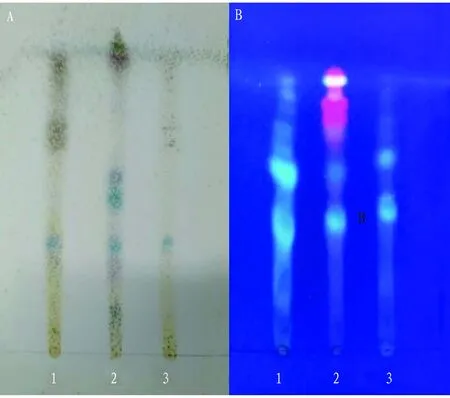
Note: 1. Sample 1; 2. Reference; 3. Sample 2.
2.4 TLC identification of P. vulgaris
2.4.1Preparation of test solution and reference solution. Accurately 5.0 g (sample 1) and 2.0 g (sample 2) of Jiedu Shengxue granules were dissolved with 30 mL water by ultrasonic sound, and extracted with equal volume of ethyl acetate for 3 times. The extracts were merged, dried, and dissolved with 1 mL of methanol (test solution). Accurately 5.0 g ofP.vulgarisreference were added with 30 mL of 70% ethanol, treated by ultrasonic sound for 30 min, and filtered. The filtrate was dried, and the residue was dissolved by adding 1 mL of methanol and used as the reference solution.
And what had become of her? Had a fierce wild beast seized her and dragged her into his lair9 in the forest? Had some bird carried her off across the wide blue sea?No, no beast had touched her, no bird had borne her away
2.4.2TLC identification. According to TLC test (Appendix VIB, 2015 Edition ofChinesePharmacopoeia). Accurately 10 μL of each of the above solutions were absorbed and dotted on the same silica gel G pre-coated plate. With cyclohexane-ethyl acetate-isopropanol-formic acid (15:3:35:0.5) as the developing agent, the samples were unfolded, taken out, dried, and examined under ultraviolet lamp (365 nm).
Fig.1B showed that two samples showed spots of the same color at points c and d of reference drug, and the two spots were completely separated, but both had a tailing phenomenon. The developing agent would be adjusted in the subsequent tests in order to solve the tailing phenomenon of spots and determine the identification method ofP.vulgaris.
3 Determination of notoginsenoside R1 content
P.notoginsengis prescribed in this product. According to literature reports,P.notoginsengis mainly composed of notoginsenoside R1, ginsenoside Rg1 and Rb1. In order to improve the quality control level of this product, the content of these components should be determined. In the test, we explored the method of determining the content of notoginsenoside R1 in the preparation, and the results showed that this product could control the quality of notoginsenoside R1 content inP.notoginseng.
3.1 Sample preparationPreparation of reference solution: Appropriate amount of notoginsenoside R1 reference was weighed accurately, and added with methanol to prepare a solution with the concentration of 0.1 mg/mL.
Preparation of test solution: Accurately 5.0 g of Jiedu Shengxue granules under the loading difference item (batch No.: 200701, 200601, 200701) were weighed and dissolved in 20 mL of pure water. Afterwards, 20 mL of sample solution was loaded into the separatory funnel which was then added with 20 mL of ethyl acetate, and shaken gently. After left undisturbed for 1 h, the water phase was removed, and the upper ethyl acetate layer was moved to an evaporating dish and evaporated in the water bath. The residues were dissolved with 2 mL of methanol, centrifuged and filtered, and moved to a 2 mL volumetric bottle. Then, methanol was added to the scale, and the test solution was obtained by filtering through microporous filter membrane (0.45 μm).
3.2 Chromatographic conditions and system suitabilityWith octadecylsilane chemically bonded silica as the filler [Inertsil-C18column (5 μm, 4.6 mm×250 mm)] and acetonitrile-water as the mobile phase, the solutions were eluted, and detected at the wavelength of 203 nm. The theoretical plate number should not be less than 4 000 according to the peak of notoginsenoside R1. Precisely 10 μL of each reference solution and test solution were absorbed, and injected into the liquid chromatograph for detection. Combined with literature data, the detection wavelength of the product was selected as 203 nm[1-2].
3.3 Method investigation and results
3.3.1Selection of mobile phase. Under the same chromatographic conditions [Inertsil-C18column (5 μm, 4.6 mm×250 mm); detection wavelength: 203 nm; flow rate: 1.0 mL/min; injection volume: 10 μL], the mobile phase systems of methanol-0.1% phosphoric acid, methanol-0.1% acetic acid, methanol-0.2% phosphoric acid, methanol-water, acetonitrile-0.1% phosphoric acid, acetonitrile-0.1% acetic acid, acetonitrile-0.2% phosphoric acid and acetonitrile-water were explored separately. Precisely 10 μL of each reference solution and test solution of notoginsenoside R1 were absorbed, and injected into the liquid chromatograph for detection. The results showed that when eluted with acetonitrile-water, the chromatographic peak of notoginsenoside R1 could achieve good baseline separation, while the separation effect of other mobile phases was relatively poor, or the analysis time was longer. Therefore, the method used acetonitrile-water as the mobile phase for gradient elution.
3.3.2Selection of flow rate. Under the same chromatographic conditions [Inertsil-C18column (5 μm, 4.6 mm×250 mm); detection wavelength: 203 nm; injection volume: 10 μL; mobile phase: acetonitrile-water], the flow rates of 0.8, 1 and 1.2 mL/min were explored separately. Precisely 10 μL of each reference solution and test solution of notoginsenoside R1 were absorbed, and injected into the liquid chromatograph for detection. The results showed that the resolution of each flow rate reached the requirement, and the conventional flow rate of 1 mL/min was adopted in the test.
3.3.3Selection of column temperature. Under the same chromatographic conditions [Inertsil-C18column (5 μm, 4.6 mm×250 mm); detection wavelength: 203 nm; injection volume: 10 μL; mobile phase: acetonitrile-water], the column temperatures of 20, 25 and 30 ℃ were explored separately. Precisely 10 μL of each reference solution and test solution of notoginsenoside R1 were absorbed, and injected into the liquid chromatograph for detection. The results showed that the resolution of each column temperature met the requirements, and this test was conducted at room temperature.
3.3.4Specificity test. Notoginsenoside R1 reference solution and test solution were prepared according to the method described in Section3.1. The negative preparation withoutP.notoginseng(except forP.notoginseng, the remaining traditional Chinese medicine were prepared into granules with 1/10 of the amount in the prescription), and negative solution was prepared according to the preparation method of test solution. Precisely 10 μL of each reference solution, test solution and negative solution were absorbed, and injected into the liquid chromatograph for detection.
The results showed that there was a chromatographic peak with the same retention time as that of notoginsenoside R1 in the chromatogram of test solution, and the chromatographic peak was separated from adjacent peaks at baseline (R>1.5). In the chromatogram of negative solution withoutP.notoginseng, there was no chromatographic peak with the same retention time at the same position as notoginsenoside R1 reference, indicating that this method is highly specific and has no negative interference.
3.3.5Determination of theoretical plate number. The test solution of 3 batches of samples was detected. The results showed that the theoretical plate number of notoginsenoside R1 was higher than 4 000. Therefore, it is determined that the theoretical plate number of this method should be equal to or higher than 4 000 when calculated according to notoginsenoside R1.
3.4 Methodological validation and results
3.4.1Linear investigation and linear range. Preparation of notoginsenoside R1 reference solution: Appropriate amount of notoginsenoside R1 reference was weighed accurately, and added with methanol to prepare a solution with the concentration of 0.1 mg/mL for later use.
Preparation of standard series solution: 0.1, 0.3, 0.5, 0.8 and 1.0 mL of the above-mentioned notoginsenoside R1 reference solutions were precisely absorbed and loaded into 10 mL volumetric flasks, then added with methanol to the scale and shaken well. The solutions obtained were used as the reference solutions of different concentrations.
Precisely 10 μL of reference solution of different concentrations were absorbed, injected and determined. With the injection volume of reference solution (μg/mL) as the abscissa and the peak area as the ordinate, the standard curve was plotted. When the injection volume of notoginsenoside R1 was in the range of 10.45-104.5 μg/mL, there was a good linear relationship between injection volume and peak area, and the regression equation wasY=82.952X-15.262 (r=0.999 7).
3.4.2Precision test. The same test solution (batch No.: 200701) was absorbed and injected continuously for 6 times according to the method described in Section3.2. The peak areas of notoginsenoside R1 were 1 163, 1 154, 1 151, 1 162, 1 167 and 1 158, respectively, with an average peak area of 1 610.5 andRSD=0.52% (Table 1). The results indicate that the method has good precision.
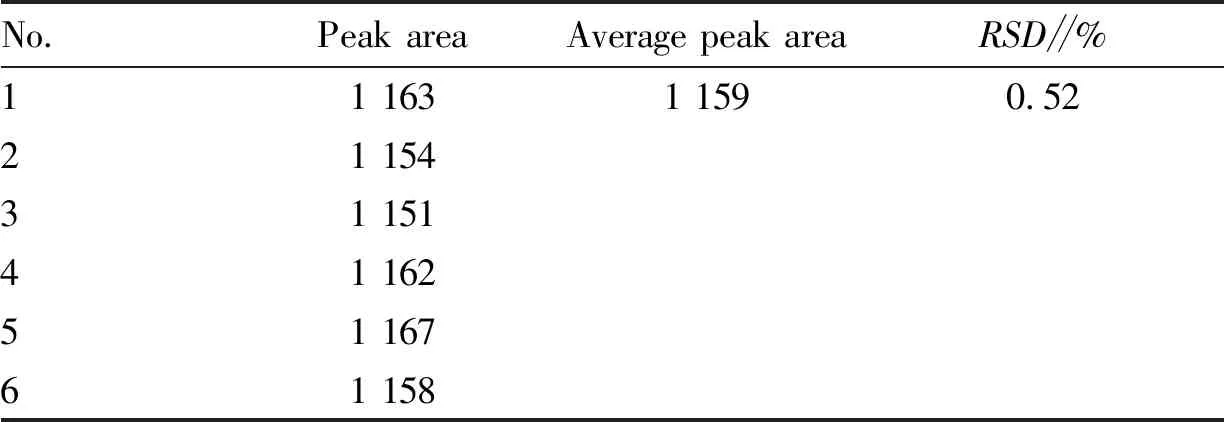
Table 1 Precision test results of Jiedu Shengxue granules (n=6)
3.4.3Repeatability test. Six copies of the sample from the same batch (batch No.: 200701) were accurately weighed, 3 mL each copy, prepared into test solutions, and injected for detection. The peak areas of notoginsenoside R1 were 1 185, 1 172, 1 161, 1 158, 1 165 and 1 170, respectively, with an average peak area of 1 169 andRSD= 0.83% (Table 2). The results show that the method has good reproducibility.
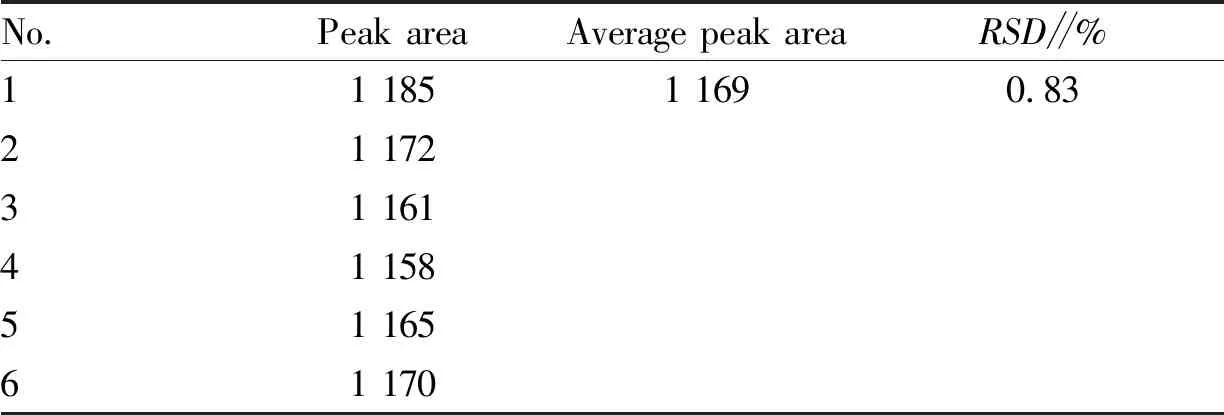
Table 2 Repeatability test results of Jiedu Shengxue granules (n=6)
3.4.4Stability test. The same sample solution (batch No.: 200701) was absorbed and injected at 0, 2, 4, 8, 12 and 24 h according to the method described in Section3.2. The peak areas of notoginsenoside R1 were 1 143, 1 165, 1 178, 1 154, 1 183 and 1 157, respectively, with an average peak area of 1 163 andRSD=1.30% (Table 3). The results showed that notoginsenoside R1 contained in the solution was stable within 24 h.
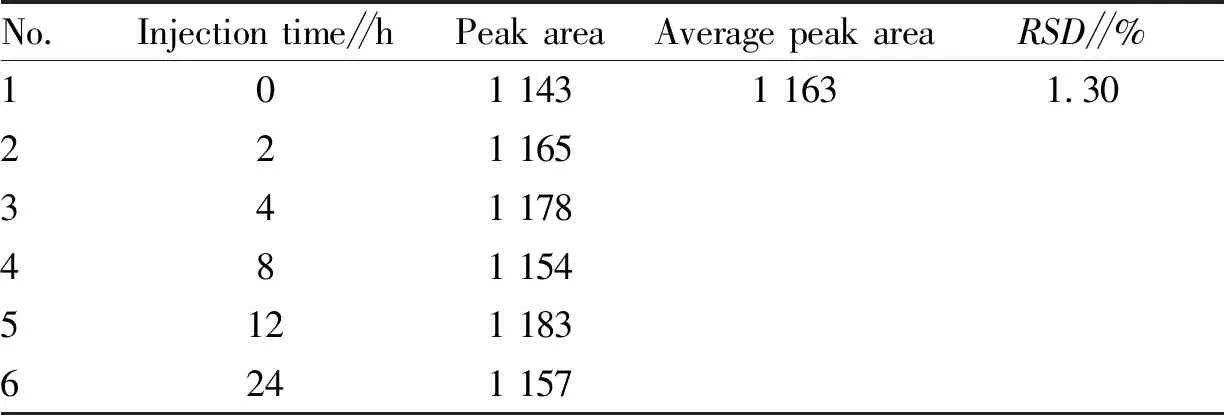
Table 3 Stability test results of Jiedu Shengxue granules (n=6)
3.4.5Recovery test. Precisely 1.5 mL of Jiedu Shengxue granules (batch No.: 200701) with known content were weighed for 6 copies, and placed into corked conical bottles. The corresponding notoginsenoside R1 reference was precisely added, prepared according to the preparation method of reference solution, and filtered. The filtrate was centrifuged at 13 000 r/min for 10 min. The supernatant was filtered by 0.45 μm microporous filter membrane and determined according to the method described in Section3.2. The average recovery andRSDvalues of notoginsenoside R1 in Jiedu Shengxue granules were 98.52% and 2.36%, respectively, indicating that the method has good accuracy.
3.5 Determination of sample contentPrecisely 5 g of Jiedu Shengxue granules (batch No.: 200701, 200702, 200703) were weighed, and prepared according to the method of preparing test solution. Precisely 10 mL of solution was injected into a high performance liquid chromatograph, and the content of notoginsenoside R1 was detected according to the method described in Section3.2(Table 4).

Table 4 Determination results of the content of Jiedu Shengxue granules (n=3)
4 Conclusions
The Jiedu Shengxue granule studied in this project is a classic hospital preparation developed on the basis of the theory of traditional Chinese medicine and gives full play to the advantages of traditional Chinese medicine. It has been clinically used in Guangxi International Zhuang Medical Hospital for many years, and has definite curative effect and reasonable price in the treatment of acute and chronic leukemia. However, this quality standard has not been systematically studied. As the basic research of this granule, this study established qualitative identification of related medicinal materials and quantitative analysis of notoginsenoside R1. However, due to the limitations of conditions, a higher quality standard system has not been developed. In the next step, we intend to study the quality standards of Jiedu Shengxue granules based on quality markers and establish a comprehensive evaluation system that is more in line with modern Chinese medicine properties.
杂志排行
Medicinal Plant的其它文章
- Effects of Exogenous Plant Hormones on Growth Status and Secondary Metabolism of Houttuynia cordata Thunb.
- Identification of Xunxi Shujin Decoction by TLC
- Preparation of 20 (S)-protopanaxadiol PLGA Nanoparticles
- A Network Pharmacology Study on Active Components and Targets of Citri Reticulatae Pericarpium for Treating Keloids
- Pharmacognostic Identification of Hedyotis auricularia and Mitracarpus villosus
- Determination of Salvianolic Acid B in Yiqi Huayu Prescription by HPLC
