Preparation of 20 (S)-protopanaxadiol PLGA Nanoparticles
2024-03-07TaoPANBaifangGONGZhixiaWANGHanyuSUNXuanleYIN
Tao PAN, Baifang GONG, Zhixia WANG, Hanyu SUN, Xuanle YIN
Key Laboratory of Molecular Pharmacology and Drug Evaluation of the Ministry of Education (Yantai University), School of Pharmacy, Yantai University, Yantai 264005, China
Abstract [Objectives] To prepare 20 (S)-protopanaxadiol PLGA nanoparticles (20(S)-PPD-PLGA-NPs). [Methods] 20 (S)-PPD-PLGA-NPs were prepared by emulsion solvent evaporation method, and the optimal formulation was screened by Box-Behnken experiment with particle size and drug loading as the indicators through single factor experiment, and the drug release in vitro was carried out. [Results] The average diameter of the nanoparticles was (119.60±2.29) nm and the polydispersity index was (0.12±0.02), the size was uniform. The encapsulation efficiency and drug loading of protopanaxadiol were (87.99±1.29)% and (14.86±0.25)%, respectively. [Conclusions] The 20 (S)-PPD-PLGA-NPs were successfully prepared by emulsion solvent evaporation method, and the 20 (S)-PPD-PLGA-NPs had good stability, to lay a foundation for the study of 20 (S)-PPD-PLGA-NPs in vitro and in vivo.
Key words 20 (S)-protopanaxadiol, PLGA nanoparticles, Emulsion solvent evaporation method
1 Introduction
Ginsenosides are the main active components of ginseng. 20 (S)-protopanaxadiol (PPD) is a metabolite of protopanaxadiol ginsenosides under the action of intestinal flora, which has neuroprotective[1], anti-inflammatory[2], antitumor[3]and antidepressant effects[4]. However, due to its poor water solubility, its equilibrium solubility in water is only 35.24 mg/L, and its oil-water partition coefficient (P) is 46.21 (logP=1.66)[5], which greatly limits its oral bioavailability (OB).
The study shows that the polymer nanocarrier has high biodegradability and biocompatibility. Poly (lactic-co-glycolic acid) (PLGA) has the characteristics of good stability and high biocompatibility, and is widely used in the research of improving drugs with poor water solubility[6]. In this study, PLGA nanoparticles were prepared from protopanaxadiol and the preparation method was optimized.
2 Materials and methods
2.1 MaterialsPPD API (Shandong Boyuan Biological Pharmaceutical Co., Ltd., batch number: 20230418); PPD reference substance (Shanghai Aladdin Biochemical Technology Co., Ltd.); PLGA (50/50, 2 A, Shandong Luye Pharma Group); polyvinyl alcohol (Sigma-Aldrich, USA); phosphomolybdic acid dye (Beijing Solarbio Technology Co., Ltd.); dichloromethane (Sinopharm Chemical Reagent Co., Ltd.); acetone (Sinopharm Chemical Reagent Company), acetonitrile (chromatographic pure, Sigma-Aldrich Company), and the rest of the reagents are analytically pure.
2.2 Methods
2.2.1Determination of PPD content. (i) Establishment of the maximum absorption wavelength of PPD. Weighed 10 mg of the prepared PPD-PLGA nanoparticle lyophilized powder, added acetonitrile to a 10 mL volumetric flask, sonicated in a water bath sonicator for 10 min, filtered through a 0.22 μm microporous membrane, and used as a test solution for later use. Prepared the blank PLGA nanoparticles without PPD prepared in the same way as the negative test solution. Took a proper amount of the above two solutions and scan the wavelength in the range of 200-400 nm to determine the maximum ultraviolet absorption wavelength of PPD.
(ii) Plotting of standard curve. Accurately weighed 5 mg of PPD, dissolved with an appropriate amount of acetonitrile and prepared into a PPD stock solution with a concentration of 1 mg/mL for later use. The above stock solutions were diluted into reference solutions with concentrations of 2.5, 5, 10, 20, 40, 80, 100 and 160 μg/mL in mobile phase, respectively, and were determined by high-performance liquid chromatography.
2.2.2Methodological investigation. The low, medium and high concentrations of PPD reference substance (1, 50, 100 μg/mL) were analyzed byinvitroHPLC within 1 day and 3 consecutive days, respectively. Prepared blank PLGA nanoparticles using the method in Section2.2.1, add low, medium and high concentration of PPD standard solution, determined the content of PPD in accordance with the treatment method of test solution, and calculated the recovery rate.
2.2.3Preparation of 20 (S)-protopanaxadiol-PLGA nanoparticles. PLGA nanoparticles loaded with PPD were prepared by emulsion solvent evaporation method[7-9]. PLGA and PPD were dissolved together in a dichloromethane/acetone (3:2) mixed solvent as the organic phase, and the aqueous solution of PVA containing 1% polyvinyl alcohol was used as the internal aqueous phase, the oil phase and the internal aqueous phase were mixed evenly under the condition of magnetic stirring, and the colostrum was obtained by ultrasonic for 3 min (power 300 W and time 3 min) in a probe ultrasound instrument under ice water bath conditions. The obtained colostrum was added to 0.3% polyvinyl alcohol PVA aqueous solution to make compound milk, and the organic solvent was evaporated by stirring (700 r/min) on a magnetic stirrer for 4 h at room temperature to obtain PPD-PLGA nanoparticles. PPD-free PLGA blank nanoparticles were prepared by the same method. The prepared PLGA nanoparticles were lyophilized, and the samples were set aside.
2.2.4Single factor investigation of PPD-PLGA nanoparticles. In this study, particle size and drug loading were selected as important evaluation indicator for the evaluation of nanoparticles, and the effects of polymer PLGA concentration, oil phase and internal aqueous phase ratio, internal aqueous phase PVA concentration, ultrasonic power and ultrasonic time on the formulation and preparation process of PPD-PLGA NPs were tested.
2.2.5Formula optimization by Box-Benken response surface methodology (RSM). In this study, we optimized the nanoparticle preparation process in the form of the Box Behnken Design-RSM[10-12].
2.2.6Determination of encapsulation efficiency, drug loading and particle size. The nanoparticles were filtered through a 0.45 μm microporous membrane, the filtrate was collected, and the volume of the collected nanoparticles was recorded. One portion of 1 mL of nanoparticle filtrate was added with an appropriate amount of acetonitrile, vortexed, ultrasonic demulsification for 20 min, and then passed through a 0.22 μm microporous filter membrane for HPLC analysis, which was recorded as the total drug dose. The other portion took 1 mL of nanoparticle filtrate in an ultrafiltration tube (M=100 KD), centrifuged at 3 000 rpm for 30 min, and an appropriate amount of acetonitrile was added to the lower filtrate, which was directly analyzed by HPLC after passing through the membrane, and was recorded as the amount of free drug. Calculate the encapsulation efficiency (EE%) and drug loading (DL%) of the nanoparticles:
EE%=(Total drug amount-Free drug amout/Total drug amount)×100%;
DL%=[Total drug amount-Free drug amount/(Total drug amount-Free drug amount)+PLGA mass]×100%.
Took 0.2 mL of the prepared nanoparticles to determine the particle size distribution on the particle size analyzer, dropped the PPD nanoparticles on a 400-mesh copper mesh, used 2% phosphotungstic acid solution for negative staining, dried the sample, and then placed it under the transmission electron microscope for observation.
3 Results and analysis
3.1 Determination of PPD content
3.1.1Determination of the maximum absorption wavelength of PPD. The results of UV-Vis spectrophotometer showed that PPD had a maximum absorption at 203 nm, while the blank PLGA nanoparticles test solution had no absorption at this wavelength.
3.1.2Plotting of standard curve. The linear regression between the chromatographic peak area (Y) and the concentration of PPD (X) is shown in Fig.1. The regression equation of the standard curve isY=6 646X-8 925,R2=0.999 3. The results show that the linear relationship of PPD is good in the range of 2.5-160 μg/mL.
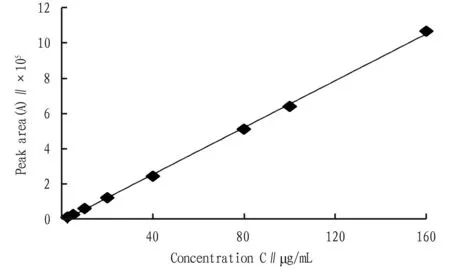
Fig.1 Standard curve of protopanaxadiol PPD reference solution
3.2 Methodological investigationThe intra-day and inter-dayRSDs were 1.52%, 0.44%, 0.30% and 1.63%, 0.66%, 0.50% (n=3), respectively. The recovery rates were 99.78%, 100.02% and 100.12% withRSDof 1.76%, 0.64% and 1.17% (n=3), respectively. The results showed that the method could be used to determine the content of PPD-PLGA nanoparticles.
3.3 Single factor investigation of PPD-PLGA nanoparticles
3.3.1Amount of PLGA. Other conditions were fixed to investigate the effect of PLGA dosage on the particle size and drug loading of PPD-PLGA nanoparticles, and the results are shown in Fig.2. When the concentration of PLGA was 5 mg/mL, the particle size was the smallest and the drug loading was the highest.
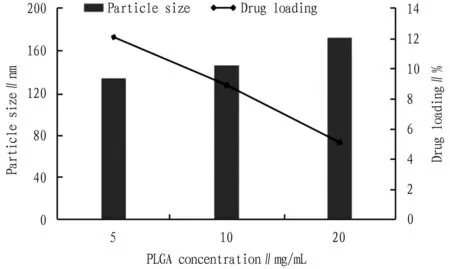
Fig.2 Effect of PLGA concentration on nanoparticle size and drug loading (n=3)
3.3.2Volume ratio of oil phase to internal aqueous phase. With other conditions fixed, the effect of the volume ratio of oil phase to internal aqueous phase on the particle size and drug loading of PPD-PLGA nanoparticles was investigated, and the results are shown in Fig.3. When the ratio of oil phase to aqueous phase was 1:3, the particle size and drug loading of nanoparticles were the highest.
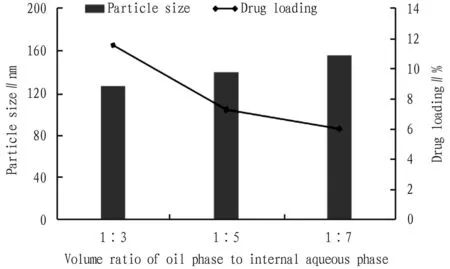
Fig.3 Effect of volume ratio of oil phase to internal aqueous phase on nanoparticle size and drug loading (n=3)
3.3.3PVA concentration in the internal aqueous phase. Other conditions were fixed to investigate the effect of PVA concentration in the internal aqueous phase on the particle size and drug loading of PPD-PLGA nanoparticles, and the results are shown in Fig.4. When the concentration of PVA in the inner aqueous phase was 1%, the particle size and drug loading of the prepared nanoparticles met the requirements.

Fig.4 Effect of PVA concentration in the internal aqueous phase on nanoparticle size and drug loading (n=3)
3.3.4Ultrasonic power. Other conditions were fixed to investigate the effect of ultrasonic power on the particle size and drug loading of PPD-PLGA nanoparticles, and the results are shown in Fig.5. When the ultrasonic power was 300 W, the nanoparticle size was the smallest and the drug loading was the highest.
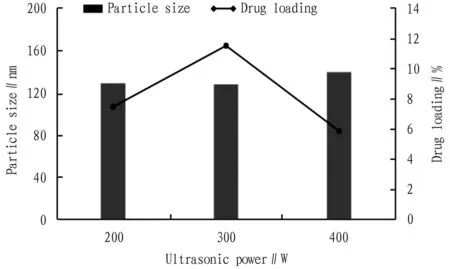
Fig.5 Effect of ultrasonic power on nanoparticle size and drug loading (n=3)
3.3.5Sonication time. Other conditions were fixed to investigate the effect of ultrasonic time on the particle size and drug loading of PPD-PLGA nanoparticles, and the results are shown in Fig.6. The particle size and drug loading were the best when the ultrasonic time was 3 min.

Fig.6 Effect of sonication time on nanoparticle size and drug loading (n=3)
3.4 Formula optimization by Box-Benken response surface methodology (RSM)Based on the results of single factor experiments, three factors were selected as factor levels, including the concentration of polymer PLGA (5-20 mg/mL,X1), the volume ratio of internal aqueous phase to organic phase (3-7,X2), and the sonication time (3-7 min,X3). Design Exper11 was used to design a 3-factor and 3-level experiment, and the particle size (Y1) and drug loading (Y2) were used as response values to optimize. The experimental design results are shown in Table 1.
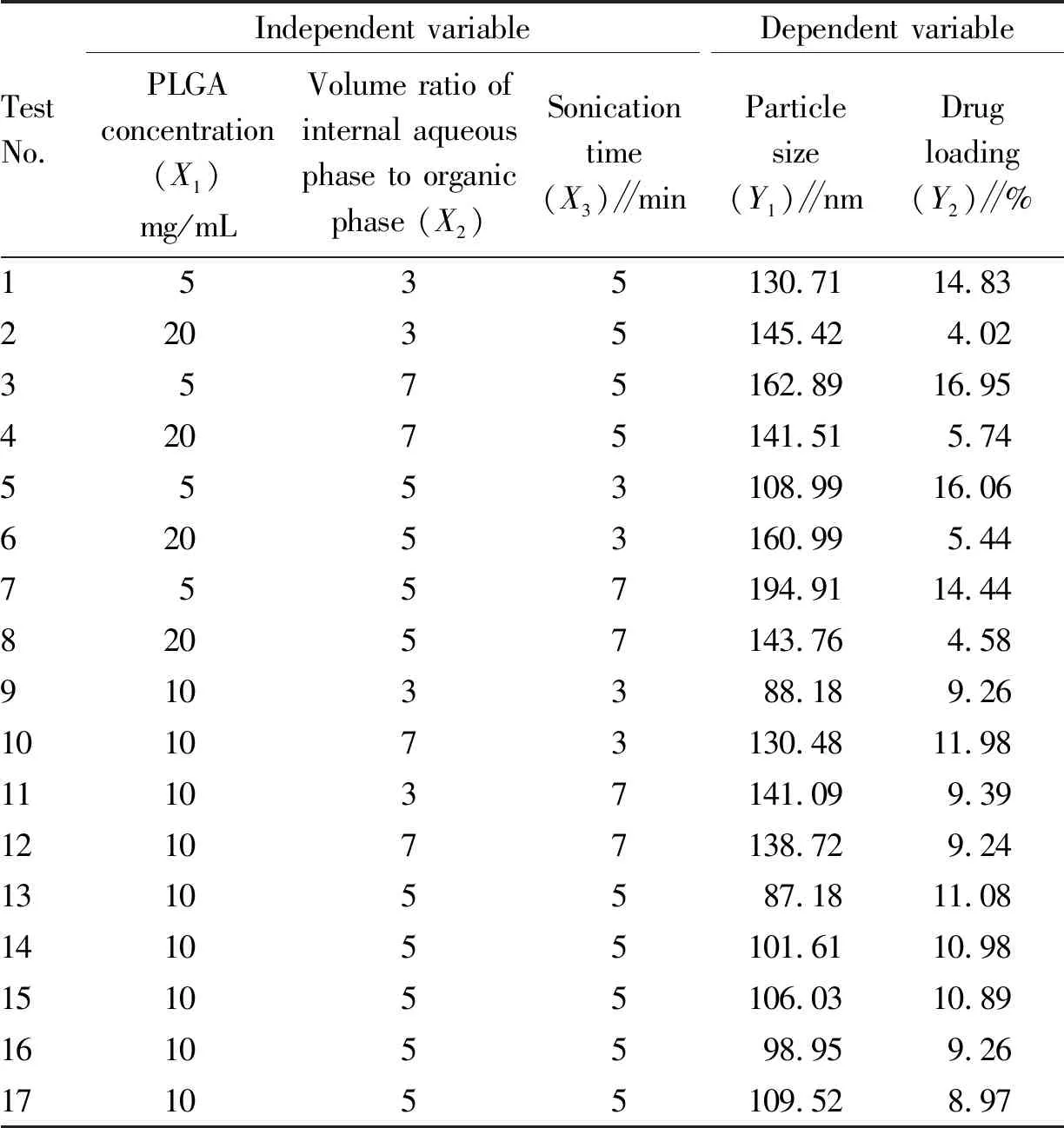
Table 1 Box-Behnken test design results
Quadratic polynomial regression fitting was performed on the experimental data in Table 1 through Design Expert 11 software to obtain the quadratic polynomial regression equation between each factor and particle size (Y1) and drug loading (Y2):Y1=95.88-0.727 5A+7.02B+12.21C-9.01AB-24.13AC-11.17BC+40.78A2+8.46B2+15.49C2,R2=0.963 1,C.V.%=6.61<10%, showing that the reliability and accuracy of the test are high.
Y2=8.22-5.31A+0.793 8B-0.605 4C-0.044 6AB+0.185 1AC-0.717 5BC+2.17A2-0.006 7B2-0.261 8C2,R2=0.980 2,C.V.%=8.12<10%, indicating that the reliability and accuracy of the experiment were high, the difference between the model and the actual value was small, the reliability of the optimized prescription process by the experimental design was high, and the fitting results of the two regression results were good (Table 2-3). The response surface diagrams of the effects of each factor on the particle size and drug loading are shown in Fig.7 and Fig.8. According to the software analysis results, the optimal parameters for the preparation of PPD-PLGA NPs are as follows:X1=5.0 mg/mL,X2=4.45, andX3=3.0 min. In theory, the nanoparticles with particle size of 119.76 nm and drug loading of 14.97% were obtained.
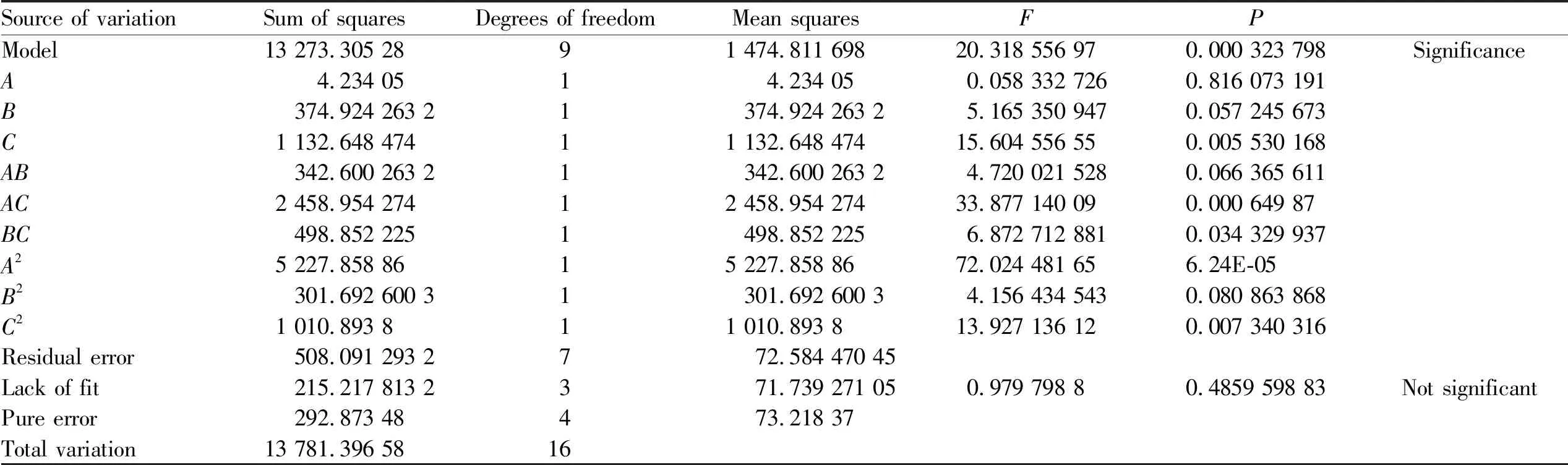
Table 2 Regression coefficient and variance significance results of particle size model

Note: A1A2: Response surfaces and contours of polymer particle size affected by the concentration of PLGA and the volume ratio of internal aqueous phase to organic phase; B1B2: polymer PLGA concentration and ultrasonic time affect the response surface and contour of particle size; C1C2: The volume ratio of the internal aqueous phase to the organic phase and the ultrasonic time affect the response surface and contour of the particle size.

Note: A1A2: The response surface and contour line of the effect of the concentration of polymer PLGA and the volume ratio of the internal aqueous phase to the organic phase on the amount of drug loading; B1B2: The response surface and contour line of the effect of the concentration of polymer PLGA and sonication time on the amount of drug loading; C1C2: Response surface and contour of the volume ratio of internal aqueous phase to organic phase and sonication time affecting the magnitude of drug loading.
3.5 Observation of particle size and morphology of nanoparticlesThree batches of PPD-PLGA nanoparticles were prepared according to the optimal prescription, and the drug loading and particle size were determined separately in accordance with the method in Section2.2.6. The results are shown in Table 4. The appearance of the prepared PPD-PLGA nanoparticles was observed by transmission electron microscopy, and the results are shown in Fig.9.

Fig.9 Particle size and TEM morphology of PPD-PLGA nanoparticles
4 Discussion
In this study, biodegradable polymer PLGA was used as carrier material by emulsion solvent evaporation method, and the formula-tion of PPD-PLGA nanoparticles was optimized by Box-behnken response surface design. The prepared PPD-PLGA nanoparticles had suitable particle size, uniform distribution, spherical morphology and stable system. It is expected to lay a foundation for further study of nanoparticlesinvitroandinvivo.
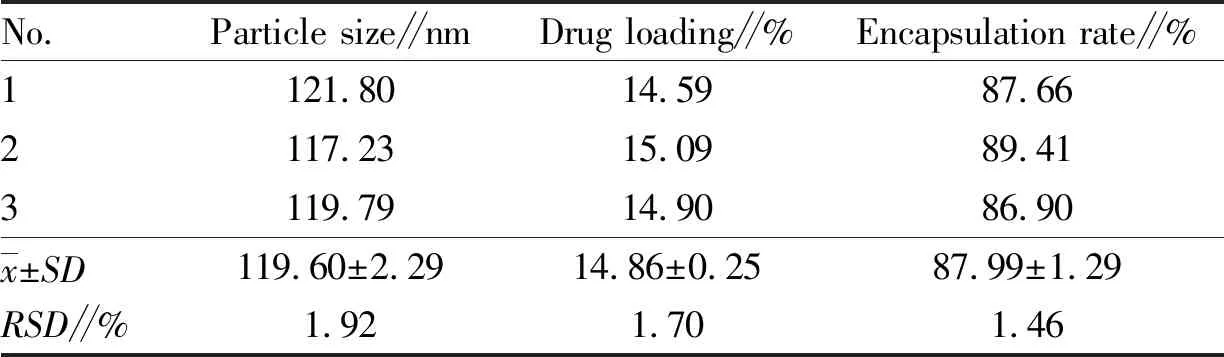
Table 4 Particle size and drug loading of PPD-PLGA nanoparticles
杂志排行
Medicinal Plant的其它文章
- Effects of Exogenous Plant Hormones on Growth Status and Secondary Metabolism of Houttuynia cordata Thunb.
- Identification of Xunxi Shujin Decoction by TLC
- A Network Pharmacology Study on Active Components and Targets of Citri Reticulatae Pericarpium for Treating Keloids
- Pharmacognostic Identification of Hedyotis auricularia and Mitracarpus villosus
- Determination of Salvianolic Acid B in Yiqi Huayu Prescription by HPLC
- Fresh Processing Technology in Polygonatum odoratum Production Area and Its Comparison with Traditional Processing
