Identification of Xunxi Shujin Decoction by TLC
2024-03-07FengxianZHAOYuanmingZHONGDerenLIYongCHENChaolanWANGJianCUI
Fengxian ZHAO, Yuanming ZHONG, Deren LI, Yong CHEN, Chaolan WANG, Jian CUI*
1. Jingxi People’s Hospital, Jingxi 533800, China; 2. Guangxi University of Chinese Medicine, Nanning 530200, China; 3. First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning 530023, China
Abstract [Objectives] To establish a TLC qualitative method for the determination of Radix Aconiti, Radix Aconiti Kusnezoffii, Caesalpinia sappan L. and Radix zanthoxyli. [Methods] TLC was used to study the quality of Radix Aconiti, Radix Aconiti Kusnezoffii and C. sappan L. in Xunxi Shujin Decoction, and the influencing factors were investigated to optimize the TLC conditions. [Results] In the TLC identification test of Radix Aconiti, Radix Aconiti Kusnezoffii, C. sappan L. and R. zanthoxyli in Xunxi Shujin Decoction, the spots were clear, the resolution was good, the specific value was moderate, the negative control was not disturbed, and the specificity was strong. [Conclusions] The established TLC method was simple, specific and reproducible, and could be used for the quality control of Xunxi Shujin Decoction.
Key words Xunxi Shujin Decoction, Radix Aconiti, Radix Aconiti Kusnezoffii, Caesalpinia sappan L., Radix zanthoxyli, Thin layer chromatography
1 Introduction
Xunxi Shujin Decoction is an external lotion for orthopedics in the First Affiliated Hospital of Guangxi University of Chinese Medicine. The prescription is composed of 11 medicinal materials, such as Radix Aconiti, Radix Aconiti Kusnezoffii,CarthamustinctoriusL.,RadixzanthoxyliandCaesalpiniasappanL., having the effects of warming meridians and dredging collaterals, promoting blood circulation and removing blood stasis, diminishing inflammation and relieving pain, and having good clinical effects in treating knee osteoarthritis and muscle contracture[1-2]. At present, the quality standard is only routine inspection of preparations, which is still at a relatively low level. In order to better control the quality of Xunxi Shujin Decoction and ensure the safety and effectiveness of clinical medication, under the guidance of traditional Chinese medicine theory, this study took principal medicine as the first choice, and took into account the adjuvant, adjuvant and conductant medicine for TLC qualitative analysis, in order to provide scientific basis for establishing and perfecting the quality standard of Xunxi Shujin Decoction.
2 Instruments and reagents
2.1 InstrumentsElectric thermostatic water bath (Shanghai Qixin Scientific Instrument Co., Ltd., DHG-9240A); SQP analytical balance (Sartorius Scientific Instrument); ultrasonic cleaning machine with stepless power adjustment (Xiaomei Ultrasonic Instrument Kunshan Co., Ltd., XM-P06H).
2.2 Drugs and reagentsMesaconitine (Chengdu Maidesheng Technology Co., Ltd., batch number: RP200325, purity: 99.83%); hypaconitine (Chengdu Maidesheng Technology Co., Ltd., batch number: RP201009, purity: 99.03%);C.sappanL. (China Food and Drug Research Institute, batch number: 121067-201807);R.zanthoxyli(China Food and Drug Research Institute, batch number: 121014-201204); Xunxi Shujin Decoction was provided by the First Affiliated Hospital of Guangxi University of Chinese Medicine.
3 TLC identification
3.1 TLC identification method of Radix Aconiti and Radix Aconiti Kusnezoffii
3.1.1Preparation of reference solution. Right amount of mesaconitine and hypaconitine was taken, and dichloromethane: isopropanol (1:1) was added to the mixed solution to prepare a mixed solution containing l mg of mesaconitine and hypaconitine per l mL, which was used as the control solution.
3.1.2Preparation of test solution. 50 mL of Xunxi Shujin Decoction solution was taken, alkalized with 2 mL of aqueous ammonia, mixed with 100 mL of dichloromethane, and treated with ultrasound for 30 min. The aqueous solution was discarded, evaporated in a water bath, and the residue was dissolved in 1 mL of mixed solution of dichloromethane: isopropanol (1:1).
3.1.3Preparation of control solution. 6 g of Radix Aconiti powder and 2 g of Radix Aconiti Kusnezoffii powder were taken, wetted with 2 mL of aqueous ammonia, 100 mL of dichloromethane was added, ultrasonic treatment was carried out for 30 min. After being filtered, evaporated in water bath, the residue was dissolved with 1 mL of mixed solution of dichloromethane: isopropanol (1:1).
3.1.4Negative solution without Radix Aconiti and Radix Aconiti Kusnezoffii. The prescription medicinal materials without Radix Aconiti and Radix Aconiti Kusnezoffii were taken, and the negative solution of Shujin Decoction without Radix Aconiti and Radix Aconiti Kusnezoffii was prepared according to the prescription process, and then treated according to the preparation method of the sample solution, to obtain the negative solution without Radix Aconiti and Radix Aconiti Kusnezoffii.
3.1.5Durability investigation. (i) Investigation of the effect of temperature. The thin layer development at low temperature (5-10 ℃) and high temperature (30-35 ℃) was investigated respectively. The results showed that there was no obvious difference between the spots of the tested samples at these two temperatures and those at normal temperature (Fig.1).
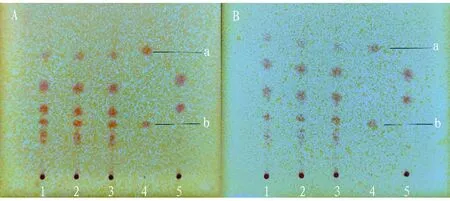
Note: A: t is 7 ℃, RH is 90%, B: t is 32 ℃, RH is 56%, 1-3: test solution, 4: mesaconitine (a), hypaconitine (b) reference solution, 5: negative control solution without Radix Aconiti and Radix Aconiti Kusnezoffii.
(ii) Investigation of the influence of humidity. It was developed under the conditions of low relative humidity and high relative humidity, and the results showed that there was no obvious difference between the spots of the tested samples at these two temperatures and those under indoor conditions (Fig.2).
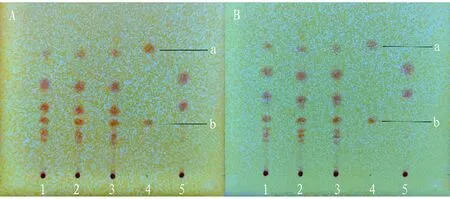
Note: A: t is 26 ℃, RH is 28%, B: t is 26 ℃, RH is 87%, 1-3: test solution, 4: mesaconitine (a), hypaconitine (b) reference solution, 5: negative control solution without Radix Aconiti and Radix Aconiti Kusnezoffii.
(iii) Investigation of thin-layer plate from different manufacturers. The influence of thin-layer plates from different manufacturers on thin-layer development was investigated. The thin-layer plates from different manufacturers showed good separation between spots, moderate Rf value and clear spots, and could be used for TLC identification of Radix Aconiti and Radix Aconiti Kusnezoffii in Xunxi Shujin Decoction (Fig.3).

Note: A: Qingdao Yonghai Silica Gel G Plate, B: Qingdao Hailang Silica Gel G Plate, C: Qingdao Ocean Silica Gel G Plate, 1-3: test solution, 4: mesaconitine (a), hypaconitine (b) reference solution, 5: negative control solution without Radix Aconiti and Radix Aconiti Kusnezoffii.
3.1.6Sample spotting and development. The experiment was carried out in accordance with the method underTLC0502inFourGeneralPrinciplesofChinesePharmacopoeia(2020Edition). Ten batches of test solution, negative solution without Radix Aconiti and Radix Aconiti Kusnezoffii, control solution of Radix Aconiti and Radix Aconiti Kusnezoffii and mixed control solution of mesaconitine and hypaconitine were absorbed and placed on the same silica gel G plate respectively. The thin-layer plate was saturated about 30 min in a developing tank containing aqueous ammonia, the developer n-hexane-ethyl acetate-methanol (8:6:1) was added, developed (distance of 8 cm), taken, dried, and sprayed with dilute potassium bismuth iodide until the spot was clear, and observed in sunlight. Ten batches of Xunxi Shujin Decoction samples had clear target spots and good resolution, and showed spots of the same color at the same horizontal position as the control medicinal materials and reference substances (Fig.4).

Note: t: 26 ℃, RH: 60%, 1-10: test solution, 11: Radix Aconiti control solution, 12: Radix Aconiti Kusnezoffii control solution, 13: mesaconitine (a), hypaconitine (b) control solution, 14: negative control solution without Radix Aconiti and Radix Aconiti Kusnezoffii.
3.2 TLC identification method of R. zanthoxyli
3.2.1Preparation of test solution. 40 mL of samples were placed in a conical bottle, 1 mL of aqueous ammonia was added, 80 mL of dichloromethane was added, and ultrasonic treatment was performed for 30 min. The liquid was separated, the dichloromethane was evaporated in a water bath, and the residue was dissolved with 1 mL of anhydrous ethanol.
3.2.2Preparation of control solution. 0.5 g ofR.zanthoxylipowder was taken, wetted with 2 mL of aqueous ammonia, mixed with 40 mL of dichloromethane, treated with ultrasound for 30 min, filtered, and evaporated to dryness in dichloromethane liquid water bath. The residue was dissolved with 1 mL of anhydrous ethanol to obtainR.zanthoxylicontrol solution.
3.2.3Negative solution withoutR.zanthoxyli. The prescription medicinal materials ofR.zanthoxyliwere taken, and the negative solution of Shujin Decoction withoutR.zanthoxyliwas prepared according to the prescription process, and then treated as negative solution withoutR.zanthoxyliaccording to the preparation method of the test solution.
3.2.4Durability investigation. (i) Investigation of the effect of temperature. Thin layer development was investigated at low temperature (5-10 ℃) and high temperature (30-35 ℃). The results showed that there was no obvious difference between the spots of the tested samples at these two temperatures and those at normal temperature, which indicated that the temperature had little influence on the thin layer development effect (Fig.5).
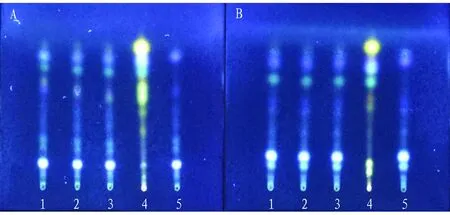
Note: A: t is 7 ℃, RH is 97%, B: t is 34 ℃, RH is 54%, 1-3: test solution, 4: control solution of R. zanthoxyli, 5: negative control solution without R. zanthoxyli.
(ii) Investigation of the effect of humidity. It was developed under the conditions of low relative humidity and high relative humidity respectively. The results showed that there was no significant difference between the spots at these two temperatures and those developed under indoor conditions, indicating that humidity had little effect on the development effect of thin layer (Fig.6).
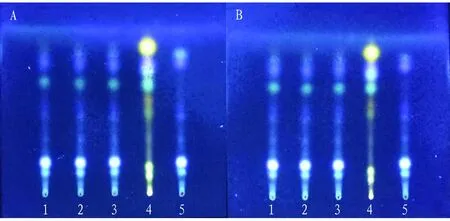
Note: A: t is 26 ℃, RH is 28%, B: t is 26 ℃, RH is 87%, 1-3: test solution, 4: control solution of R. zanthoxyli, 5: negative control solution without R. zanthoxyli.
(iii) Investigation of thin-layer plates from different manufacturers. The influence of thin-layer plates from different manufacturers on thin-layer development was investigated. The thin-layer plates from different manufacturers showed good separation between spots, moderate Rf value and clear spots, and they can be used for thin-layer identification ofR.zanthoxyliin Xunxi Shujin Decoction (Fig.7).
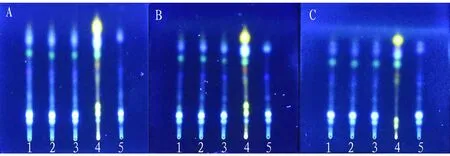
Note: A: Qingdao Yonghai Silica Gel G Plate, B: Qingdao Hailang Silica Gel G Plate, C: Qingdao Ocean Silica Gel G Plate, 1-3: test solution, 4: control solution of R. zanthoxyli, 5: negative control solution without R. zanthoxyli.
3.2.5Sample spotting and development. The experiment was carried out in accordance with the method underTLC0502inGeneralTechnicalRequirementsofVolumeIVofChinesePharmacopoeia(2020Edition). Ten batches of test solution, negative solution withoutR.zanthoxyli, and control solution ofR.zanthoxyliwere absorbed and placed on the same silica gel G plate respectively. Trichloromethane:methanol:aqueous ammonia (30:1:0.2) was used as developing agent, saturated for 20 min, developed with a distance of 8 cm, taken out, dried, sprayed with 10% sulfuric acid ethanol, and examined under ultraviolet lamp at 365 nm. Ten batches of samples had clear spots and good separation, and showed spots of the same color at the same horizontal position as the control medicinal materials and reference substances (Fig.8).
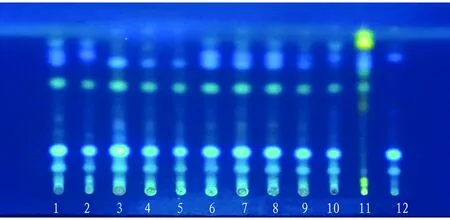
Note: t: 24 ℃, RH: 65%, 1-10: test solution, 11: control solution of R. zanthoxyli, 12: negative control solution without R. zanthoxyli.
3.3 TLC identification method of C. sappan L.[3-4]
3.3.1Preparation of test solution. 10 mL of samples were taken, evaporated in a water bath, mixed with 40 mL of anhydrous ethanol, and treated with ultrasound for 30 min. After filtration, the anhydrous ethanol liquid was evaporated in a water bath, and the residue was dissolved with 3 mL of anhydrous ethanol.
3.3.2Preparation of control solution. 0.2 g ofC.sappanL. powder was taken, mixed with 30 mL of anhydrous ethanol, and treated with ultrasound for 30 min. After filtration, anhydrous ethanol was evaporated in water bath, and the residue was dissolved with 1 mL of anhydrous ethanol to obtain control solution ofC.sappanL. medicinal materials.
3.3.3Negative solution withoutC.sappanL. The prescription medicinal materials withoutC.sappanL. were taken, and the negative Shujin Decoction solution withoutR.zanthoxyliwas prepared according to the prescription process, and then treated according to the preparation method of the test solution, to obtain the negative solution withoutC.sappanL.
3.3.4Durability investigation. (i) Investigation of the effect of temperature. The thin layer development at low temperature (5-10 ℃) and high temperature (30-35 ℃) was investigated respectively. The results showed that there was no obvious difference between the spots of the tested samples under these two temperature conditions and those at normal temperature, indicating that temperature had little influence on the thin layer development effect (Fig.9).

Note: A: t is 7 ℃, RH is 98%, B: t is 33 ℃, RH is 57% , 1-3: test solution, 4: control solution of C. sappan L., 5: negative control solution without C. sappan L.
(ii) Investigation of the effect of humidity. It was developed under the conditions of low relative humidity and high relative humidity, and the results showed that there was no obvious difference between the spots of the tested samples under these two temperature conditions and those under indoor conditions, indicating that humidity had little influence on the thin layer development effect (Fig.10).
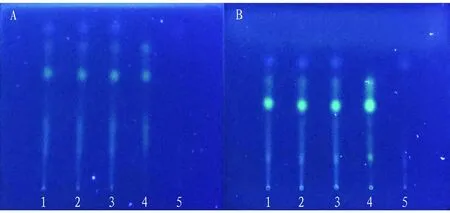
Note: A: t is 24 ℃, RH is 26%, B: t is 24 ℃, RH is 90% , 1-3: test solution, 4: control solution of C. sappan L., 5: negative control solution without C. sappan L.
(iii) Investigation of thin-layer plates from different manufacturers. The influence of thin-layer plates from different manufacturers on thin layer development was investigated. The thin-layer plates from different manufacturers showed good separation between spots, moderate Rf value and clear spots, and they can be used for TLC identification ofC.sappanL. in Xunxi Shujin Decoction (Fig.11).
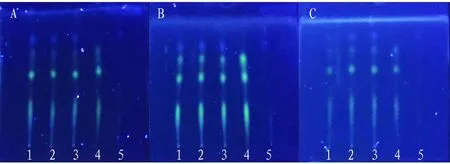
Note: A: Qingdao Yonghai Silica Gel G Plate, B: Qingdao Hailang Silica Gel G Plate, C: Qingdao Ocean Silica Gel G Plate, 1-3: test solution, 4: control solution of R. zanthoxyli, 5: negative control solution without R. zanthoxyli.
3.3.5Sample spotting and development. The experiment was carried out in accordance with the method underTLC0502ineneralTechnicalRequirementsofVolumeIVofChinesePharmacopoeia(2020Edition). Ten batches of test solution, negative solution withoutC.sappanL., and control solution ofC.sappanL. were absorbed and placed on the same silica gel G plate separately. Dichloromethane-acetone-formic acid (8:4:1) was used as developing agent, saturated for 20 min, developed with a distance of 8 cm, taken out, dried, and examined under ultraviolet lamp at 365 nm. Ten batches of samples had clear spots and good separation, and showed spots of the same color at the same horizontal position as the control medicinal materials and reference substances (Fig.12).
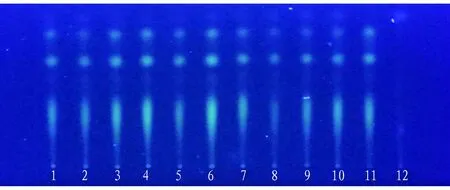
Note: t: 25 ℃, RH: 60%, 1-10: test solution, 11: control solution of R. zanthoxyli, 12: negative control solution without R. zanthoxyli.
4 Discussion and conclusion
4.1 TLC optimization of Radix Aconiti and Radix Aconiti Kusnezoffii[5]The effects of three developing agents on TLC identification of Radix Aconiti and Radix Aconiti Kusnezoffii in Xunxi Shujin Decoction were investigated. The results showed that toluene-ethyl acetate-diethylamine (7:2:0.5) as developing agent produced edge effect and the spots were not on the same horizontal line; n-hexane-ethyl acetate-methanol (6.4:3.6:1) as developing agent had high Rf value of hypaconitine; n-hexane-ethyl acetate-methanol (8:6:1) had the best Rf value and separation, so n-hexane-ethyl acetate-methanol (8:6:1) was selected as developing agent for TLC identification of Radix Aconiti and Radix Aconiti Kusnezoffii. Two developing methods, iodine vapor and dilute bismuth potassium iodide, were investigated respectively. The results showed that the development of iodine vapor was negative and disturbed. Spraying dilute bismuth potassium iodide as chromogenic agent in daylight showed high spot resolution and good separation, which could meet the test conditions.
4.2 TLC optimization ofR.zanthoxyli[6-7]Referring to related literature, when petroleum ether-trichloromethane-methanol-aqueous ammonia (2:13:1:0.1) was used as developing agent, the spot resolution effect was poor, the Rf value was low, and the spot fluorescence was weak; when xylene:ethyl acetate:methanol:isopropanol:aqueous ammonia (10:2.5:2.5:0.5:0.05) was used as developing agent, the spot color of the tested sample was different from that of the control medicine; when chloroform:methanol:aqueous ammonia (30:1:0.2) was used as developing agent, the spot resolution was good, Rf value was moderate, the tested sample and the control medicine ofR.zanthoxylishowed the same spot at the same horizontal position, and the fluorescence color was consistent and clear, indicating that it was the best developing agent for TLC identification. In terms of color development conditions, after drying, the spot fluorescence was very light and not obvious when observed directly with ultraviolet lamp at 365 nm; after spraying 10% sulfuric acid ethanol, the fluorescence spots were clear and the specificity was strong when observed with ultraviolet lamp at 365 nm.
4.3 TLC optimization ofC.sappanL.[3-4]The effects of three developing systems on TLC were compared. It was found that the upper solution of toluene:ethyl acetate:water:formic acid (20:10:1:1) was negative and disturbed, which could not meet the experimental conditions. When trichloromethane-acetone-formic acid (8:4:1) was used as developing agent, it could meet the TLC identification requirements ofC.sappanL., but the spots had tailing phenomenon, Rf value was low, and the effect was not good; using dichloromethane-acetone-formic acid (8:4:1) as developing agent, the spot resolution was good and Rf value was moderate. The test sample andC.sappanL. control medicine showed the same spots at the same horizontal position, and the fluorescence color was consistent and clear; when investigating the influence of color development methods on TLC, it was found that the samples and control materials had the same color spots at the same horizontal position under sunlight, but the Rf value was low. Under the ultraviolet lamp at 365 nm, the spot fluorescence was clear, the Rf value was moderate, and it was negative without interference.
The TLC qualitative identification method for Radix Aconiti and Radix Aconiti Kusnezoffii,C.sappanL. andR.zanthoxyliin Xunxi Shujin Decoction was established in this study, and it was simple, reproducible and specific, and could provide experimental basis and reference for the quality control of Xunxi Shujin Decoction.
杂志排行
Medicinal Plant的其它文章
- Effects of Exogenous Plant Hormones on Growth Status and Secondary Metabolism of Houttuynia cordata Thunb.
- Preparation of 20 (S)-protopanaxadiol PLGA Nanoparticles
- A Network Pharmacology Study on Active Components and Targets of Citri Reticulatae Pericarpium for Treating Keloids
- Pharmacognostic Identification of Hedyotis auricularia and Mitracarpus villosus
- Determination of Salvianolic Acid B in Yiqi Huayu Prescription by HPLC
- Fresh Processing Technology in Polygonatum odoratum Production Area and Its Comparison with Traditional Processing
