Cactus-like NC/CoxP electrode enables efficient and stable hydrogen evolution for saline water splitting
2024-03-07CHENXuZHAOJinyuZHANGWenshengWANGXiaomin
CHEN Xu,ZHAO Jin-yu,ZHANG Wen-sheng,WANG Xiao-min
(College of Materials Science and Engineering,Taiyuan University of Technology,Taiyuan 030024, China)
Abstract:Designing efficient and robust catalysts for hydrogen evolution reaction (HER) is imperative for saline water electrolysis technology.A catalyst composed of CoxP nanowires array with N-doped carbon nanosheets (NC) was fabricated on Ni foam(NF) by an in-situ growth strategy.The material is designated as NC/CoxP@NF.In the preparation process,Co(OH)2 nanowires were transformed into a metal organic framework of cobalt (ZIF-67) on NF by the dissolution-coordination of endogenous Co2+ and 2-methylimidazole.The resulting cactus-like microstructure gives NC/CoxP@NF abundant exposed active sites and ion transport channels,which improve the HER catalytic reaction kinetics.Furthermore,the interconnected alternating nanowires and free-standing nanosheets in NC/CoxP@NF improve its structural stability,and the formation of surface polyanions (phosphate) and a NC nanosheet protective layer improve the anti-corrosive properties of catalysts.Thus,the NC/CoxP@NF has an excellent performance,requiring overpotentials of 107 and 133 mV for HER to achieve 10 mA cm−2 in 1.0 mol L−1 KOH and 1.0 mol L−1 KOH+0.5 mol L−1 NaCl,respectively.This in-situ transformation strategy is a new way of constructing highly-efficient HER catalysts for saline water electrolysis.
Key words: Hydrogen evolution reaction;Nanoarchitecture;Transition metal phosphide;Chlorine-corrosion resistance;Saline water splitting
1 Introduction
Currently,the vast and inexhaustible reserves of seawater have made it a more appealing option for large-scale hydrogen production through water splitting[1–4].Unfortunately,the complex composition of seawater has rendered direct seawater electrolysis with high selectivity and stability a highly challenging endeavour[5–6].Particularly,the sluggish hydrogen evolution reaction (HER) kinetics significantly compromises the efficiency of seawater electrolysis[7–9].
However,the scarcity and inferior stability severely limits the large-scale applications of Pt-based catalysts in seawater conditions[10–11].Therefore,developing highly active and robust noble-metal-free electrocatalysts is urgently needed for sustainable hydrogen production in seawater electrolysis[12–13].Among the various materials,transition metal phosphides (TMPs) demonstrate superior activity for HER due to the easily adjustable electronic structure and improved conductivity.Most importantly,the negatively charged P sites can capture protons and modify the adsorption energy of intermediate products on the catalyst surface to realize accelerated HER kinetics[14–17].Particularly,the Gibbs free energy(△GH*) of hydrogen adsorption on CoP surface is close to 0,demonstrating the moderate interaction with the intermediates on its surface,making it a promising candidate for efficient HER catalysts[18–19].However,due to the insufficiency of active sites and poor stability,its catalytic activity and stability must be optimized further to meet the demands for practical application.Some constructive strategies such as morphological engineering[15],electronic structure engineering[20]and protective layer construction[21–23],have been proposed to enhance the electrocatalytic activity and increase tolerance of CoP.Especially,a porous carbon layer with high specific surface area in principle should be able expose more active sites.More importantly,it can protect the components from dissolution by the physical barrier effect[24–26].Therefore,the combination of phosphides and carbon materials shows advantages in enhancing activity and durability of TMPs-based electrocatalysts[24].Metalorganic frameworks (MOFs) assembled by metal nodes and organic ligands,featuring high porosity and well-defined and tailorable structures,are considered as ideal candidates for the construction of TMPs.
Inspired by the previous studies,a self-supporting phosphide electrode combined with carbon nanosheets (NC/CoxP@NF) was successfully constructed via in-situ formation of ZIF-67 on nickel foam (NF) and subsequent phosphorization approach.The cactus-shaped microstructure endows NC/CoxP@NF-10 with large surface areas and more available active sites.As a result,NC/CoxP@NF-10 exhibits the excellent HER activity in 1 mol L−1KOH and 1.0 mol L−1KOH+0.5 mol L−1NaCl with the overpotential of 107 and 133 mV at the current density of 10 mA cm−2,respectively.Furthermore,the NC/CoxP@NF-10 catalyst also displays superior durability in alkaline simulated seawater due to the enhanced mechanical strength and the repulsion of chlorine ions by polyanions.
2 Experimental
2.1 Chemicals
NaH2PO2·H2O (AR,99%),urea (CO(NH2)2,99%),NH4F (AR,96%),Co(NO3)2·6H2O (AR,99%),2-methylimidazole (2-MeIM,98%),potassium hydroxide (KOH,≥ 85%),HCl (3 mol L−1),nickel foam(NF thickness: 1.5 mm),sodium chloride (NaCl,AR,≥99.5%) and anhydrous ethanol (≥ 99.5%) were obtained from Aladdin.20% (mass fraction) commercial Pt/C was purchased from Johnson Matthey.All reagents and chemicals purchased and utilized without further purification treatment.Distilled water was utilized in all experiments.
2.2 Material synthesis
2.2.1 Synthesis of Co(OH)2@NF
The oxide layer on NF surface was eliminated through sequential sonication in 3 mol L−1HCl solution,distilled water and ethanol,respectively.Each sonication step took 15 min.Co(NO3)2·6H2O,NH4F and CO(NH2)2were dissolved in distilled water and continuously stirred to form a homogeneous solution.Subsequently,the cleaned NF was immersed in the above solution and then transferred to a 50 mL Teflon-lined stainless-steel autoclave and heated in an oven at 120 °C for 12 h.After cooling down naturally to room temperature,a pink-colored thin film formed on the NF substrate.Then,the products were rinsed alternately with deionized water and ethanol,and dried at 60 °C for 12 h in vacuum.The products are noted as Co(OH)2@NF.
2.2.2 Synthesis of Co-MOF@NF
Co(OH)2@NF was soaked in 2-MeIM solution(1 mol L−1) for 5,10 and 15 h.During the process,Co2+in Co(OH)2coordinated with 2-MeIM ligand to convert Co(OH)2@NF to Co-MOF.Then,the obtained samples were thoroughly cleaned with ethanol and distilled water,and then were dried at 60 °C for 12 h to form the target products.They were marked as Co-MOF@NF-5,Co-MOF@NF-10,Co-MOF@NF-15,respectively.
2.2.3 Synthesis of NC/CoxP@NF
The dried Co(OH)2@NF and NaH2PO2·H2O were placed into 2 separate porcelain boats,and then calcined at 350 °C for 2 h with the heating rate of 2 °C min−1under N2atmosphere.Afterwards it was cooled and washed with ultrapure water and ethanol,then dried at 60 °C and labelled as CoxP@NF.The fabrication process of NC/CoxP@NF is similar to that of the CoxP@NF.Co-MOF@NF-5,Co-MOF@NF-10 and Co-MOF@NF-15 after phosphorization were labelled as the NC/CoxP@NF-5,NC/CoxP@NF-10 and NC/CoxP@NF-15,respectively.
2.3 Material characterization
Scanning electron microscopy (SEM,ZEISS Gemini 300) was used to analyze the microstructure of materials.Transmission electron microscopy(TEM) images were obtained using JEOL JEM 2100F.X-ray diffraction diffractometer (XRD,Smart Lab) was conducted to investigate the crystal structure of samples.Raman spectra of catalysts were recorded using Renishaw with the excitation wavelength of 532 nm.X-ray photoelectron spectroscopy (XPS,Thermo Fisher) was employed to reveal the valence states of samples’ surfaces.The surface area of the samples was analyzed by nitrogen adsorption-desorption isotherms (ASAP-2460).Zeta potential measurements were carried out using DTS1070 cell on Malvern Zetasizer Pro.
2.4 Electrochemical test
The electrochemical measurements of samples were conducted on the CHI760E electrochemical workstation.The obtained electrocatalysts (2 cm ×1 cm) were directly used as a working electrode,an Ag/AgCl as the reference electrode and graphite rod was served as the counter electrode.The electrochemical tests were performed in 2 different electrolytes,including 1 mol L−1KOH,1 mol L−1KOH+0.5 mol L−1NaCl (alkaline simulated seawater).For comparison,the Pt/C (5 mg,20% mass fraction) was dispersed in a mixture of ethanol (980 μL),Nafion solution (20 μL)and ultrasonicated for 30 min to form a uniform catalyst ink.Then,this catalyst ink was loaded on the clean NF (0.5 mg cm−2) to obtain the working electrode.
The linear sweep voltammetry (LSV) curves were obtained at a scan rate of 5 mV s−1with 90% IR correction in the range 0-1 V (vs Ag/AgCl).Before the LSV measurements,30 cyclic voltammetry (CV)cycles were performed to make the catalysts more active and stable.Cyclic voltammetry (CV) curves were recorded in the potential range from 0.2 to 0.3 V with a sweep rate from 20-120 mV s−1.The double-layer capacitance (Cdl) values were derived from the CV curves in non-Faradaic potential region.The electrochemical impedance spectroscopy (EIS) measurements were performed at the frequency from 100 kHz to 0.01 Hz at an AC amplitude of 5 mV.The longterm durability test was evaluated by chronoamperometry at a current density of −100 mA cm−2.
3 Results and discussion
As illustrated in Fig.1a,the synthesis process of NC/CoxP@NF electrocatalyst was proceeded by hydrothermal and phosphating processes.Firstly,the Co(OH)2nanowires grow on the surface of NF by hydrothermal method.Then,Co(OH)2/NF was soaked in 2-MeIM solution,and Co-MOF nanosheets were formed in situ on NF.Subsequently,it was subjected to phosphorization in a tube furnace to obtain NC/CoxP@NF[18].The N-doped carbon nanosheets derived from the Co-MOF after the low-temperature phosphating process is conducive to the charge transfer and the improvement of conductivity of catalysts[25].The SEM images of CoxP@NF (Fig.1b-d) demonstrate that nanowires are uniformly and densely growing on NF.Additionally,Fig.1e-m demonstrates Ndoped carbon nanosheets formed and increased gradually with extended soaking duration in 2-MeIM solution.The edge with the strongest electric field attracts more Co2+than other areas,forming CoxP nanowires on the edge[27].Hence,the unique cactus-like structure composed of CoxP nanowires and NC nanosheets was achieved due to the electric field distribution.As shown in Fig.1h-j,NC/CoxP@NF-10 exhibits the densely aligned nanosheets with needle-like CoxP nanowires,creating a hierarchical interconnected structure.Additionally,the EDS mapping images in Fig.S1 verify the uniform distribution of P,N,Co and C elements in NC/CoxP@NF-10,in which the nitrogen content is 1.42% (mass fraction) (Table S1).Of note,the nanosheets are interspersed with CoxP nanowires and interlinked,which further increase the specific surface area,providing more active sites and ion transport channels to facilitate the catalytic reactions.Furthermore,the interconnected carbon layer can protect the inner core from corrosion of simulated seawater and consequent aggregation.Fig.1k-m shows that further prolonging the soaking time results in superfluous carbon nanosheets that completely fill the void within the CoxP nanowires.This is detrimental to the availibity of exposed active sites and mass transfer during catalysis.The mechanical strength is a critical factor in catalyst stability.Therefore,catalysts were immersed in water and sonicated for 1.5 h to assess the mechanical strength of materials.It is noteworthy that more CoxP@NF peeled from the surface of NF,while the NC/CoxP@NF-10 electrocatalyst maintained the initial dark color after sonication for 30 min (Fig.S2),implying the high mechanical strength of NC/CoxP@NF-10.
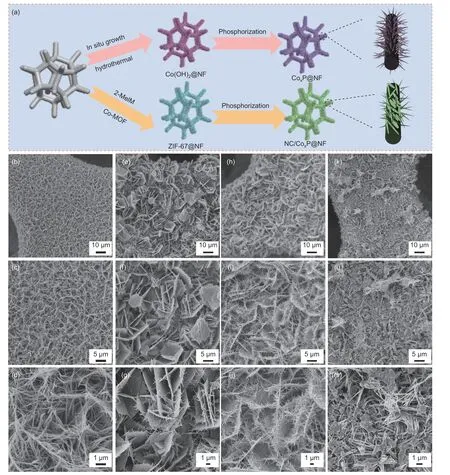
Fig.1 (a) Schematic illustration of catalysts synthesis.SEM images at different magnifications: (b-d) CoxP@NF;(e-g) NC/CoxP@NF-5;(h-j) NC/CoxP@NF-10 and (k-m) NC/CoxP@NF-15
Transmission electron microscopy (TEM,Fig.2a)further verifies the cactus-like structure of NC/CoxP@NF-10.High-resolution TEM (HR-TEM)of NC/CoxP@NF-10 is shown in Fig.2b,where lattice spacings of 0.22 and 0.20 nm are well indexed to the (111) and (021) crystal planes of Co2P,while 0.18 and 0.27 nm lattice spacings corresponds to the (202)and (011) crystal planes of CoP,indicating the co-existence of Co2P and CoP in the catalyst.The corresponding elemental maps of NC/CoxP@NF-10(Fig.2c) confirm the uniform distributions of the Co,P,N and C elements,which is consistent with Fig.S1.The X-ray diffraction (XRD) patterns were employed to clarify the crystal structures of as-prepared catalysts.As displayed in Fig.2d-e,for CoxP@NF (ignoring the peaks of NF),the peaks at 40.84°,44.86° and 48.38° can be attributed to (111),(021) and (120)crystal planes of Co2P (PDF#54-0413).Besides,the diffraction peaks at 31.60°,48.40° and 56.78° corresponds to the crystal planes of (011),(202) and (301)of CoP (PDF#29-0497)[28–30].For NC/CoxP@NF samples,a characteristic peak centered at 2θof 25°corresponds to the (002) plane of C,indicating the successful conversion of Co-MOF to N-doped carbon layer during phosphating process.Furthermore,the intensity of diffraction peak of carbon increases with increased impregnation time.Raman spectra in Fig.2f revealed the graphitization degree of the carbon framework.Two distinct peaks at 1 350 cm−1and 1 580 cm−1can be assigned to theDband (disordered sp3defective graphite carbon) andGband (ordered sp2graphitic carbon),respectively[31–32].TheID/IGratio is a reliable indicator of graphitic degree.For the spectrum of CoxP@NF,no obvious graphitic peaks appeared.While,NC/CoxP@NF-10 exhibited the expectedDandGpeaks,with anID/IGvalue of 0.98,indicating the appropriate graphitization degree relative to other control samples.N2adsorption-desorption measurements were applied to analyze the BET surface area and pore structure of the catalysts.The isotherm of NC/CoxP@NF-10 features type-IV curves,exhibiting the significant hysteresis loops at higher relative pressure,suggesting the presence of mesopores(Fig.S3).The hierarchical structure (Fig.2g) of NC/CoxP@NF-10 (as will be discussed later) favors adequate contact with the electrolyte,accelerating the mass transfer of the HER.
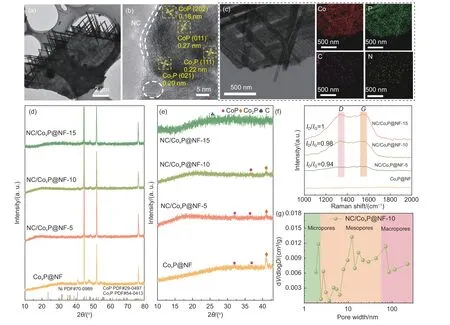
Fig.2 (a) TEM image;(b) HRTEM image and (c) the corresponding elemental mappings of NC/CoxP@NF-10.(d) XRD patterns and(e) the magnified diffraction peaks at the range of 10°-43° of all catalysts.(f) Raman spectra of NC/CoxP@NF-5,NC/CoxP@NF-10 and NC/CoxP@NF-15.(g) Pore distribution curve of NC/CoxP@NF-10
The elemental composition and surface valence state of the catalyst were investigated by the X-ray photoelectron spectroscopy (XPS).The obvious peaks of Co,P,N and C elements manifest in the full survey of XPS spectra of NC/CoxP@NF-10 (Fig.S4).As revealed in Fig.3a,the Co 2p spectra of NC/CoxP@NF-10 presents 3 pairs,which can be attributed to Co3+(781.36 and 796.36 eV),Co2+(782.95 and 797.95 eV) and Co―P bond (778.72 and 793.72 eV).Besides,the two peaks located at 783.81 and 802.4 eV are assigned to satellite peaks[33–34].However,for CoxP@NF,the binding energies of 782.1 and 798.71 eV are attributed to Co2+,while the two other peaks located at 778.63 and 793.63 eV are assigned to Co―P bond.It should be noted that the peak position of Co 2p3/2and Co 2p1/2of all samples with N-doped carbon layer exhibit a negative shifted compared with CoxP@NF.This means that when the carbon layer is introduced,the electronic coupling and charge density of CoxP are redistributed[25].The significant electrical interactions between CoxP and NC is beneficial for HER activity due to the accelerated electron transfer[35].For the high-resolution P 2p spectra of NC/CoxP@NF-10 (Fig.3b),two peaks at 129.47 and 130.31 eV correspond to the P 2p3/2and P 2p1/2,and the binding energy of 134.69 eV can be assigned to P―O bonds,which derives from the surface oxidation.Compared with that of CoxP@NF,NC/CoxP@NF-10 (129.47 eV) exhibits a negative shift of 0.76 eV (130.23 eV)[36–37].In addition,the intensity of the M―P peak in the CoxP@NF spectrum is relatively lower than that for NC/CoxP@NF-10,suggesting the stronger combination of P with Co in NC/CoxP@NF-10.Fig.3c reveals the high-resolution C 1s peak of NC/CoxP@NF-10,which can be fitted with 3 peaks,originating from the C―C,C―N/C―P and O―C=O group[38–39].Moreover,Fig.3d presents the N 1s high-resolution spectra of NC/CoxP@NF-10.The peaks located at 398.09 and 401.37 eV are deconvoluted into the pyridinic N and graphitic N,respectively[40–41].The above results put together confirm the construction of N-doped carbon structures in NC/CoxP@NF-10.

Fig.3 High-resolution XPS spectra of (a) Co 2p,(b) P 2p,(c) C 1s and (d) N 1s in catalysts
The electrocatalytic HER activities of all catalysts were estimated in 1 mol L−1KOH.As observed in Fig.4a,except for the Pt/C (29 mV),NC/CoxP@NF-10 shows the optimum activity with overpotential of 107 mV at 10 mA cm−2,which is lower than those of CoxP@NF (170 mV),NC/CoxP@NF-5 (113 mV),NC/CoxP@NF-15 (109 mV) and bare NF (235 mV),suggesting the advantage of carbon layer coupled with phosphide in enhancing the HER catalytic activity.Besides,NC/CoxP@NF-10 exhibits the superior electrocatalytic HER activity compared to catalysts reported in the literature in 1 mol L−1KOH (Table S2).As shown in Fig.4b,the Tafel slope value of NC/CoxP@NF-10 is 97 mV dec−1,lower than those of NC/CoxP@NF-15 (106 mV dec−1),CoxP@NF (150 mV dec−1),NC/CoxP@NF-5 (127 mV dec−1) and bare NF (160 mV dec−1),demonstrating its superior HER catalytic kinetics.To prove the intrinsic activity of catalysts,the double-layered capacitance (Cdl) were measured,which were derived from the CV curves at different scan rates (Fig.4c and Fig.S5).TheCdlvalues of CoxP@NF,NC/CoxP@NF-5,NC/CoxP@NF-10,NC/CoxP@NF-15,Pt/C and bare NF are 24.9,20.2,27.9,25.1,13.7 and 0.7 mF cm−2,respectively.The higherCdlof NC/CoxP@NF-10 indicates optimization of the nanostructure which makes more active sites available[42].Hence,NC/CoxP@NF-10 exhibits the largest ECSA,implying its high intrinsic activity.Electrochemical impedance spectroscopy (EIS) was performed to assess charge transfer resistance (Rct)from the Nyquist plots,where the smaller semicircle generally represents a smallerRctat the interface between the catalysts’ surface and electrolyte.Obviously,NC/CoxP@NF-10 shows the lowestRctvalues for HER (1.8 Ω,Fig.4d),revealing lower charge transfer resistance and fast charge transfer kinetics,which is in good agreement with the Tafel slope values in Fig.4b.This is mainly because the internally connected transport channels and hierarchical porous structure provided by NC/CoxP@NF-10 reduce the barriers to electron transport,facilitating the mass transfer during HER reactions.

Fig.4 HER performance of all catalysts in 1 mol L−1 KOH solution.(a) Polarization curves.(b) The corresponding Tafel plots.(c) Scan rate dependence of the current densities.(d) EIS Nyquist plots of all electrodes (Inset is the equivalent circuit diagram)
To simulate an application-relevant environment,the HER activity of the catalysts was also characterized in 1 mol L−1KOH+0.5 mol L−1NaCl electrolyte.In 1 mol L−1KOH+0.5 mol L−1NaCl electrolyte,the HER activity of NC/CoxP@NF-10 remained superior to that in 1 mol L−1KOH,requiring 133 mV to reach the current density of 10 mA cm−2(Fig.5a).Moreover,compared with CoxP@NF (194 mV),NC/CoxP@NF-5(148 mV) and NF (267 mV),the as-prepared NC/CoxP@NF-10 demonstrates lower overpotential at 10 mA cm−2in simulated alkaline seawater.Moreover,the performance of NC/CoxP@NF-10 is superior to other reported materials in 1 mol L−1KOH+0.5 mol L−1NaCl (Table S2).The NC/CoxP@NF-10 exhibits Tafel slope (Fig.5b) of 102 mV dec−1,implying the promoted HER kinetics for NC/CoxP@NF-10 in alkaline simulated seawater.TheCdlvalue of NC/CoxP@NF-10 is much larger than those of other samples (Fig.5c and Fig.S6),suggesting the enhanced possibilities of exposing more active sites for NC/CoxP@NF-10.Furthermore,the Nyquist plots reveal that the charge transfer resistance of NC/CoxP@NF-10 in 1.0 mol L−1KOH+0.5 mol L−1NaCl solutions is 2.2 Ω during HER process,which is lower than that of CoxP@NF (3.2 Ω),NC/CoxP@NF-15 (2.4 Ω),NC/CoxP@NF-5 (2.6 Ω),Pt/C (6.0 Ω) and NF (11.0 Ω) (Fig.5d),confirming the fast charge transfer rate and high electrical conductivity for NC/CoxP@NF-10 during HER process.

Fig.5 Electrocatalytic HER performance of all catalysts in 1 mol L−1 KOH+0.5 mol L−1 NaCl.(a) LSV polarization curves.(b) The corresponding Tafel plots.(c) Scan rate dependence of the current densities.(d) EIS Nyquist plots of all electrodes
The HER durability of catalysts in different electrolytes were evaluated.As displayed in Fig.6a and d,compared with the CoxP@NF (91 and 114 mV),the potentials of NC/CoxP@NF-10 features enhancements of 17 and 67 mV under continuous operations for 55 h in alkaline freshwater and simulated alkaline seawater,respectively.The LSV curves before and after stability test also exhibit the same trend in both the electrolytes (Fig.6 b-c and e-f),consolidating the promising application potential of NC/CoxP@NF-10 in hydrogen production from seawater.Of note,CoxP nanowires and NC nanosheets brace each other in NC/CoxP@NF-10 manifesting enhanced mechanical strength,which consequently protects the catalyst from chemical corrosion during the HER.The XPS spectrum of NC/CoxP@NF-10 after long-term electrochemical cycling experiment were obtained,and its valence state changes were studied.As shown in Fig.S7a,for Co 2p,the peak Co-P intensity of the sample after HER test was significantly reduced.In addition,the peak of Co 2p is negatively shifted,indicating the formation of economically valuable Co substances[43].For P 2p (Fig.S7b),the intensity of P―M peaks(127.39 and 128.23 eV) is reduced.The peak at 132.76 eV is attributed to the surface oxidation of phosphates,implying the formation of PO43−,which act as stabilizers against chlorination chemistry.In addition,the binding energy of P―O peaks shifted negatively,indicating the formation of low valence state P species[7,17].Hence,polyanionic electrostatic repulsion effect from phosphate ions play an important role in enhancing the corrosion resistance of the NC/CoxP@NF-10[44–45].Most importantly,to assess the chloride exclusion and corrosion resistance of the obtained catalysts in saline water electrolyte,their zeta potentials were measured (Fig.S8).Among all catalysts,NC/CoxP@NF-10 showed the highest zeta negative potential (−18.30 mV).From literature it is known that the more negative surface charge,the stronger the repulsion ability and the better the corrosion resistance to Cl−[46–47],which is also the reason why NC/CoxP@NF-10 has superior stability during the saline water splitting process.

Fig.6 1 mol L−1 KOH electrolyte: (a) Chronopotentiometric curves conducted at a constant current density of -100 mA cm−2.The LSV curves before and after stability tests for (b) NC/CoxP@NF-10 and (c) CoxP@NF.1 mol L−1 KOH+0.5 mol L−1 NaCl solution: (d) Stability tests of NC/CoxP@NF-10 and CoxP@NF.LSV curves before and after stability tests for (e) NC/CoxP@NF-10 and (f) CoxP@NF
4 Conclusion
In summary,a self-supporting electrode composed of CoxP nanowires and N-doped carbon nanosheets has been synthesized by a novel in-situ construction strategy.The NC/CoxP@NF-10 exhibited excellent catalytic activity,efficiently and stably catalyzing HER not only in alkaline electrolyte but also in simulated alkaline seawater.It requires an overpotential of 107 mV in 1 mol L−1KOH to achieve a current density of 10 mA·cm−2and 133 mV to deliver 10 mA·cm−2in simulated alkaline seawater.The excellent anti-corrosion properties are attested by its stability for 50 h during operation.The reasons for the enhanced electrochemical activity and chloride corrosion resistance can be ascribed to the synergistic coupling effect between carbon layer and CoxP nanowires.The cactus-shaped microstructure of NC/CoxP@NF-10 exposes plenty of active sites and ensures adequate contact with the electrolyte,while the presence of N atoms simultaneously reduce charge transfer resistance and the reaction barrier of HER.Most importantly,the exceptional corrosion resistance ability can be attributed to the combined blocking effect of high mechanical strength and repelling effect of the PO43−to Cl−.This work provides a new avenue for designing highly efficient and robust HER catalytic material for seawater electrolysis.
Acknowledgements
The authors appreciate the support from National Natural Science Foundation of China (52072256),Key R &D program of Shanxi Province(202102030201006,202202070301016),Central guide local science and technology development funding program (YDZJSX2021B005),Shanxi Province Science and Technology Program Unveiled Bidding Program (20201101016),Science and Technology Innovation base Construction Project of Shanxi Province(YDZJSX2022B003),Natural Science Foundation of Shanxi Province (20210302124308),Shanxi Province Teaching Reform Project (2021YJJG046).
杂志排行
新型炭材料的其它文章
- Carbon-based electrocatalysts for water splitting at high-current-densities: A review
- 序
- Defect engineering of carbon-based electrocatalysts for the CO2 reduction reaction: A review
- Carbon-based metal-free nanomaterials for the electrosynthesis ofsmall-molecule chemicals: A review
- A review of carbon-based catalysts and catalyst supports for simultaneous organic electro-oxidation and hydrogen evolution reactions
- MOF-derived nanocarbon materials for electrochemical catalysis and their advanced characterization
