Carbon-based electrocatalysts for water splitting at high-current-densities: A review
2024-03-07CHENYuxiangZHAOXiuhuiDONGPengZHANGYingjieZOUYuqinWANGShuangyin
CHEN Yu-xiang ,ZHAO Xiu-hui ,DONG Peng ,ZHANG Ying-jie ,ZOU Yu-qin ,WANG Shuang-yin
(1.Faculty of Material Science and Engineering,Kunming University of Science and Technology,Kunming 650093, China;2.National and Local Joint Engineering Laboratory for Lithium-ion Batteries and Materials Preparation Technology,Key Laboratory of Advanced Battery Materials of Yunnan Province,Kunming University of Science and Technology,Kunming 650093, China;3.College of Aerospace Science and Engineering,National University of Defense Technology,Changsha 410073, China;4.State Key Laboratory of Chemo/Bio-Sensing and Chemometrics,College of Chemistry and Chemical Engineering,Advanced Catalytic Engineering Research Center of the Ministry of Education,Hunan University,Changsha 410082, China)
Abstract:Electrocatalytic water splitting is a promising strategy to generate hydrogen using renewable energy under mild conditions.Carbon-based materials have attracted attention in electrocatalytic water splitting because of their distinctive features such as high specific area,high electron mobility and abundant natural resources.Hydrogen produced by industrial electrocatalytic water splitting in a large quantity requires electrocatalysis at a low overpotential at a large current density.Substantial efforts focused on fundamental research have been made,while much less attention has been paid to the high-current-density test.There are many distinct differences in electrocatalysis to split water using low and high current densities such as the bubble phenomenon,local environment around active sites,and stability.Recent research progress on carbon-based electrocatalysts for water splitting at low and high current densities is summarized,significant challenges and prospects for carbon-based electrocatalysts are discussed,and promising strategies are proposed.
Key words: Electrocatalytic water splitting;Carbon-based material;Bubble;High current density;Decouple
1 Introduction
Hydrogen emerges global wide applications in petroleum refining,industrial production of ammonia and methanol,fuel cells,and metallurgical industry[1–2].Compared with steam reforming of methanol and coal gasification that produce hydrogen,electrochemical water splitting has various advantages[3],such as high purity carbon-free hydrogen,use of renewable sources,nontoxic reagent and scale flexibility[4–6].Typically,hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) are the two steps in electrocatalytic water splitting[7].OER requires 4 electrons during the process,while 2 electrons are needed in HER[8].Besides,a larger than the thermodynamic potential should be required in electrolysis due to the intrinsic barriers[9].Reducing the overpotential and enhancing the stability is of enormous economic benefit to realize industrial applications[10–11].
In fundamental laboratory research at low current densities[12],the charge state of carbon-based catalysts can be easily regulated through engineering structure defects,heteroatom engineering,heterojunction engineering,and structure regulation[13].For example,defective carbon materials and carbon-based noble/non-noble metal materials are potential substitutes for noble metal catalysts due to their multiple superiorities[14],such as excellent electronic conductivity,high surface area,excellent resistance to corrosion,low cost and structural flexibility.Breaking the sp2carbon lattice with electroneutrality can lead to uneven charge distribution in the carbon matrix that serve as new electrocatalytic active sites.A series of defective carbon electrocatalysts exhibiting superior HER or OER performance have been synthesized by plasma etching,oxidation,irradiation,heteroatom removal[10],etc.The carbon-based catalysts are able to achieve more excellent electrocatalytic performance by the modification with metal species.Besides,the charge transfer between the carbon host and metal or metal compounds can regulate the electronic structure of each component[15–16].Owing to the modification by metal species,carbon-based catalysts can achieve increased electrocatalytic performance.
The interconnection of the charge transfer path on carbon-based materials and the electrocatalytic water splitting behavior have been reviewed previously[12].Although great progress in advanced electrocatalysts under laboratory conditions has been achieved[17–19],the laboratory research is usually carried out in dilute solution at a low current density[20].Actually,the optimized electrocatalysts,exhibiting an excellent HER and OER performance at a low current density usually,do not display an expected performance at a high current density[21].Because current density greatly impacts the electron transfer at the interface of electrocatalyst-electrolyte and electrocatalyst-support[22].For example,MoS2/Mo2C catalyst delivers a commonplace electrocatalytic performance compared with Pt at a low current density.In contrast,MoS2/Mo2C catalyst requires much lower overpotentials to realize high current densities (191 mV @500 mA cm−2) than commercial Pt foil (567 mV @500 mA cm−2)[22].The above tremendous performance differences demonstrate that the anode and cathode processes of water splitting become complicated at large current densities[23–29].
The difference in testing current density leads to a significant discrepancy in electrocatalyst design and electrode design[30].In a general test,an electrocatalyst mixed with Nafion is usually loaded on a glass carbon substrate to form a thin film.The thin film configuration usually employs low loading levels of less than 1 mg cm−2,as a result well mass transfer and fluent release of generated hydrogen and oxygen bubbles can be realized when the current density is low (<10 mA g−1)[31].When the current density is much higher,the mass transfer resistance,bubble adhesion,the exfoliation of the electrocatalyst from the support,the corrosion and dissolution of the carbonbased electrocatalyst,and the local environment may limit its original performance of gas-involving electrocatalytic water splitting[32].Creating a unique super aerophobic feature at the catalyst surface by rational engineering nano-microstructure and chemical coating is favorable to enhance the HER and OER performance at high current densities[33],which leads to a low interfacial adhesion on the interface of electrolyte-catalyst,enhances the release of generated hydrogen bubbles from the electrocatalyst electrode surface,realizes the effective exposure of electrocatalytic active sites to the surrounding electrolyte.While in industry,the efficient formation of oxygen and hydrogen is of little use unless generated oxygen and hydrogen can be kept separate totally[34].
In this review,the gaps in the fundamental research between low and current densities are summarized.Special attention is paid to discussing the impact of nano-micro structure of electrocatalysts,bubble,binder,electrode local microenvironment,and the decoupling of HER and OER on the electrocatalytic performance of water splitting (Fig.1).Finally,the challenges,strategies and perspectives are proposed to achieve high activity and durable carbon-based materials for electrocatalytic water splitting at high-currentdensities.

Fig.1 Schematic diagram of the topics covered in this review
2 Electrocatalytic water splitting
HER and OER occur at the surface and interfaces of anode-electrolyte and cathode-electrolyte,respectively (Fig.2).And the reactions at acidic,basic,and neutral electrolytes are listed as follows[35]:

Fig.2 Typical water electrolysis cell
HER is a two-step reaction.Two mechanisms of Volmer-Tafel and Volmer-Heyrovsky mechanisms are presented to describe HER in an aqueous electrolyte.The high H+content and fast dynamic of intermediate hydrogen atoms in an acidic solution make it more favorable for HER,while a basic electrolyte is more favorable for OER.
3 Advance in carbon-based electrocatalysts at low current densities
Benefiting from the low cost,environmental friendliness,and strong resistance to the harsh acid and alkali environment,carbon-based materials have rapidly been developed for electrocatalytic water splitting by engineering defective carbon materials[36],non-metal[37]and metal doping[38],and anchoring/loading metal species on carbon-based substrates[39].
Defective carbon materials with topological defects,edge defects,vacancy defects,and complex defects deliver increased electrocatalytic activities benefiting from the uneven electronic distribution in carbon lattice[40].Extensive research reported that inducing structure defects by breaking the electroneutrality of the sp2carbon lattice could lead to new active sites for electrocatalytic reactions[41].Different topological defects generally deliver different application advantages[36],and several topological defects generally co-exist in one sample.The electron transfer and the adsorption energy on a series of intermediates in HER and OER are affected by the charge/spin distributions on carbon lattice[42].Engineering vacancy defects in the carbon matrix can greatly adjust the coordination degree to accelerate the adsorption,transformation,and desorption of reactants and intermediates,thus decrease the energy consumption in HER and OER[43].Different topological defects could be effectively introduced in carbon lattice by the nitrogen removal strategy[36].The 5-1 and 585-1 defects exhibited the lowest theoretical overpotential for basic OER among the 4 models of 5-1,585,5775 and 7557[36].Additionally,engineering vacancy defects in carbon lattice can also be used to adjust the charge distributions on the surface of carbon catalysts to regulate the electrochemical adsorption/desorption of H+.The 7557-4 model displayed the lowest free energy of 0.187 eV,and the defective graphene with the above topological defects demonstrated superior HER performance.
The vacancy defects and edge defects commonly co-exist.The absence of a carbon atom in a carbon lattice leads to a vacancy defect,and the number of the lacking atoms is related to the activity of a vacancy defect[43].When the lacking atoms are located along the same direction,it may be the edge defect.For both vacancy defects and edge defects,the unsaturated coordination can also lead to charge polarization[44].The edge-rich graphene obtained by plasma etching exhibited a higher ORR electrocatalytic performance than the un-modified one[45].And the edgeenrich graphene (Fig.3) also showed higher HER performance than that of graphene[36].

Fig.3 Computational simulation of specific N-doping and removing process in different carbon models[36].(A) Schematic and formation energy calculation of transformation from edge-deficient carbon to GN-dominated and divacancy-rich carbon.(B) Schematic and formation energy calculation of transformation from edge-rich carbon to PDN-dominated carbon and then to pentagon-rich carbon.(C) Schematic and formation energy calculation of transformation from pentagon-edge-rich carbon to PON-dominated carbon and then to unique carbon reconstruction.Reproduced with permission
For complex defects,heteroatom doping is a common and effective strategy[46].Tremendous attention has been paid to the heteroatom-doped carbonbased electrocatalysts since the first report of the superior oxygen reduction reaction performance realized by the nitrogen-doped carbon nanotubes[37].And various heteroatoms[36],including nonmetal species and metal species[38],have been used as dopants by direct carbonization of the selected precursor[47]or by the post-treatment method[48].
The heteroatom doping can induce surface charge redistribution in the whole carbon lattice[37].Some carbon atoms on the lattice and N atoms are negative,and others are positive.Thus,the positive carbon atoms easily adsorb negative species.The armchair nanoribbon carbons near the N atoms are identified as the active sites for OER[49].Besides,the Ndoped graphene demonstrated excellent OER electrocatalytic performance[49],N,P co-doped nanocarbon and surface oxidized carbon nanotubes also exhibited superior OER in basic media[50].Additionally,benefiting from the synergistic effects of heteroatom doping,topological defects and edge defects,the local electronic structures can be effectively adjusted,and the Gibbs free energy of adsorption for H+is close to that of Pt catalysts.Thus,the excellent HER performance could be realized by 3D graphene with rich N atoms and edge defects[51].
Compositing carbon materials with metallic active components such as metal single atom[36],cluster[47]and oxide[1]can significantly enhance the holistic electrocatalytic activity.For a Pt single-atom catalyst,it is essential to adjust the adsorption energy of intermediate species[38].An enhanced bond strength between Pt-adsorbate could be obtained by a decreased electron population,thus achieving an increasing electrocatalytic performance for HER.Edgerich graphene could adjust the electronic structure of the Pt by the hybridization,attributing to the highly localized density of state near the Fermi level at the edge-rich graphene.When the Pt single atom was anchored on the graphene edges,superior HER electrocatalytic performance was achieved in basic media,with a turnover frequency (TOF) of 22.6 at an overpotential of 150 mV.
Like metal single-atom modified carbon materials,Me-C and Me-N coordination adjusts the charge distribution and enhances the electron transfer in Me-N-C catalysts[52].As a result,Co-C-N catalysts exhibited high HER activity and stability in acidic media,with a high current density of 100 mA cm−2at a quite low overpotential of 212 mV.
In addition,introducing another metal into the single metal catalyst could reduce the amount of the original noble metal and modify the original electrocatalytic properties due to the strain effect and coordination effect[53].The Ru3Co model exhibits the lowest among all models.When anchoring the Ru3Co on the defective graphene with N doping,more electrons transferred from the Ru3Co to the carbon lattice of the defective graphene[54],and thus RuCo-NC exhibited superior HER electrocatalytic performance[54],with an overpotential of 28 mV at a current density of 10 mA cm−2.Uniform non-noble metal CoNi alloys anchoring on the graphene also delivered an excellent HER performance in acidic media[55],with an overpotential of 142 mV at a current density of 10 mA cm−2.
Different from the metal atom,their corresponding metal oxides[56],carbides[57],borides[58],sulfides[59],nitrides[60],phosphides[61]and selenides[62]usually have low electronic conductivities,low surface areas and unstable mechanical and chemical structure for OER/HER in acid/basic media.The interaction between metal metallic compounds and carbons can influence the charge transfer of both components and increase the utilization rate of metal species[56–62].Oxygen-vacancy-rich CoOxanchored on the N-doped graphene catalyst displayed increased OER and HER activity than those of Co@N-doped carbon,CoOx@C and N-doped carbon[56].
Considering the powder morphology of experimental electrocatalysts,conductive substrates are needed to load these powder catalysts in an electrolytic cell,which employs substrates with high electrical conductivity,strong tolerance to harsh basic or acidic environments and excellent mechanical stability,including carbon paper[63],glass carbon[64]and carbon cloth[65].When a Ni/Mo2C-N-doped porous carbon was loaded on the carbon cloth,low overpotentials of 130 and 297 mV at a current density of 10 mA cm−2were realized for HER and OER,respectively[65].
4 Advance in carbon-based electrocatalysts at high current densities
4.1 Superaerophobic structure
The mass transfer process exists due to the ions transfer in the electrolyte,membranes,and the interface of electrolyte-electrocatalyst[66],which is related to the electrolyte,additives,electrolysis cell structure,and the nano-microstructure of the electrocatalyst[67].Besides,the adhesion of generated oxygen and hydrogen bubbles on the surface of the electrocatalyst is also a challenge[68].
The oxygen and hydrogen bubbles must depart timely after their generation at the surface of the electrocatalyst.Once the bubbles cannot be removed from the surface,the surface active sites of the electrocatalyst would be blocked,resulting in an increased overpotential[69].Since the bubble can only be expelled when it grows up to a defined size,the adhesive bubbles keep covering the interface of the electrocatalyst-electrolyte,reducing the accessible electrochemical active sites,limiting the mass transfer between the electrocatalyst and electrolyte[70].As a result,the overall catalytic efficiency is severely hampered.Hence,understanding the features of bubbles is vital for the gas-evolving HER and OER at high current densities.
The separation ability of the oxygen and hydrogen bubbles from the electrocatalyst electrode surface depends on its wettability[71].The movement of bubbles is determined by the concentration difference between gas bubbles and electrolytes.As shown in Fig.2,a low separation rate of oxygen and hydrogen bubbles will lead to a thick bubble layer at the surface of the electrocatalyst,which is extremely disadvantageous to industrial electrocatalytic water splitting at high current densities.
Similarly,within the interface of air-liquid,the bubble contact angle on the flat surface of liquid-solid can be predicted[72].The bubble competes with liquid to stick to the interface of liquid-solid in the liquid medium.A superhydrophilic surface is an aerobic surface in a water medium[73].Besides,hysteresis contact angels can also be used to describe the wettability of the bubble[74],the superaerophilic surface delivers high hysteresis angels and small bubbles contact angels[75–76].The superaerophobic surfaces make an adhesion force on the surface of the oxygen and hydrogen bubbles,which can be determined by the hysteresis contact angle and the volume of the bubble[72].The adhesion force may lead to a desquamation of electrocatalysts from the support when the giant bubble departs away from the surface of the electrocatalysts[77].Engineering superaeropholic surface can be used to reduce the accumulation and restrain the growth of the bubbles,enhance the separation of bubbles from the solid in a liquid medium,and result in a reduced adhesion force on the surface of electrocatalytic electrode.Similarly to the mass transfer process,engineering catalysts with a rational nano-micro structure can be a simple and effective strategy to create a superaerphobic surface on the electrocatalyst electrode (Fig.4).Benefiting from the porous structure and the surface hydrophilic groups of CoOOH nanosheets,hydrogen bubble contact angle measurements in water medium indicate that the surface has stronger adsorption of electrolyte than a bubble.Compared with carbon foams,smaller hydrogen bubbles depart away from the Ni2P-CoOOH electrode quickly[78],leading to a sustaining exposure of electrocatalytic active sites,low interfacial adhesion,and durable stability at a 100 mA cm−2-level current density in seawater water over 100 h.

Fig.4 Digital images showing the bubble generation behavior difference on (a) flat MoS2 film and (b) nanostructured MoS2 film.Scale bar: 500 μm.The corresponding statistics of the size distribution of releasing bubbles are shown on the right.These data clearly evidence that the size of bubbles released from nanostructured electrodes is only 1/10 of that of a flat surface.By alleviating the bubble adhesion issue and reducing the “dead area,” the lower-adhesion “superhydrophobic” nanostructured MoS2 electrodes exhibit higher electrolysis efficiency[79].Reproduced with permission
Inducing non-super aerophobic catalysts on the support with superhydrophobicity is another efficient strategy for gas evolution reactions[80].Compared with the continuous three-phase contact line (TPCL),the discontinuous TPCL is more favorable for the release of bubbles from the electrode due to the low contact angles and the low adhesion force[81].WS2,a semiconductor lacking conductivity,has a high charge-transfer resistance and a low onset potential for HER.Graphene with excellent conductivity delivers a small transfer resistance.Different from flat or planar graphene,vertically grown graphene nanohills with nanorough surfaces exhibit superhydrophobic properties.When depositing nonsuperaerophobic WS2on the superaerophobic vertically grown graphene nanohills,hydrogen bubbles with smaller size can release quickly from the electrocatalyst,and a lower onset potential of 36 mV can be achieved[82].Combining the superaerophobic vertically 2D carbon materials with nanorough surfaces with non-noble metals or non-precious metal compounds can pave the way for synergetic integrated super aerophobic electrocatalyst systems for gas-involving reactions,which is crucial to achieving superior electrocatalytic activity.
4.2 Stability
Apart from the activity,stability is also a key issue for evaluating the electrocatalytic performance,which includes the mechanical and chemical aspects.As shown in Fig.4,in the binder and binder-free systems,the mechanical stability is greatly affected by the adhesion strength on the support,the electrocatalysts and the bubble[83].The chemical stability is greatly affected by the resistance to corrosion of the support and the electrocatalyst in a liquid medium under high oxidation,corrosion and reduction voltage[84].The mechanical stability of the electrocatalytic electrode in binder systems is governed by the chemical stability of the ionomer binders and the mass loading of the catalyst[85].
The adhesion strength between the support and electrocatalysts can be greatly influenced by the binder content and binder kinds[86].A higher binder content may lead to a worse electrical conductivity and severely block exposed active sites.The optimized binder content should be obtained by balancing the catalytic activity and stability.Exploring advanced binders is vital for the powder-support system.The Co3O4-C catalyst with paraffin oil as the binder delivered superior stability of 40 h in an acidic medium,which is much longer than that of the same catalyst with the Nafion binder[87].
The choice of conductive support is also critical,where the electrochemical active sites can homogeneously be anchored[88].The interaction of the catalysts and the support materials can be used to tailor the electronic structure and suppress the aggregation or peeling of the electrocatalytic active sites[88–89].Benefiting from the structural diversity,high electrical conductivity,large surface area and abundant defects,carbon-based materials have been used as support materials in various electrocatalytic systems[90].Unfortunately,conventional carbon-based supports and carbon-based catalysts are prone to passivation or dissolution under an oxidative environment,especially at high potentials,which reduce electron transfer pathways[91–92].Subsequently,parts of the electrocatalytic electrode are inactivated.
Carbon-based supports and carbon-based catalysts can be electrochemically oxidized to release CO,resulting in carbon corrosion and the disappearance of active sites.Many strategies have been proposed to enhance the catalyst resistance to corrosion and dissolution,such as crystallinity engineering,surface group engineering,and doping[15].Based on the controllable surface oxidation,the amino-rich carbon exhibits superior stability in acidic OER.With controllable oxidation of RuO2nanopowder encapsulated in graphene in Fig.5,RuNi2©G-250 catalyst delivered superior stability for OER of 24 h in an acidic medium[88].The modified graphene with controllable oxidation can serve as an electron reservoir to modify charge transfer to noble metal electrochemical active sites and resist the corrosion in a harsh acidic medium.Similarly,N-doped carbon supports displayed robust chemical stability with no carbon oxidation.However,the above improvements are all achieved at a low current density (10 mA g−1),when the current density is increased to above 100 mA g−1,more severe corrosion and dissolution may occur.
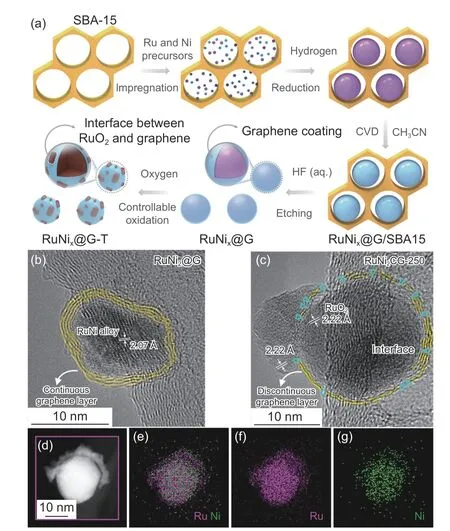
Fig.5 Schematic synthesis process,morphology,and structural characterizations of RuNi2©G-250 and RuNi2@G[88].(a) Schematic illustration of the preparation process for RuNi2©G-250 with the interface between RuO2 and graphene,where the symbol “T” represents the different oxidation temperatures,and “X” represents the molar ratio of Ru and Ni precursors.(b) High resolution transmission electron microscope (HRTEM) image of RuNi2@G.(c) HRTEM image of RuNi2©G-250 with a unique interface between RuO2 and graphene.(d–g) High angle annular dark field scanning transmission electron microscope (HAADF-STEM) image and the corresponding energydispersive spectroscopy maps of RuNi2©G-250 for Ru,Ni and the combined image.Reproduced with permission
The bubbles exert strong adhesion force on the mini-surface of the electrocatalyst,and some electrocatalysts may be peeled off when the huge bubbles separate from the electrocatalysts.Much more and larger bubbles are usually generated at the high current densities,which have a much stronger interfacial adhesion force on the electrocatalyst.Apart from engineering nano-micro rough structures to reduce the contact angles of bubbles in a liquid medium,the disturbance of hydrogen and oxygen bubbles can also be reduced by surface chemical modification.Controlling the polytetrafluoroethylene (PTFE) coverage can create a superhydrophobic surface of the electrocatalyst[93].As a result,bubble nucleation and release at the same position,reducing the desquamation of the electrocatalysts from the support materials.
4.3 Interface micro-environment
HER and OER are both interfacial sensitive reactions,and the interfacial micro-environment varies along the depth of the electrocatalytic electrode.Substantial work has been carried out to regulate the interfacial micro-environment of the electrocatalysts[94].
The kinetics of HER in acidic conditions is much faster than in alkaline/neutral due to the many more protons in the acidic medium.As a result,the state-ofthe-art noble metal electrocatalysts display 2 to 3 orders of magnitude higher activity in the acidic medium than in neutral/alkaline conditions[95].Apart from the nature and the nano-micro structure of the catalyst,the kinetics of HER in liquid media is governed by the local interfacial micro-environment around the electrochemical active sites at electrolyte-electrocatalyst.Substantial conventional strategies have been made to enhance the kinetics of the HER and OER,such as straining engineering,heterostructure engineering,and doping engineering[11].However,these attempts cannot change the feature of low concentration of protons in non-acidic medium.Hence,generating a local acidic environment by a favorable electrode system in non-acidic media is more effective for enhancing the electrocatalytic performance of the same catalyst[94].
Hydrogen-doped WO3could act as a proton reservoir and electron sponge to form an acid-local microenvironment on the surface of the catalyst[95].But,the surface H―H coupling process and the strong adsorption of hydrogen by the basic oxygen centers severely consume the local acidic species.Benefiting from the metal Ir revitalizing the lattice hydrogen species in hybridized Ir-HxWO3on carbon fiber host,Ir-HxWO3is expected to deliver superior HER performance in the non-acidic environment[95–96].Based on a series of experiments,the HER kinetics and activity of Ir-HxWO3/carbon fibers in a 1.0 mol L−1PBS neutral medium were similar to those under acidic conditions.Compared with the HER performance of 277 mA cm−2in acidic medium,Ir-HxWO3/carbon fibers delivered 256 mA cm−2in neutral medium at 150 mV overpotential (Fig.6).And the current densities of reported catalysts Pt/C and Ir/C in acidic medium were respectively 2.6 and 6.2 times higher than those in neutral media at the same overpotential.The significantly decreased Tafel slopes demonstrate that HER kinetics is strongly associated with the local proton concentration.Besides,the super hydrophilic feature of Ir-HxWO3/carbon fibers was proven by highspeed imaging experiments.And Ir-HxWO3/carbon fibers exhibit less shift after accelerating degradation test for 10 000 times.Ir-HxWO3/carbon fibers also displayed superior stability at a high current density of 400 mA cm−2for 40 h.Both the composition and structure of Ir-HxWO3/carbon fibers remained unvaried before and after the HER tests.Selective poisoning,kinetic isotope effect experiments,and operando Raman measurements confirmed the synergism between lattice-hydrogen species and Ir in Ir-HxWO3/carbon fibers.The Volmer process is entirely enhanced at the Ir site to form Ir-H*,accompanied by spontaneous recombination of Ir-H*and WO-H*species to form H2molecular by interfacial Tafel step,as proven by Density Functional Theory (DFT) calculation,thereby keeping the HER reaction in neutral medium at a high rate.

Fig.6 Schematic diagram of local acid-like microenvironment generation on HxWO3 cathode[96].Reproduced with permission
4.4 Decoupling water splitting
Based on the electrocatalytic water-splitting reaction mechanisms of HER and OER,the anodic and cathodic pressures in each chamber must be well controlled to suppress the hydrogen and oxygen permeation across the membranes.To date,substantial attempts have been contributed to develop new and advanced solid polymer membranes[85].Benefiting from the advantages of anion,a proton solid exchange membrane,water electrolysis can be carried out at huge pressure differences.However,it is still challenging to realize the adequate separation of oxygen and hydrogen upon electrolysis.Hydrogen and oxygen gas crossover through the membrane to generate a mixture of hydrogen and oxygen gases,which is considered a safety issue.In addition,the mixture may also damage the membrane.The high applied potential also requires more compatible and economical water electrolysis systems.Decoupling HER and OER offers a solution to avoid the above challenges by generating oxygen and hydrogen in different electrochemical cells at different times and at different rates[97].The reduction of some mediators can be put to displace the generation of hydrogen gas,while the OER can be substituted by the oxidation of other mediators[97–99].The oxygen evolution reaction at the anode needs more than 1.23 V potential.It is more attractive to replace OER with a more thermodynamically favorable and economically organic oxidation reaction such as 5-hydroxymethyl furfural[100–101].The generation of high-value organic products is more economical rather than that of oxygen gas[102].Ascorbic acid can be oxidized to the high-value dehydroascorbic acid.The conventional conversion strategy includes the oxidation of chlorine and aerobic,several concerns need to be solved,such as the low efficiency,the toxic oxidant residues and the low purity of dehydroascorbic acid.The electrocatalytic oxidation of ascorbic acid to dehydroascorbic acid provides a facile and efficient method with water and electricity under mild conditions[103].The ascorbic acid electrocatalytic oxidation can be coupled with hydrogen evolution reaction at a low bath potential due to the low theoretical electrochemical oxidation potential of ascorbic acid.The continuous and efficient production of high-value chemicals and hydrogen gas can be achieved in one electrocatalysis cell at a low bath voltage,which is of great economic value[104].Benefiting from the oxygen plasma-engraved carbon paper electrode,an excellent performance of the electrochemical oxidation of ascorbic acid to dehydroascorbic acid could be realized on the oxygen-engraved carbon paper.Unlike other carbon modification methods,the plasma strategy is a non-contact method[105].The oxygen at the surface of the carbon electrode enhances the hydrophilicity of the carbon-based electrocatalytic electrode.The adsorption kinetics of hydrophilic ascorbic acid at the surface of the carbonbased electrode can be greatly increased[106].Based on the experiments and theoretical calculation,the carboxyl group on the engraved carbon paper can increase the dehydrogenation kinetics of the electrocatalytic oxidation of ascorbic acid to dehydroascorbic acid and then improve the overall electrocatalytic activity.A high current density of 1.0 A cm−2at 0.98 V was realized in the coupled system between the electrochemical oxidation of ascorbic acid to dehydroascorbic acid and HER in an acid medium.
As displayed in Fig.7,apart from the electrochemical oxidation reactions of biomedicines and HER[107–108],numerous kinds of new coupled electrolysis systems can be explored[109].The expensive solid membrane systems can be replaced or removed.
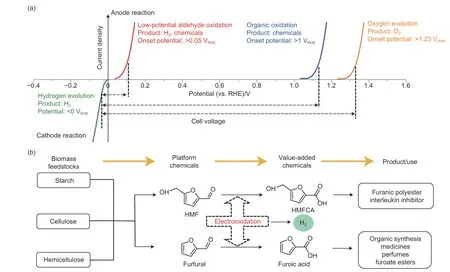
Fig.7 Water electrolysis systems with various anode reactions[107].(a) Comparison of various anode reactions for water electrolysis.(b) Electrochemistry-involved conversion path from biomass feedstock into value-added chemicals.Reproduced with permission
5 Summary and prospects
Electrocatalytic water splitting is one of the most promising strategies to generate green hydrogen gas by renewing energy sources[89,110].Carbon and carbonbased catalysts hold great superiority over other types of materials owing to their large surface area,superior electrical conductivity and thermal conductivity,and excellent chemical resistance.Numerous types of defective carbon materials and carbon-based materials have been explored in HER and OER in different media,including acidic,basic and neutral media[111–112].As two surface-dominated processes,the catalyst activity for HER and OER is governed by the adsorption/desorption features of the critical intermediates on the electrochemically active sites in laboratory research at low current densities[113].And the activity of the carbon-based catalyst can be enhanced by regulating the charge state.The electrocatalytic active sites and their numbers have great impact on the electrocatalytic activity of the catalysts.
Commercial water electrolysis is carried out at a high voltage and a high current density (above 1.6 A cm−2in PEM water electrolysis,above 0.5 A cm−2in alkaline OER).The optimized electrocatalyst with a single superior physical property are favorable to catalytic kinetics at low current densities usually.However,it does not exhibit a superior electrocatalytic water splitting performance due to several factors[114],such as the electron transfer process,the amount and exposure of electrochemical active sites,mass transfer,bonding strength,local environment,bubble phenomena and water dissociation kinetics[115–116].And huge knowledge gaps still exist between the low and high current densities in typical electrocatalytic water splitting systems and decoupled water splitting systems.
Apart from regulating the charge state of the defective carbons and carbon-based materials to engineer electrocatalytic active sites and increasing the exposure of active sites,the transfer of proton and reaction-formed intermediates at solid-liquid interface has a significant impact on the performance at a high current density[117].Specifically,the formed hydrogen bubbles tend to adhere tightly to the surface of the electrocatalytic catalyst due to the interface force,especially at high current densities,leading to reduced accessible solid-liquid interface contact area and limited mass and electron transfer.Rational engineering,nano-micro structure and chemical coating to create unique super aerophobic features at the catalyst surface are favorably used to enhance the HER performance at high current densities[118],which leads to a low interfacial adhesion on the interface of electrolytecatalyst,enhances the release of generated hydrogen bubbles from the electrocatalyst electrode surface and realizes the increased exposure extent of electrocatalytic active sites to the surrounding electrolyte[79].
Stability is another key concern for evaluating the electrocatalyst performance,including mechanical and chemical stabilities.In the binder and binder-free systems,the mechanical stability is greatly influenced by the adhesion strength among the support,the electrocatalysts and the bubble[119].The chemical stability is greatly affected by the resistance to corrosion of the support and the electrocatalyst in a liquid medium under oxidation[120],corrosion and reduction[121].Besides,conventional carbon-based supports and carbon-based catalysts are prone to passivation or dissolution under an oxidative environment,especially at high potentials.Exploring advanced binder and surface chemical modification method is vital for the powder-support system electrocatalytic electrode in electrochemical water splitting[122].
HER and OER are both interfacial sensitive reactions.The interfacial microenvironment is involved in the electrocatalytic process[123].The fast decreased reactant concentration near the electrocatalysts may depress the stability under high current densities.Substantial conventional strategies have been made to enhance the kinetics of the HER and OER.However,these attempts cannot change the feature of the low concentration of protons in a non-acidic medium[124].Hence,generating a local acidic environment by a favorable electrode system in non-acidic media is more effective for enhancing the electrocatalytic performance of the same catalyst[125].Engineering rational and multifunctional cocatalysts in carbon-based materials effectively solves the above points[126].
To date,substantial attempts have been contributed to develop new and advanced solid polymer membranes.Benefiting from the advantages of anion or proton solid exchange membrane,water electrolysis can be carried out at huge pressure differences.In recent years,substantial works have been done on electrocatalytic water splitting,but most of them focused on increasing the efficiency of HER and OER themselves.However,the efficient formation of oxygen and hydrogen is of little use unless generated oxygen and hydrogen can be kept separate totally.And it is still challenging to realize the adequate separation of oxygen and hydrogen upon electrolysis[127].Decoupling HER and OER offer a solution to avoid these challenges by generating oxygen and hydrogen in different electrochemical cells[128]at different times and at different rates[129].Much more attention should be paid to shortening the gap between industrial application and fundamental research.
Apart from the above issues,continued attention is worth to be paid to other vital concerns to increase the activity and stability of carbon-based materials for electrocatalytic water splitting[130],such as the electrolyte[131–132],environment conditions including the pressure[133]and temperature[134–135],flow cell[136]and heat management[137–138].
Acknowledgements
This work was supported by China Postdoctoral Science Foundation (2020M672471).
杂志排行
新型炭材料的其它文章
- 序
- Defect engineering of carbon-based electrocatalysts for the CO2 reduction reaction: A review
- Carbon-based metal-free nanomaterials for the electrosynthesis ofsmall-molecule chemicals: A review
- A review of carbon-based catalysts and catalyst supports for simultaneous organic electro-oxidation and hydrogen evolution reactions
- MOF-derived nanocarbon materials for electrochemical catalysis and their advanced characterization
- 石墨烯基二氧化碳还原电催化材料研究进展
