Carbon-based metal-free nanomaterials for the electrosynthesis ofsmall-molecule chemicals: A review
2024-03-07SHILeiLIYanzheYINHuajieZHAOShenlong
SHI Lei ,LI Yan-zhe ,YIN Hua-jie ,ZHAO Shen-long,
(1.CAS Key Laboratory of Nanosystem and Hierarchical Fabrication,National Center for Nanoscience and Technology,Beijing 100190, China;2.CAS Key Laboratory of Materials Physics,Institute of Solid State Physics,Hefei Institutes of Physical Science,Chinese Academy of Sciences,Hefei 230031, China)
Abstract:Electrocatalysis is a key component of many clean energy technologies that has the potential to store renewable electricity in chemical form.Currently,noble metal-based catalysts are most widely used for improving the conversion efficiency of reactants during the electrocatalytic process.However,drawbacks such as high cost and poor stability seriously hinder their large-scale use in this process and in sustainable energy devices.Carbon-based metal-free catalysts (CMFCs) have received growing attention due to their enormous potential for improving the catalytic performance.This review gives a concise comprehensive overview of recent developments in CMFCs for electrosynthesis.First,the fundamental catalytic mechanisms and design strategies of CMFCs are presented and discussed.Then,a brief overview of various electrosynthesis processes,including the synthesis of hydrogen peroxide,ammonia,chlorine,as well as various carbon-and nitrogen-based compounds is given.Finally,current challenges and prospects for CMFCs are highlighted.
Key words: Electrosynthesis;Electrocatalysis;Carbon-based nanomaterials;Metal-free electrocatalysts;Small-molecule chemicals
1 Introduction
Chemical manufacturing heavily relies on fossil fuels for its energy needs,which constitutes a significant portion of the world’s energy demand[1].Given the escalating energy crisis and environmental concerns,there is an urgent need to develop clean,low-cost and efficient renewable energy technologies to replace the traditional chemical manufacturing processes.Electrosynthesis emerges as a promising green strategy,utilizing clean electricity to drive electrochemical reactions for chemical synthesis.Unlike conventional industrial synthesis methods with high energy consumption,electrosynthesis technologies effectively reduce the energy barriers of electrochemical reactions,thus enabling the synthesis of valuable chemicals under milder conditions.As a result,the direct electrochemical transformation of abundant raw ingredients,such as H2O,CO2,O2and N2,into high-value-added chemicals and fuels has attracted increasing attention[2].In these systems,electrocatalysts play a pivotal role in increasing reaction efficiency and regulating product selectivity[3–5].This inherent capability makes catalysts indispensable in various electrochemical reactions,including the oxygen reduction/hydrogen oxidation reaction (ORR/HOR) in fuel cells[6–9],hydrogen and oxygen evolution reaction (HER/OER) in photo-/electro-water splitting[10–14],carbon dioxide reduction reaction (CO2RR) in the artificial carbon cycle[15–16],nitrogen reduction reaction (NRR) in artificial nitrogen fixation[17–19],and other electrosynthesis processes for generating high-value-added chemicals[20–22].Currently,noble metal-based catalysts are commonly employed to enhance the conversion efficiency of reactants to products during the electrocatalytic process.However,the drawbacks such as high cost and poor stability seriously hinder their large-scale applications in electrosynthesis and sustainable energy devices[23–25].Therefore,it is highly desirable to develop cost-effective electrocatalysts for overcoming these challenges and advancing the progress of electrochemical technologies.
Carbon-based metal-free catalysts (CMFCs) exhibit unique physical and chemical properties,including controllable dimensions,large surface area,exceptional conductivity,substantial porosity,and excellent chemical stability.Their tunable structures,ranging from 0D to 3D,offer an ideal platform for precise design and effective integration of dynamic reaction interfaces.Additionally,their compositions can be precisely controlled through element doping or chemical functionalization,resulting in improved catalytic activity and enabling exploration of their structureactivity relationships at the atomic/molecular level.The strong covalent bonds in CMFCs grant exceptional chemical stability which ensures long-term catalytic performance.Furthermore,various effective methodologies,such as ball milling,chemical vapor deposition and chemical modification,have been established for creating CMFCs,providing an optimal foundation for the design of high-performance electrocatalysts.These features enable CMFCs to be a highly promising alternative to noble or transition metal-based nanomaterials[26].
The development of highly efficient CMFCs holds great promise for making a significant breakthrough in electrocatalysis.In 2009,Dai and colleagues introduced a new branch of metal-free catalysis by developing nitrogen-doped vertically-aligned carbon nanotubes (N-doped VA-CNTs) as a high-performance CMFC for ORR[27].The enhanced ORR activity is attributed to charge transfer induced by Ndoping,altering the adsorption mode of oxygen molecules and facilitating the ORR process (Fig.1a).Subsequently,this principle of modifying intrinsic catalytic properties has been widely applied in designing efficient CMFCs to enhance the catalytic performance in electrocatalysis and sustainable energy devices[28–32].For instance,the carbon-based metalfree nanomaterials have demonstrated promising performance in ORR[26,33–35],HER[36–37],OER[38–39],CO2RR[40],NRR[41],bi-functional[42–49]and multi-functional catalysis[50–52].Moreover,many CMFCs have been proven to be stable and effective multifunctional electrodes in applications such as hydrogen peroxide photo-electrochemical production[53–54],Zn-air batteries[55–56],and water splitting[9,50].These breakthroughs in CMFCs hold great promise for the development of affordable and durable catalysts for various key reactions involved in energy conversion and electrosynthesis technologies[31,57].

Fig.1 (a) Calculated charge density distribution of N-doped CNTs and the corresponding adsorption modes of oxygen molecule[27].Reproduced with permission from AAAS.(b) Reaction process between pyridinic N and OH species.(c) Proposed mechanism for ORR on nitrogen-doped carbon materials[59].Reproduced with permission from AAAS.(d) Heteroatom-doping mechanism in CMFCs[60].Reproduced with permission from Wiley-VCH
Considering the comprehensive reviews on CMFCs for HER,four-electron ORR and OER[26,32–37],this review shifts the focus toward the application of CMFCs for advanced chemical electrosynthesis,such as hydrogen peroxide,multi-carbon fuels,ammonia,urea,and other small-molecule chemicals.Based on the broad interest in CMFCs for electrocatalysis,we first discuss the mechanism understanding,and design strategies related to CMFCs.Subsequently,it delves into the application of CMFCs in various electrosynthesis reactions,involving two-electron transfer ORR and water oxidation reaction (WOR) for hydrogen peroxide (H2O2) production,multi-electron transfer CO2RR and NRR for carbon-and N-based chemicals,and other electrosynthesis of emerging small-molecule chemicals such as chlorine and urea.Finally,we propose the emergent challenges and future developments of CMFCs.This review aims to provide readers with deeper insight into the intelligent design of CMFCs with high activity,exceptional selectivity and long-term stability.
2 Mechanism understanding
To modulate the electrocatalytic activity of CMFCs,a variety of approaches have been developed,including heteroatom doping,intermolecular charge transfer and defect generation[32].These strategies have demonstrated effectiveness in altering charge/spin distribution within carbon-based nanomaterials.Specifically,catalytic centers bearing positive charge and/or higher spin density play a pivotal role in modifying the chemical adsorption of reactants and intermediates.The fine-tuned electron configurations can enhance the compatibility between active sites and adsorbed reactants,promoting the adsorption of reaction intermediates and facilitating electron transfer.Therefore,understanding these mechanisms is crucial for the precise design of highly efficient CMFCs.In this section,we delve into the origins of catalytic performance through experiments and density functional theory (DFT) calculations,focusing on two-electron ORR and WOR for hydrogen peroxide production,CO2RR and NRR for carbon-and nitrogen-based chemicals production.
2.1 Two-electron oxygen reduction/water oxidation for H2O2
The catalytic mechanism of metal-free catalysis has been proposed based on the discovery of N-doped VA-CNTs catalyst.However,there is some controversy regarding the potential role of metal impurities in ORR.To address this,positively charged polyelectrolytes,exemplified by poly(diallyldimethylammonium chloride) (PDDA),have been employed to verify the charge-transfer mechanism for ORR[58].As a result,the ORR performance of PDDA-modified CNT is significantly enhanced,whereas the activity of the electron-donated polyethyleneimine functionalized CNT electrode is inferior to that of bare CNT electrode.This finding revealed that the ORR activity in CMFCs is attributed to doping-induced charge redistribution rather than the presence of metal impurities.Subsequently,the active site is conclusively confirmed using a suite of model N-doping carbon catalysts,including pyridinic N-/graphitic N-/edges-/clean-highly oriented pyrolytic graphite(HOPG)[59].X-ray photoelectron spectroscopy (XPS) studies of the pyridinic N-doped HOPG demonstrated that carbon atoms adjacent to pyridinic N can react with OH species,leading to a transformation of the pyridinic N to pyridonic N (Fig.1b).This indicated that the carbon atoms adjacent to pyridinic N were the real active sites.Experiments combined with DFT highlighted that pyridinic N-doping could significantly promote the chemisorption of O2at the adjacent carbon atom and the subsequent protonation process (Fig.1c).
Considering the ORR reaction pathway,an oxygen molecule accepts 2 electrons and combines with 2 protons to form a reaction intermediate (OOH*),which can be directly reduced to form H2O2(Fig.1c).Therefore,the two-electron ORR pathway has been considered a promising approach for green H2O2production[54].The CMFCs have demonstrated great potential in the electrosynthesis of H2O2due to their high tunability and chemical stability.The activity and selectivity of H2O2production are strongly correlated to heteroatomic species,oxygen groups,and structural defects[54].Heteroatom-doped CMFCs generally cause a redistribution of charge density and/or spin density due to differences in electronegativity/spin density between carbon atoms and heteroatoms,thereby regulating the adsorption of reactants and intermediates(Fig.1d)[60].Moreover,the difference in heteroatom species leads to a tunable activity and selectivity toward ORR.For example,among various N species in CMFCs,the pyrrolic N species have shown high performance toward two-electron ORR due to the suitable adsorption strength with*OOH species[61].The pyrrolic N configuration has the closest adsorption energy compared with the ideal value,thus being considered a suitable species for electrochemical H2O2synthesis.In addition,two-electron ORR performance is strongly linearly correlated to oxygen content,as demonstrated by Cui and co-workers[62].DFT calculations demonstrate that excellent activity and selectivity originated from the ―COOH and C―O―C functional groups in CMFCs.Furthermore,the synergistic effect between heteroatom and defect has also been proven effective for two-electron ORR.Most recently,cooperation between pentagon defect and Ndoping can effectively regulate the geometric and electronic properties of the carbon structure,resulting in a high affinity for O2and suitable strength of*OOH species[63].Similarly,an O-modified defective CMFC exhibits high activity for H2O2electrosynthesis.Comprehensive experiments and calculations reveal that the combination of defects and O-groups is the key to the two-electron pathway of ORR[64].
In addition to ORR,WOR involving a two-/fourelectron transfer process is also a complex yet crucial reaction for both water electrolysis and H2O2electrosynthesis.Theoretical calculations suggest that Ndoped CMFCs can significantly reduce the reaction energy of the rate-limiting step (from O*to OOH*),demonstrating the optimal catalytic activity for WOR[65].For instance,the pyridine N-oxide sites induce enough positive charge separation through π-π delocalization,facilitating the initial hydroxyl ion adsorption and enhancing the WOR process.Moreover,the charge redistribution caused by pyridinic N also can optimize the thermodynamic energy barrier of the*OOH intermediate.As a result,the as-prepared Ndoped CMFC exhibits the excellent WOR performance,surpassing the commercial RuO2[66].According to the charge redistribution principle,O-/S-doped CMFCs were also successfully synthesized to reduce the overpotential or energy barrier of WOR[67–69].
The electrosynthesis of H2O2through WOR is gaining increasing attention because water is the sole raw reactant in the electrochemical process.However,owing to the significantly lower theoretical potential of oxygen evolution compared to hydrogen peroxide formation (1.23 V vs.1.76 V),the introduction of heteroatoms,defects,and functionalization often generates a high activity for the four-electron OER.In addition,the high oxidation potential of WOR to H2O2also leads to the inevitable self-oxidation of CMFCs themselves during the reaction,resulting in structural changes and unclear identification of active sites.Therefore,the CMFCs used in H2O2electrosynthesis must possess highly intrinsic stability,such as nanodiamond,graphyne,and HOPG-based materials[70–71].Consequently,the two-electron WOR for H2O2production by CMFCs poses a significant challenge.To date,there are very few reports on CMFCs for twoelectron WOR to H2O2.Recent findings show that boron-doped diamond (BDD) nanomaterials exhibit impressive activity for H2O2production,with performance highly correlated to the doping level of boron atoms[70].This discovery paves the way for new research in the design of sp3-structured carbonaceous materials for H2O2electrosynthesis.Furthermore,acetylene-based CMFCs have also shown great potential for photo/electrocatalytic two-electron WOR for H2O2electrosynthesis.The acetylene species could significantly promote charge separations and electronic modulation,thereby facilitating the formation of*OOH intermediate for H2O2production[71].
2.2 Electrocatalytic CO2RR for chemicals
The electrocatalytic reduction of CO2to produce valuable chemicals and fuels,including CO,HCOOH,CH4,C2H4,and CH3COOH,involves complex multielectron transfer pathways[40].Carbon-based metalfree nanomaterials can disrupt the scaling relationship and modulate the adsorption/desorption of intermediates,demonstrating comparable CO2RR performance to traditional metal-based catalysts.CO2is a thermodynamically stable molecule with inherently low electron affinity.The dissociation energy of the C=O bond surpasses that of various other carbon-based chemical bonds.Consequently,the electrochemical reduction of CO2demands a considerable input of energy.The resulting products in CO2RR always depend on the catalysts and experimental parameters.In general,electrocatalytic CO2RR involves 3 steps: initial CO2adsorption onto the carbon-based electrode surface,charge transfer for the breakage of C=O bonds and the formation of C―H bonds,and subsequent desorption of the products[72].The substantial overpotential in CO2RR primarily arises from the initial step,where the CO2molecule undergoes rearrangement to form CO2·–intermediate.This CO2·–radical is highly reactive,initiating a series of protoncoupled multiple-electron-transfer reactions instantly.Notably,the equilibrium potentials of CO2RR for various valuable fuels closely approach 0 V (vs.RHE).An undesirable side-reaction of HER (2H++2e−→ H2,E0=−0.42 V) often occurs during the CO2RR process in aqueous electrolytes.Therefore,the development of highly selective CMFCs becomes imperative for the electrosynthesis of valuable chemicals through CO2RR[72].
Since the introduction of N-doped carbon nanofibers (N-CNF) as the pioneering catalyst for CO2RR to CO in 2013[73],various N-doped CMFCs have been developed[40].It is found that N-doped CMFCs exhibit the potential to significantly reduce the formation energy of CO2·–,thus improving the catalytic activity of CO2reduction.As a result,the prepared N-CNF exhibited a high current density for CO2RR,which is 13 times higher than that of bulk Ag catalysts.In particular,pyridinic N-doping has been considered an attractive approach to promote CO2RR to produce CO,as the lone pair in s orbital of pyridinic N acts as Lewis base site to enhance CO2adsorption,thereby significantly enhancing the CO2RR activity[59,74–75].Additionally,the type and level of N dopants can be precisely controlled to optimize electrocatalytic performance[76].
Beyond CO2RR to CO,heteroatom and/or defectdoped CMFCs present potential for electrochemical CO2RR to diverse value-added chemicals,including single-carbon (C1),multi-carbon (C2+) oxygenates and hydrocarbons.For example,heteroatom B-doped diamond has exhibited an effective CO2RR performance to produce formic acid by optimizing the reaction pathway[77].More interestingly,B,N co-doped nanodiamonds have shown high catalytic activity in CO2RR to ethanol.DFT calculations highlighted that the synergism of B and N co-doping enables C―C coupling to form C2+intermediates (*COCO)[78].
2.3 Electrocatalytic NRR for ammonia
The electrochemical reduction of N2to produce ammonia (NH3) through metal-free catalysis has gathered much attention due to abundant raw materials and mild reaction conditions.Understanding the reaction mechanisms and optimizing the catalytic activity of CMFCs are crucial for the development of efficient and sustainable NRR catalytic systems.In general,the catalysis of NRR on heterogeneous surfaces involves 2 primary mechanisms: associative and dissociative[79].The associative mechanism follows the hydrogenation based on N-N bonding and subsequent N―N bond cleavage to release ammonia molecules.The hydrogenation process can occur through potential distal and alternating pathways.In the distal pathway,hydrogenation takes place preferentially on the outside nitrogen site,leading to the release of ammonia and the generation of a site-nitrido unit to yield the second NH3molecule.The alternating pathway involves each of the 2 nitrogen centers undergoing single hydrogenation in turn until the cleavage of N≡N bond and conversion into NH3molecule.In contrast,the dissociative mechanism involves the cleavage of N≡N bond before any hydrogenation occurs,resulting in 2 individual N centers on the electrode surface and independent formation of ammonia molecules.
3 Design strategies
Understanding mechanisms from the above-discussed studies is pivotal for designing and constructing novel CMFCs.Although carbon nanomaterials have unique physicochemical and structural properties,including excellent conductivity,diversity of molecular architectures,and abundant porosity,the catalytic activity on pristine carbon atoms is negligible.Fortunately,carbon-based materials like graphene and CNTs possess conjugated C―C and C=C bonds,allowing for π-electrons delocalization and modifications.
The introduction of heteroatoms can induce charge redistribution and/or lattice distortion,thereby altering the physicochemical properties of carbon atoms (Fig.2a)[26,80].In general,heteroatoms with electron-donating or electron-withdrawing characteristics can greatly modulate the electronic properties of carbon atoms,thus influencing the catalytic activity of CMFCs.The guiding principle behind this modulation varies and depends on the dopant species.Notably,nitrogen emerges as an ideal dopant for CMFCs due to its similar atom size and a relatively higher electronegativity of 3.07 compared to carbon atoms(2.55).N-doped CMFCs induce a sufficiently high positive charge density on surrounding carbon atoms,thus helping reactant chemisorption and electron transfer during the catalytic process[27].Additionally,various nitrogen configurations,such as pyridinic N,pyrrolic N,graphitic N and pyridine-N-oxide,have different impacts on the catalytic activity of electrocatalytic processes.Moreover,the precursor amount,pyrolysis temperature,and doping time play a crucial role in controlling the configuration,location,and content of dopants of CMFCs (Fig.2b)[81].For instance,the pyrolysis of graphene oxides using ammonia as a N source preferentially creates graphitic and pyridinic N species whereas the polyaniline and polypyrrole-based precursors mostly generate pyridinic and pyrrolic N species,respectively[81].The obtained pyridinic N-based CMFC exhibited the best ORR catalytic activity,suggesting that pyridinic N is the dominating active species for oxygen reduction.In the case of B-doping,the electro-affinity between B and C atoms allows the retention of a planar structure.The greater electro-positivity induces charge polarization in CMFCs,thereby stabilizing the negatively polarized oxygen atoms in O2,CO2and reaction intermediate.Conversely,S-doped CMFCs generally induce electron spin redistribution rather than charge transfer due to the close electronegativity of sulfur (2.58),which is also beneficial to enhance the electrocatalytic performance of CMFCs.In addition,P-and F-doping can create defect-induced or charge-polarized carbon atoms,thereby optimizing reactant adsorption and catalytic activity.For the carbon nanomaterials doped with large-size atoms,such as selenium,bromine and iodine,the enhanced catalytic activities are generally attributed to the unique spatial distortion effect.In summary,the type,location,and concentration of doping can lead to modulation in electronic and/or geometric structures,hence fine-tuning the electrocatalytic performance of CMFCs[32].

Fig.2 (a) Heteroatoms-doping strategies and the electronegativity of various elements[26].Reproduced with permission from American Chemical Society.(b) Schematic diagram of preparation for controlled N-doped graphene[81].Reproduced with permission from The Royal Society of Chemistry.(c,d) Design principles for various CMFCs[87].Reproduced with permission from Wiley-VCH
Furthermore,structural defects,including high curvature regions,vacancies,edges,and non-hexagonal topologies like pentagon and heptagon defects,have demonstrated effectiveness in regulating the electron distribution of carbon nanomaterials.Topological defects,resulting from lattice mismatch,missing atoms,and/or adding atoms to the pristine carbon hexagonal ring,can induce the rehybridization of electron orbitals and the adjustment of bond lengths,thus regulating the electrocatalytic activity[32–33].Charge delocalization occurring over the edge carbon atoms,such as pentagon defects and zigzag edges,often leads to much higher charge densities,which is an effective approcah to enhance the catalytic activity of CMFCs[82–83].For example,the high local curvature of the carbon matrix in our previous work effectively facilitates O2adsorption and the subsequent O―O bond-breaking process,consequently enhancing the intrinsic ORR activity[7].Moreover,our recent research demonstrates that vacancy-defect engineering noticeably regulates the adsorption model of O2,transitioning from “side-on” to “end-on” configuration.This promotes the two-electron ORR pathway,helping in H2O2electrosynthesis[23].
In addition,dual-/multi-doping with different heteroatoms and defects emerges as a novel method for improving the catalytic activities of CMFCs,thus leading to the synergistic effect between the different doping heteroatoms and surrounding carbon atoms[28,84–85].First-principles calculations combined with experiments have demonstrated that B,N codoped CMFCs exhibit higher spin and charge densities compared to pristine and single-doped CMFCs,benefiting the regulation of catalytic activity[86].Our recent research demonstrated that the remarkable enhancement of electrocatalytic CO2RR was achieved through the incorporation of B/N dopants in porous carbon nanorods.The syngas generation is linked to the adjustable concentrations of B/N dopants and the corresponding formation of B-N pairs[16].
Apart from controlled heteroatom and defect doping,structural control and surface modification of CMFCs offer an alternative route to enhance catalytic performance by increasing the exposed density of active centers,mass transfer,and diffusion of reactants[25–26].In this regard,various dimensional carbon nanomaterials have been used for constructing high-performance carbon-based electrocatalysts(Fig.2c,d)[87].The tailorable porous structures of CMFCs play an important role in facilitating the diffusion of relevant molecules involved in reactants and products.In general,pore size,pore volume,and pore dispersion significantly affect the adsorption and diffusion dynamics of gas molecules,such as CO2,O2and N2.Both experimental and theoretical studies have proven the importance of porous structures,especially well-suited micropore and mesopore geometries,in optimizing the supply of reactants and facilitating the conductance of electrolyte ions and electrons during the electrocatalytic processes.Lu et al.reported that the 3D-ordered porous carbon-based electrodes with precisely tunable bimodal mesopores,provide a substantial tri-phase interface for electrocatalytic reactions.This design ensures excellent electrical conductivity and ion diffusibility,thereby resulting in outstanding CO2and O2electroreduction with enhanced selectivity and current density[88–89].Additionally,the chemical surface functionalization of CMFCs also could enhance electron transfer by introducing groups along the organic molecule backbone with strong electron-accepting abilities,which can greatly enhance the catalytic activities of the carbon atoms in CMFCs through the intermolecular charge transfer[59,90].Furthermore,since many small-molecule reactions take place in an aqueous solution,the hydrophobic nature of the surface results in a low utilization efficiency and accessibility of active centers in CMFCs.Therefore,chemical surface functionalization is of great importance in altering the wettability properties of CMFCs,a critical factor in their high electrocatalytic activity.Notably,gas-consuming reactions such as CO2RR,ORR and NRR often need relatively hydrophobic gas-liquid-solid tri-phase interfaces.This ensures an efficient gas supply and subsequent coupling of protons and electrons with reactants toward the active centers[89,91].In our recent work,we demonstrated that a mesostructured polyacrylate hydrogel,coating on a conductive substrate as a metal-free electrocatalyst,exhibited a superhydrophobic surface.This unique surface property facilitates the transport of electrolytes,expediting OER kinetics and resulting in an unexpected and exceptional catalytic performance[13].Furthermore,hybridizing carbonbased materials with other conducting substrates can improve the conductivity of CMFCs,which is an important factor in determining electrocatalytic performance.For example,graphitic-C3N4/graphene hybridization exhibited significantly improved catalytic activities for both ORR and HER,superior to the individual graphitic-C3N4or graphene catalysts[92–93].Similarly,partially oxidized CNTs created abundant oxygen functional groups while maintaining high conductivity,demonstrating an excellent performance for ORR[94].
4 Electrosynthesis of small-molecule chemicals
Carbon-based metal-free nanomaterials have received significant attention due to their unique physicochemical properties,holding great promise for diverse applications.Since previous reviews extensively covered the research and development of CMFCs for electrocatalysis such as water splitting and four-electron transfer ORR[51,57],the following section specifically focuses on recent progress in the field of CMFCs for the electrosynthesis of small-molecule chemicals.This includes two-electron transfer ORR and WOR for H2O2production,multi-electron transfer CO2RR and NRR for generating carbon-and nitrogen-based chemicals,as well as emerging electrochemical processes like chlorine evolution and the production of multi-carbon compounds.
4.1 CMFCs for ORR and WOR
The production of H2O2serves as a prominent example of electrochemical synthesis,encompassing diverse applications in industries such as textiles,pulp-and-paper,and chemical synthesis[95].However,its industrial production heavily relies on the energyintensive anthraquinone process.Developing sustainable and highly efficient electrocatalytic methods for synthesizing H2O2under ambient conditions has received significant interest.To date,various metalbased nanomaterials,including noble metals and nonprecious metal catalysts,have been widely explored.However,the high cost and poor stability seriously hinder their large-scale application in H2O2electrosynthesis.Recently,CMFCs have emerged as a promising solution due to their low cost,high tunability,and excellent chemical stability under required conditions.In this context,we focus on the recent progress of CMFCs for H2O2production via two-electron transfer ORR and WOR.
Among various CMFCs,N-doped and O-doped carbons generally exhibit the highest activity and selectivity for H2O2electrosynthesis.For instance,Sa et al.reported that edge-rich and oxygen-functional carbon exhibited significantly enhanced performance compared to basal plane-rich carbon nanotubes(Fig.3a,b)[96].It also demonstrated excellent stability during long-time continuous H2O2production with a high FE of~99% (Fig.3c).Similarly,oxidized carbon nanotubes (o-CNTs) with abundant C―O and C=O functional groups were prepared as efficient CMFCs for H2O2production through electrochemical oxygen reduction (Fig.3d)[62].Comprehensive experiments showed the positive role of oxygen functional groups for activity and selectivity toward two-electron transfer ORR (Fig.3e).Calculated volcano plot revealed that carbon atoms adjacent to specific oxygen functional groups,such as ―COOH and C―O―C,serve as the active centers for the two-electron ORR,thus providing significant theoretical guidance for future catalyst design (Fig.3f)[62].Most recently,Lu and colleagues also confirmed that the carbon atoms adjacent to the oxygen-functional carbon edges can be considered active centers for two-electron transfer ORR.A series of comparative experiments demonstrated that the activity and selectivity are influenced not only by oxygen-containing functional groups but also by the pore structure and specific surface area[97].Furthermore,N-doped graphitized single-wall carbon nanohorns (CNHs) were synthesized as two-electron transfer ORR electrocatalysts for direct electrosynthesis of H2O2(Fig.3g)[98].The asprepared CNH catalyst demonstrated excellent performance in a wide pH range and a high FE of 98%,which is attributed to its microporosity and specific pyridinic N/pyrrolic N ratios[96].The high activity,selectivity,and stability of this CMFC pave the way for reliable strategies in the commercial production of
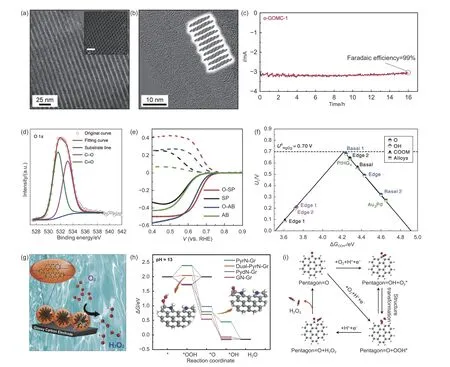
Fig.3 (a,b) TEM and HRTEM images of edge-rich graphitic ordered mesoporous carbon (GOMC),(c) The stability test of o-GOMC-1 for H2O2 electrosynthesis[96].Reproduced with permission from Wiley-VCH.(d) O 1s spectrum of O-CNTs.(e) ORR polarization curves for SP and AB-based CMFCs.(f) Calculated two-electron ORR-related volcano plot via the function of ΔGOOH*[62].Reproduced with permission from Nature Publishing Group.(g) The mechanism of H2O2 electrosynthesis for N-doped CNH[98].Reproduced with permission from Elsevier Inc.(h) Free energy profile of two-and four-electron ORR pathways for different N-doped CMFCs[63].Reproduced with permission from Nature Publishing Group.(i) The possible two-electron ORR mechanism of O-DG-30[64].Reproduced with permission from Nature Publishing Group
Recently,multi-site CMFCs have shown great potential in H2O2electrosynthesis by optimizing the adsorption configuration of the intermediate.For instance,a carbon-based metal-free nanomaterial with multiple pyrrolic-N sites was fabricated for electrocatalytic H2O2production,achieving a high selectivity and FE,as well as excellent durability[63].Theoretical calculations demonstrated that the synergy of multiple pyrrolic N sites can regulate the adsorption configuration of the*OOH species,thereby reducing the reaction energy barrier and creating a kinetically favorable pathway for two-electron transfer ORR (Fig.3h)[63].Similarly,Yao and co-workers demonstrated the dynamic identification of active centers for two-electron pathway ORR in oxygen-modified defective CMFCs[64].By controlling the density and types of defect and oxygen-functional groups,they investigated the nature of defect sites,revealing a positive correlation relationship between the defect density and the FE of H2O2.The oxygen-modified CMFC with the highest defect density exhibited an excellent FE of nearly 100% for H2O2production.Insitu attenuated total reflectance infrared,Raman spectra,and DFT calculations demonstrated that the high defect density could promote the modification of Ogroups,serving as highly efficient active centers to maximize the two-electron ORR performance(Fig.3i)[64].
In addition to ORR,two-electron transfer WOR is another interesting approach to producing H2O2from water.It is well known that carbon-based nanomaterials are widely employed in the four-electron transfer process of OER.However,there are limited reports on their use for water oxidation to H2O2.Recently,Chen et al.reported that an acetylene-based CMFC has shown significant promise in two-electron WOR for H2O2electrosynthesis (Fig.4a)[71].As shown in Fig.4b,c,ORR polarization curves reveal that the carbon-based metal-free polymer without acetylene only oxidizes water into O2,whereas acetylene-based CMFCs tend to directly oxidize water into H2O2.The enhanced performance is attributed to the introduction of acetylene or diacetylene moieties into carbonbased metal-free polymer.The introduced acetylene groups could modulate electronic structures and suppress charge recombinations.This significantly reduces the energy associated with OH*formation,enabling a highly efficient two-electron oxidation pathway toward H2O2production.In another study,Wang and co-workers used an interfacial engineering approach to increase the intrinsic H2O-to-H2O2selectivity by coating the catalyst with a hydrophobic polytetrafluoroethylene (PTFE) polymer (Fig.4d)[99].The PTFE coating traps in-situ produced O2gas close to active sites,decreasing the reaction pathway toward the O2molecule.More importantly,the reaction mechanisms study proven that the locally confined O2gas alters the*OH binding on carbon atoms and further promotes H2O2production (Fig.4e,f).As a result,the H2O2selectivity was enhanced six fold,with a high production rate and current density,as well as a small overpotential,outperforming most reported catalysts[99].
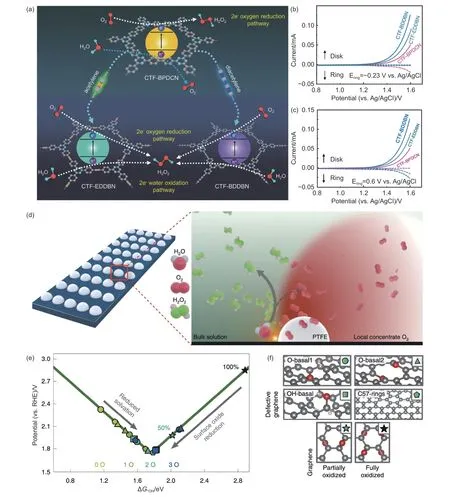
Fig.4 (a) Schematic illustration of reaction pathways for different acetylene-based polymer,(b,c) WOR polarization curves of different acetylene-based polymer,the potential of Pt ring were set to -0.23 V and 0.6 V vs. Ag/AgCl to detect O2 and H2O2,respectively[71].Reproduced with permission from Wiley-VCH.(d) The possible reaction pathway of PTFE-coated carbon electrode.(e) The data points depict *OH binding energies on different carbon-based metal-free nanomaterials.(f) The coverages of oxygen atoms in different carbon-based defected structures surface[99].Reproduced with permission from Nature Publishing Group
4.2 CMFCs for CO2 reduction
Electrochemical CO2RR emerges as a promising strategy for recycling CO2into renewable carbonbased fuels[100–101].Unlike water molecules,CO2possesses a linear structure,demanding substantial energy input to generate its radical.Furthermore,the high selectivity of CO2RR is challenging because of the closely distributed thermodynamic energies of competing reactions like the HER.To enhance the efficiency and selectivity of this transformation,various carbon-based metal-free nanomaterials have been explored.These exhibited great potential for electrochemical CO2RR to high value-added chemicals.
Cui and colleagues have developed a steam etching strategy for controlling the types and concentrations of nitrogen dopants in CMFCs (Fig.5a,b)[76].As H2O molecules prefer to bind with the carbon atoms surrounding graphitic and pyridinic N,this steam etching strategy significantly increases the content of pyrrolic N in the N-doped carbon network.The obtained CMFC exhibited excellent CO2RR performance with a maximum FE of~88% for CO production,while simultaneously suppressing HER activity[76].Moreover,dual-doping with different heteroatoms can also improve the catalytic performance of CO2RR.For instance,N,S-codoped hierarchically porous carbon nanofiber (NSHCF) has demonstrated outstanding catalytic activity for CO2to CO conversion with a high FE and current density (Fig.5c-e),which was far superior to the individual N-or Sdoped CMFCs.The excellent CO production performance is attributed to the synergism between pyridinic N and the C―S bond,which effectively decreases the Gibbs free energy of*COOH intermediate,as supported by DFT calculations (Fig.5f)[102].

Fig.5 (a,b) The synthesis illustration of N-doped CMFCs and the corresponding catalytic ability for CO2RR[76].Reproduced with permission from Wiley-VCH.(c) Schematic diagram of the structure of NSHCF.(d-f) Polarization curves,FE,and CO2RR free energy of NSHCF900 catalyst[102].Reproduced with permission from Wiley-VCH
Considering the high value of formic acid,carbon-based metal-free nanomaterials have also been employed for CO2reduction to synthesize formate.For instance,the conductive-conjugated polydopamine (PDA) catalyst exhibited a total faradaic efficiency of 95.8% for C1 products (CO and formate)[103].The strong electron-phonon coupling,along with the conductive-conjugated materials caused by doping-induced distortions,contributes to the enhanced electrical conduction ability (~ 0.43 S cm–1at 300 K) and catalytic activity.Operando measurements and DFT calculations show that CO2initially attaches to the carbonyl-PDA,then is preferentially reduced to CO and formate due to hydrogen bonding effects.Additionally,N-doped PC61BM ([6,6]-phenyl-C61-butyric acid methyl ester,termed as N-C61) was also fabricated as CO2RR electrocatalysts to produce formate by a simple pyrolysis process (Fig.6a)[104].The resultant N-C61 exhibited excellent activity and stability for formate production (Fig.6b,c).DFT calculation shows that the high catalytic performance toward formate production is due to the special active center of graphitic N-defects (Fig.6d)[104].

Fig.6 (a,b) Schematic illustration of synthesis procedures,the stability and corresponding formate FE of N-C61 catalyst.(c) Tafel plots comparison for NC61-800 and pristine C61.(d) The proposed mechanism of N-C61 electrocatalysts for CO2RR[104].Reproduced with permission from Royal Society of Chemistry.(e) TEM and HRTEM images,(f,g) FE of CH4 and jCH4 for GQD-NH2-H and GQD-NH2-L electrocatalysts[105].Reproduced with permission from Nature Publishing Group
CMFCs have also been applied to CO2RR for methane production,exemplified by the functionalized graphene quantum dots (GQDs) (Fig.6e)[105].Wu et al.discovered that electron-donating groups (e.g.,NH2) functionalized GQDs exhibited remarkable performance for CO2RR to CH4with a high FE of 70.0%at 200 mA cm−2(Fig.6f,g).DFT calculation indicated that both N and C near the electron-donating groups are potential active sites,these can stabilize the key intermediates and maintain a greater charge density,thus leading to a high CH4productivity and selectivity[105].Beyond C1 products,significant progress has been made in the electrosynthesis of C2+products through the rational design of carbon-based metal-free nanomaterials[106–108].For example,Ndoped nanodiamond/Si rod array (NDD/Si RA) composites have been developed for converting CO2into acetate with a maximum FE of 78%[106].Similarly,Ndoped GQDs have proven to be effective in reducing CO2into multi-carbon products with high FEs and current densities.The N-doped GQDs exhibited a total FE of up to 90% for CO2RR,and the high selectivities for ethylene and ethanol (~45%) are comparable to those of copper-based nanomaterials[107].
4.3 CMFCs for NRR to ammonia
Ammonia stands as a vital industrial chemical with wide-ranging applications in various fields,including agriculture,plastics,pharmaceuticals,and textiles.It is considered an excellent hydrogen carrier for efficient energy storage due to its volumetric energy density being twice as high as that of liquid hydrogen.NH3synthesis primarily occurs through 3 routes: natural azotobacter nitrogen fixation via nitrogenase enzymes,the industrial-scale Haber–Bosch process and electrocatalysis.While the conventional Haber-Bosch method employs H2and N2at high pressures and temperatures,the electrocatalysis method offers compelling advantages,such as energy efficiency and cost-effectiveness.However,activating N2by electrochemical approach poses a great challenge in chemistry due to the remarkably robust N≡N bond and the absence of a dipole moment in N2molecules[109].Electrochemical NRR for NH3production has been developed using metal-based electrocatalysts like Ru,Au,and Fe/CNT under mild conditions[110–112].Unfortunately,these catalysts suffer from large overpotential and poor N2adsorption,resulting in low NRR efficiencies and NH3yields.Conversely,CMFCs are promising for electrochemical NRR for NH3production owing to their exceptional surface areas,tunable charge densities,and structural diversities.
Numerous CMFCs have been investigated for efficient NRR,including N-doped porous carbon derived from Metal-Organic Frameworks (MOFs)[18],Bdoped graphene[19],boron carbide (B4C) nanosheet[113],and defect-rich polymeric C3N4[114].For instance,MOF-derived N-doped porous carbons (NPCs) have demonstrated impressive ammonia production rates and energy conversion efficiency (Fig.7a,b)[18],hence outperforming most previously reported electrocatalysts at identical conditions.DFT calculations revealed that pyridinic N and pyrrolic N sites are the active sites for ammonia synthesis on NPC,with very similar maximum reaction free energy.Similarly,Bdoped graphene (BG) with various boron structures has been prepared for catalyzing the reduction of N2molecules (Fig.7c)[19].A resulting BG catalyst,with a 6.2% B-doping level,exhibited an outstanding NH3production rate of 9.8 mg h−1cm−2.DFT calculations indicated that B-doping induces an electron density redistribution,driven by the larger electronegativity difference between carbon and boron atoms,enhancing N2adsorption.Among these B-doped carbon structures,BC3species show the lowest energy barrier for the rate-determining step (N*― NH*),making it highly favorable for electrocatalytic NRR.In another reported work,a boron carbide (B4C)nanosheet has been fabricated as a high-performance nitrogen fixation electrocatalyst at ambient conditions[113],achieving an average NH3formation rate of 26.57 μg h−1mg−1cat.along with an impressive FE of 15.95%,and excellent stability(Fig.7d).According to DFT calculations,the end-on configuration of molecular nitrogen adsorption is a significant factor in optimizing the rate-limiting step,hence promoting the NRR process (Fig.7e)[113].

Fig.7 (a,b) The performance of ammonia production for different N-doped porous carbons[18].Reproduced with permission from American Chemical Society.(c) Structural illustration of B-doped graphene and the corresponding BC3 sites for adsorbing N2[19].Reproduced with permission from Elsevier Inc.(d,e) NH3 yields,FEs and DFT calculations for NRR on the B4C (110) surface[113].Reproduced with permission from Nature Publishing Group.(f,g) NH3 yields of PCN-based catalysts.(h-j) N2 adsorption model and charge density difference on “N” vacancy of PCN.(k) Free energy diagram toward NRR for PCN[114].Reproduced with permission from Wiley-VCH
In addition to doping technologies,defect engineering strategies have been employed to create metalfree catalysts for NRR,exemplified by nitrogen vacancy-based polymeric carbon nitride (PCN-NV)[114].By regulating the degree of nitrogen vacancy,the optimized PCN-NV4 exhibited an outstanding NRR performance,with high NH3yield and FE (Fig.7f,g).DFT calculations supported that nitrogen vacancies in PCN enhance the chemisorb of N2molecules and form a binuclear “end-on” bonded structure,facilitating subsequent electron transfer from nearby carbon atoms.Notably,adsorbed N2experiences a substantial increase in N≡N bond length,further promoting the electrocatalytic reduction of N2into NH3(Fig.7h-k)[114].With these strides in carbon-based metal-free materials and catalytic strategies,the electrocatalytic NRR process holds tremendous promise for NH3production.
In addition to the electrochemical reduction of N2to ammonia,there is a growing focus on nitrate(NO3−) reduction for electrosynthesis of ammonia due to the relatively low dissociation energy of the N=O bond and the increased solubility of nitrate.While current nitrate reduction catalysts predominantly involve metals,alloys and metal oxides,high-performance CMFCs for nitrate-to-ammonia conversion are reported less.Ye’s group recently introduced the preparation of amorphous graphene with a tailorable heterophane,exhibiting a topological structure distinct from crystalline graphene and amorphous carbon.The optimized CMFCs exhibited a high FE (83.7%) for direct conversion of NO3−to NH3[115].Furthermore,their recent findings showed that the amorphous graphene with disordered pentagons,hexagons,and heptagons can catalyze the NO3−reduction to NH3with a FE approaching 100%,achieving an impressive ammonia production rate of 2 859 μg cm−2h−1.In situ Fourier-transform infrared spectroscopy and DFT calculations highlighted the crucial role of amorphous carbon in directing catalytic selectivity[116].The outstanding electrocatalytic performance for NO3− to NH3conversion holds significant promise for the electrosynthesis of ammonia.
4.4 CMFCs for electrosynthesis of other advanced chemicals
In addition to the previously discussed electrosynthesis for common chemicals,CMFCs hold great promise for the electrosynthesis of emerging smallmolecule chemicals such as chlorine,urea,and highvalue-added chemicals.In this section,we also overview recent progress in employing CMFCs as efficient and stable electrode materials for chlorine evolution reaction (CER) and urea electrosynthesis.
The earliest metal-free catalyst for chlor-alkali process was graphite,dating back to 1950[117–118].While carbon-based metal-free nanomaterials exhibited good chemical stability in strong acidic or alkaline media,graphite electrodes often need a high overpotential,leading to a substantial self-oxidation process.The inefficiency and instability have severely limited the practical application lifespan of graphite electrodes.Consequently,substantial efforts have been devoted to developing highly efficient metal-free electrodes for CER in recent years[119].One prominent strategy for creating CMFCs involves heteroatom doping.In 2000,boron-doped diamond electrodes(BDD) demonstrated exceptional stability and higher selectivity compared to pure graphite in dilute chlorine media.Extensive electrochemical characterization studies revealed that a degree of oxidation may contribute to the catalytic activity in BDD-based CMFCs for the CER process.More recently,Wang and colleagues introduced a hollow porous carbon nanocage,investigating the bonding effect between the oxygen atom and carbon topology defect for CER[120].Theoretical calculation indicated that an unsaturated O atom bonded to a low-defective carbon site exhibits the lowest Cl−adsorption,hence benefiting the CER process (Fig.8a).By controlling the oxygen electronic state around designed carbon defects,the resulting CMFC exhibited an outstanding electrocatalytic performance,with a low overpotential of 94 mV at the current density of 10 mA cm−2,surpassing commercial dimensional stable anode and most previously reported electrocatalysts[120].

Fig.8 (a) The Cl adsorption structure at different O sites[120].Reproduced with permission from Wiley-VCH.(b,c) Schematic illustration and performance of urea synthesis on the F-CNT[121].Reproduced with permission from Elsevier Inc.(d) The FE of electrochemical formaldehyde reduction for various metal/metal-free catalysts[123].Reproduced with permission from Nature Publishing Group
The electrocatalytic synthesis of urea has long been considered a promising alternative to traditional energy-consuming industrial processes.CMFCs also exhibit substantial potential for highly selective urea electrosynthesis.Recently,a fluorine-dopant carbon nanotube (F-CNT) was fabricated to synthesize urea from nitrate and CO2under moderate conditions(Fig.8b)[121].The resultant F-CNT achieved a high urea yield rate of 6.36 mmol h−1g−1,nearly 4 times higher than that of pristine CNT-based catalyst(Fig.8c).DFT calculations revealed that the produced “CF2” units could promote the formation of intermediates*COOH and*NH2,thus facilitating the C―N coupling.In a similar study,a porous N-doped CMFC with various types of nitrogen species was controllably synthesized via pyrolysis strategy,leading to a high urea yield rate (up to 610.6 mg h−1gcat−1).The different nitrogen species played a synergistic role,with pyridinic-N and pyrrolic-N inducing the active sites for NO3RR activity,while graphitic-N served as the active centers for CO2RR,suggesting the significant potential of CMFCs in multi-site electrocatalytic processes[122].Most recently,Jiao and co-workers found that CMFCs were effective in electrochemical reduction of formaldehyde into ethylene glycol.Impressively,carbon is the only catalyst that favors the C―C coupling,while most metal-based materials only catalyze the formaldehyde hydrogenation to methanol (Fig.8d)[123].This substantial progress in biomass upgrade electrosynthesis is expected to gain intensive attention and promote the development of electrochemical synthesis toward high-value-added chemicals.
5 Summary and outlook
Over the past decade,remarkable progress has been made in the field of CMFCs,hence making the possibilities for the electrosynthesis of diverse advanced chemicals.However,despite this advancement,the catalytic activity of CMFCs is still inferior to those of metal-based catalysts.Bridging this gap and realizing practical applications for CMFCs require further concerted research efforts.
Among the reported strategies,heteroatom and/or defect-doping have demonstrated obvious advantages in optimizing the catalytic performance of CMFCs.Nevertheless,it remains a great challenge to precisely control the doped content,species,and position at the molecular or atomic level.Additionally,the development of bi-or multi-functional CMFCs poses a significant challenge but is essential for practical multisite/functional electrocatalytic processes.Despite devoted efforts to understand the mechanisms in various electrocatalytic reactions,gaining molecule or atomlevel insight into the interplay between active sites and performance remains a considerable challenge.Therefore,there is an urgent need for advancements in the semi-quantification/quantification and imaging of the structure-activity relationships in CMFCs.Furthermore,greater attention should be focused on designing suitable in-situ experiments combined with advanced characterization techniques (e.g.,X-ray absorption spectra,scanning tunneling microscopy,and atomic force microscopy) to pinpoint the precise location of active sites and provide a visual representation of the catalytic process.It is noteworthy that the combination of theoretical studies and experimental work is crucial for the rational design of high-performance CMFCs.Establishing a feedback loop between theory and experiments plays a pivotal role in systematically understanding the structure,mechanism,and kinetics of the catalytic centers.This comprehensive approach can effectively guide the design and development of high-performance CMFCs for large-scale electrosynthesis and sustainable energy devices.
Declaration of interests
The authors declare no competing interests.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China(22373027,52102325).
杂志排行
新型炭材料的其它文章
- Carbon-based electrocatalysts for water splitting at high-current-densities: A review
- 序
- Defect engineering of carbon-based electrocatalysts for the CO2 reduction reaction: A review
- A review of carbon-based catalysts and catalyst supports for simultaneous organic electro-oxidation and hydrogen evolution reactions
- MOF-derived nanocarbon materials for electrochemical catalysis and their advanced characterization
- 石墨烯基二氧化碳还原电催化材料研究进展
